Abstract
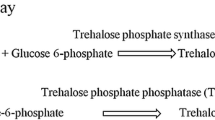
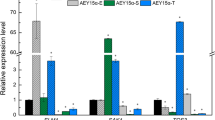
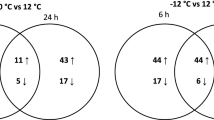
The psychrotolerant yeast Guehomyces pullulans 17-1 grows the best at 15 °C. When the yeast cells grown at 15 °C for 48 h were transferred to new medium and grown at 10, 15, and 25 °C, respectively, trehalose-6-phosphate synthase (Tps1) activity and trehalose content of the yeast cells grown at 25 °C were higher than those of the yeast cells grown at 10 and 15 °C. However, Tps1 activity and trehalose content of the yeast cells grown at 10 °C were lower than those of the yeast cells grown at 15 °C. This may suggest that trehalose synthesized by G. pullulans 17-1 only can play more important role in its adaption to high temperature than in its adaption to low temperature. After the GPTPS1 gene encoding trehalose-6-phosphate synthase was cloned from the psychrotolerant yeast, it was found that the promoter of the gene contained several stress–response elements such as C4T and AG4, indicating that the gene expression might be regulated by heat shock. It was also found that the transcriptional level of the GPTPS1 gene in the yeast cells grown at 25 °C was higher than that of the GPTPS1 gene in the yeast cells grown at 10 and 15 °C. However, the transcriptional level of the GPTPS1 gene in the yeast cells grown at 10 °C was lower than that of the yeast cells grown at 15 °C. This meant that expression of the GPTPS1 gene was constant with the changes in Tps1 activity and trehalose content of the yeast cells.






Similar content being viewed by others
References
Al-Fageeh MB, Smales CM (2006) Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J 397:247–259
Bell W, Klaassen P, Ohnacker M, Boller T, Herweijer M, Schoppink P, Vanderzee P, Wiemken A (1992) Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur J Biochem 209:951–959
Benaroudj N, Lee DH, Goldberg AL (2001) Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J Biol Chem 276:24261–24267
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–253
Cai Z, Peng G, Cao Y, Liu Y, Jin K, Xia Y (2009) Trehalose-6-phosphate synthase 1 from Metarhizium anisopliae: clone, expression and properties of the recombinant. J Biosci Bioeng 107:499–505
Casanueva A, Tuffin M, Cary C, Cowan DA (2010) Molecular adaptations to psychrophily: the impact of ‘omic’ technologies. Trend Microbiol 18:374–381
Chi ZM, Liu J, Ji J, Meng Z (2003) Enhanced conversion of soluble starch to trehalose by a mutant of Saccharomycopsis fibuligera sdu. J Biotechnol 102:135–141
Chi ZM, Liang LK, Zhu KL, Zhang FL (2006) Advances in metabolism and regulation of trehalose in yeasts. J Ocean Univ Chin 26:209–219
Chi ZM, Zhe Chi Z, Liu GL, Wang F, Ju L, Zhang T (2009) Saccharomycopsis fibuligera and its applications in biotechnology. Biotechnol Adv 27:423–431
Deegenaars ML, Watson K (1998) Heat shock response in psychrophilic and psycrotrophic yeast from Antarctica. Extremophiles 2:41–49
Gerday C (2000) Cold-adapted enzymes: from fundamentals to biotechnology. TBTECH 18:103–107
Herdeiro RS, Pereira MD, Panek AD, Eleutherio ECA (2006) Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim Biophys Acta 1760:340–346
Hottiger T, Schmutz P, Wiemken A (1987) Heat-induced accumulation and futile cycling of trehalose in Saccharomyces cerevisiae. J Bacteriol 169:5518–5522
Hua MX, Chi Z, Liu GL, Buzdar MA, Chi ZM (2010) Production of a novel and cold-active killer toxin by Mrakia frigida 2E00797 isolated from sea sediment in Antarctica. Extremophiles 14:515–521
Kobayashi N, McEntee K (1993) Identification of cis and trans components of a novel heat shock stress regulatory pathway in Saccharomyces cerevisiae. Mol Cell Biol 13:248–256
