Abstract
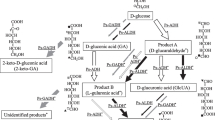
During a screening for novel microbial trehalose phosphorylase three Pichia strains were identified as producers of this particular enzyme that have not yet been described. To our knowledge, this is the first time that this enzyme activity has been shown in yeasts. Pichia fermentans formed trehalose phosphorylase when cultivated on a growth medium containing easily metabolizable sugers such as glucose. Addition of NaCl (0.4 M) to the medium increased the synthesis of the enzyme significantly. Production of trehalose phosphorylase was found to be growth-associated with a maximum of activity formed at the transition of the exponential to the stationary phase of growth. Trehalose phosphorylase catalyzes the phosphorolytic cleavage of trehalose, yielding glucose 1-phosphate (glucose-1-P) and glucose as products. In vitro the enzyme readily catalyzes the reverse reaction, the synthesis of trehalose from glucose and glucose-1-P. For this reaction, the enzyme of P. fermentans was found to utilize α-glucose-1-P preferentially. A partially purified enzyme preparation showed a pH optimum of 6.3 for the synthesis of trehalose. The enzyme was found to be rather unstable; it was easily inactivated by dilution unless Ca2+ or Mn2+ were added. This instability is presumably caused by dissociation of the enzyme. In contrast to other yeasts, P. fermentans rapidly degraded intracellularly accumulated trehalose when the carbon source in the medium was depleted. Trehalose phosphorylase seems to be a key enzyme in the degradative pathway of trehalose in P. fermentans. Additional enzymes in this catabolic pathway of trehalose include phosphoglucomutase, glucose-6-phosphate dehydrogenase, and gluconolactonase.
Similar content being viewed by others
References
Belocopitow E, Maréchal LR (1970) Trehalose phosphorylase from Euglena gracilis. Biochim Biophys Acta 198:151–154
Blumenthal HL (1976) Reserve carbohydrates in fungi. In: Smith JE, Berry DR (eds) The filamentous fungi, vol 2. Edward Arnold, London, pp 292–307
Booth IR, Higgins CF (1990) Enteric bacteria and osmotic stress: intracellular potassium glutamate as a secondary signal of osmotic stress. FEMS Microbiol Rev 75:239–246
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Chandrasekar I, Graber BP (1988) Stabilization of the biomembrane by small molecules: interaction of trehalose with the phospholipid bilayer. J Biomol Struct Dvn 5:1163–1171
Crowe LM, Mouradian R, Crowe JH, Jackson SA, Womersley C (1984) Effect of carbohydrates on membrane stability at low water activities. Biochim Biophys Acta 769: 141–150
Dellweg H, Schmid RD, Trommer WE (1992) Römpp Lexikon Biotechnologie. Thieme, Stuttgart New York, pp 782
Deutsche Sammlung von Mikroorganismen und Zellkulturen (1989) Catalogue of strains
Elbein AD (1974) The metabolism of α,α-trehalose. Adv Carbohydr Chem Biochem 30:227–256
Fischer EH, Pocker A, Saari JC (1970) The structure, function and control of glycogen phosphorylase. In: Campbell PN, Dickens F (eds) Essays in biochemistry, vol 6. Academic Press, New York London, pp 23–68
Fosset M, Muir LW, Nielsen LD, Fischer EH (1971) Purification and properties of yeast glycogen phosphorylase a and b. Biochemistry 10:4105–4113
Fukui T, Shimomura S, Nakano K (1982) Potato and rabbit muscle phosphorylases: comparative studies on the structure, function and regulation of regulatory and nonregulatory enzymes. Mol Cell Biochem 42:129–144
Gadd GM, Chalmers K, Reed RH (1987) The role of trehalose in dehydration resistance of Saccharomyces cerevisiae. FEMS Microbiol Lett 48:249–254
Gélinas P, Fiset G, LeDuy A, Goulet J (1989) Effect of growth conditions and trehalose content on cryotolerance of baker's yeast in frozen doughs. Appl Environ Microbiol 55:2453–2459
Guibert A, Monsan P (1988) Production and purification of sucrose phosphorylase from Leuconostoc mesenteroides. Ann NY Acad Sci 542:307–311
Haug I (1991) Glucose-Fructose-Oxidoreduktase und Gluconolactonase für die simultane enzymatische Synthese von Gluconsäure und Sorbitol. PhD Thesis Universität Hohenheim, Germany