Kwon HB, Yeo ET, Hahn SE, Bae SC, Kim DY, Byun MO (2003) Cloning and characterization of genes encoding trehalose-6-phosphate synthase (TPS1) and trehalose-6-phosphate phosphatase (TPS2) from Zygosaccharomyces rouxii. FEMS Yeast Res 3:433–440
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Magan M (2007) Fungi in extreme environments. In: The Mycota IV Kubicek CP and Druzhinina IS (eds) Environmental and microbial relationships, 2nd edn. Springer, Berlin, pp 85–103
Murata Y, Homma T, Kitagawa E, Momose Y, Sato MS, Odani M, Shimizu H, Hasegawa-Mizusawa M, Matsumoto R, Mizukami S, Fujita K, Parveen M, Komatsu Y, Iwahashi H (2006) Genome-wide expression analysis of yeast response during exposure to 4 °C. Extremophiles 10:117–128
Rossi M, Buzzini P, Cordisco L, Amaretti A, Sala M, Raimondi S, Ponzoni C, Pagnoni UM, Matteuzzi D (2009) Growth, lipid accumulation, and fatty acid composition in obligate psychrophilic, facultative psychrophilic, and mesophilic yeasts. FEMS Microbiol Ecol 69:363–372
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Beijing, pp 367–370 (Chinese translated edn)
Schick I, Haltrich D, Kulbe KD (1995) Trehalose phosphorylase from Pichia fermentans and its role in the metabolism of trehalose. Appl Microbiol Biotechnol 43:1088–1095
Song CL, Chi ZM, Li J, Wang XH (2010a) β-Galactosidase production by the psychrotolerant yeast Guehomyces pullulans 17-1 isolated from sea sediment in Antarctica and lactose hydrolysis. Bioprocess Biosyst Eng 33:1025–1031
Song CL, Liu GL, Xu JL, Zhen-Ming Chi ZM (2010b) Purification and characterization of extracellular β-galactosidase from the psychrotolerant yeast Guehomyces pullulans 17-1 isolated from sea sediment in Antarctica. Proc Biochem 45:954–960
Stewart PR (1982) In: Prescott DM (ed) Methods in cell biology, vol 12. Academic Press, London and New York, pp 111–147
Tibbet M, Sanders FE, Cairney JWG (1998) The effect of temperature and inorganic phosphorous supply on growth and acid phosphatase production in arctic and temperate strains of ectomycorrhizal Hebeloma spp. Mycol Res 102:129–135
Walker GM, Dijck PV (2006) Physiological and molecular responses of yeasts to the environment. In: Graham H. Fleet (eds) The Yeast Handbook Amparo Querol, Yeasts in food and beverages © Springer, Berlin Heidelberg, pp 111–152
Weinstein RN, Palm ME, Johnstone K, Wynn-Williams DD (1997) Ecological and physiological characterization of Humicola marvinii, a new psychrophilic fungus from fellfields in the maritime Antarctic. Mycologia 89:706–711
Weinstein RN, Montiel PO, Johnstone K (2000) Influence of growth temperature on lipid and soluble carbohydrate synthesis by fungi isolated from fellfield soil in the maritime Antarctic. Mycologia 92:222–229
Wolschek MF, Kubicek CP (1997) The filamentous fungus Aspergillus niger contains two “differentially regulated” trehalose-6-phosphate synthase-encoding genes, tpsA and tpsB. J Biol Chem 272:2729–2735
Wu JCF, Hamada M (2000) Experiments planning analysis and parameter design optimization. Wiley, New York, pp 23–34
Zaragoza O, Blazquez MA, Gancedo C (1998) Disruption of the Candida albicans TPS1 Gene encoding trehalose-6-phosphate synthase impairs formation of hyphae and decreases infectivity. J Bacteriol 180:3809–3815
Acknowledgments
The authors would like to thank the State Oceanic Administration People’s Republic of China for providing financial support to carry out this work. The grant number is 201005032.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Driessen.
Rights and permissions
About this article
Cite this article
Zhang, F., Wang, ZP., Chi, Z. et al. The changes in Tps1 activity, trehalose content and expression of TPS1 gene in the psychrotolerant yeast Guehomyces pullulans 17-1 grown at different temperatures. Extremophiles 17, 241–249 (2013). https://doi.org/10.1007/s00792-013-0511-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0511-2