Haynie SL, Whitesides GM (1990) Enzyme-catalyzed organic synthesis of sucrose and trehalose with in situ regeneration of UDP-glucose. Appl Biochem Biotechnol 23:155–170
Heinzler A, Haug I, Lutz S, Scholze HA, Stolz P, Wiesner W, Kulbe KD (1991) Enzymatic synthesis of polyhydroxy alcohols by new NAD(P)H-dependent microbial enzymes. In: Reuss M, Chmiel H, Gilles E-D, Knackmuss H-J (eds) Biochemical engineering-Stuttgart. Fischer, Stuttgart New York, pp 158–161
Hottinger T, Boller T, Wiemken A (1987) Rapid changes of heat and desiccation tolerance correlated with changes of trehalose content in Saccharomyces cerevisiae cells subjected to temperature shifts. FEBS Lett 220:113–115
Kitamoto Y, Akashi H, Tanaka H, Mori N (1988) α-Glucose-1-phosphate formation by a novel trehalose phosphorylase from Flammulina velutipes. FEMS Microbiol Lett 55:147–150
Labat-Robert J (1982) Trehalases. Dev Food Carbohydr 3:81–106
Maréchal LR, Belocopitow E (1972) Metabolism of trehalose in Euglena gracilis. J Biol Chem 247:3223–3228
Meikle AJ, Chudek JA, Reed RH, Gadd GM (1991) Natural abundance 13C-nuclear magnetic resonance spectroscopic analysis of acyclic polyol and trehalose accumalation by several yeast species in response to salt stress. FEMS Microbiol Lett 82:163–168
Murao S, Nagano H, Ogura S, Nishino T (1985) Enzymatic synthesis of trehalose from maltose. Agric Biol Chem 49:2113–2118
Panek AD, Panek AC (1990) Metabolism and thermotolerance function of trehalose in Saccharomyces: a current perspective. J Biotechnol 14:229–238
Piper PW (1993) Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 11:339–356
Roser B (1991) Trehalose, a new approach to premium dried foods. Trends Food Sci Technol 2:166–169
Salminen SO, Streeter JG (1986) Enzymes of α,α-trehalose metabolism in soybean nodules. Plant Physiol 81:538–541
Sasaki T, Tanaka T, Nakagawa S, Kainuma K (1983) Purification and properties of Cellvibrio gilvus cellobiose phosphorylase. Biochem J 209:803–807
Schick I, Fleckenstein J, Weber H, Kulbe KD (1991) Coenzyme-independent enzymatic synthesis of α,α-trehalose. In: Reuss M, Chmiel H, Gilles E-D, Knackmuss H-J (eds) Biochemical Engineering-Stuttgart. Fischer, Stuttgart New York, pp 126–129
Shimomura S, Nagai M, Fukui T (1982) Comparative glucan specificities of two types of spinach leaf phosphorylase. J Biochem (Tokyo) 91:703–717
Tagaya M, Shimomura S, Nakano K, Fukui T (1982) A monomeric intermediate in the reconstitution of potato apophosphorylase with pyridoxal 5′-phosphate. J Biochem (Tokyo) 91:589–597
Tanabe S, Kobayashi M, Matsuda K (1987) Yeast glycogen phosphorylase: characterization of the dimeric form and its activation. Agric Biol Chem 51:2465–2471
Thevelein JM (1984) Regulation of trehalose mobilization in fungi. Microbiol Rev 48:42–59
Tu J, Jacobson GR, Graves DJ (1971) Isotopic effects and inhibition of polysaccharide phosphorylase by 1,5-gluconolactone. Relationship to the catalytic mechanism. Biochemistry 10:1229–1236
Vandamme EJ, Van Loo J, Machtelinckx L, De Laporte A (1987) Microbial sucrose phosphorylase: fermentation process, properties, and biotechnical applications. Adv Appl Microbiol 32:163–201
Van Laere A (1989) Trehalose, reserve and/or stress metabolite? FEMS Microbiol Rev 63:201–210
Weinhäusel A, Nidetzky B, Rohrbach M, Blauensteiner B, Kulbe KD (1994) A new maltodextrin-phosphorylase from Corynebacterium callunae for the production of glucose-1-phosphate. Appl Microbiol Biotechnol 41:510–516
Wiemken A (1990) Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie van Leeuwenhoek 58:209–217
Author information
Authors and Affiliations
Additional information
This contribution is part of the Ph.D. thesis of Ingrid Schick
Rights and permissions
About this article
Cite this article
Schick, I., Haltrich, D. & Kulbe, K.D. Trehalose phosphorylase from Pichia fermentans and its role in the metabolism of trehalose. Appl Microbiol Biotechnol 43, 1088–1095 (1995). https://doi.org/10.1007/BF00166930
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00166930