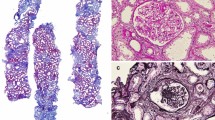
Abstract
Fabry disease (FD) is a rare X-linked lysosomal storage disorder of glycosphingolipids, mostly globotriaosylceramide (Gb3). Proteinuric chronic kidney disease develops frequently, and recognition of Fabry nephropathy on a kidney biopsy may be the first clue to the underlying diagnosis. Since the accumulated glycosphingolipids are largely extracted by the paraffin-embedding procedure, the most characteristic feature of Fabry nephropathy on routine light microscopy (LM) is nonspecific cell vacuolization. To test whether residual Gb3 in kidney tissue might be exploited for the specific diagnosis of Fabry nephropathy, paraffin-embedded kidney biopsies of nine FD patients (one boy, four men, four women) and of a female carrier of a mild genetic mutation, with no evidence of Fabry nephropathy, were immunostained with an anti-Gb3 antibody. The adult biopsies were additionally co-stained with a lysosomal marker (anti-lysosomal-associated membrane protein 2 (anti-LAMP2) antibody). The distribution of Gb3 deposits was scored per cell type and compared to the histological scorings of glycosphingolipid inclusions on semi-thin sections. FD patients had residual Gb3 in all types of glomerular, tubular, interstitial and vascular kidney cells. The highest expression of LAMP2 was seen in tubular cells, but there were no meaningful associations between LAMP2 expression and prevalence of Gb3 deposits on different kidney cell types. The histological scorings of glycosphingolipid inclusions were relatively higher than the corresponding immunohistochemical scorings of Gb3 deposits. In the mildly affected female, Gb3 expression was limited to tubular cells, a pattern similar to controls. Gb3 immunostaining allows the specific diagnosis of Fabry nephropathy even in kidney biopsies routinely processed for LM.




Similar content being viewed by others
References
Desnick RJ, Ioannou YA, Eng CM (2001) α-Galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet AL, Sly WS et al (eds) The metabolic and molecular bases of inherited disease, 8th edn. McGraw Hill, New York, pp 3733–3774
Zarate YA, Hopkin RJ (2008) Fabry’s disease. Lancet 372:1427–1435
Wilcox WR, Oliveira JP, Hopkin RJ et al (2008) Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab 93:112–128
Scriba K (1950) Zur Pathogenese des Angiokeratoma corporis diffusum Fabry mit cardio vasorenalem symptomenkomplex. Verhandlg Deutsch Path GesellschVerhandlg Deutsch Path Gesellsch 34:221–226
Henry EW, Rally CR (1963) The renal lesion in angiokeratoma corporis diffusum (Fabry’s disease). Canad Med Ass J 89:206–213
Gubler MC, Lenoir G, Grunfeld JP et al (1978) Early renal changes in hemizygous and heterozygous patients with Fabry’s disease. Kidney Int 13:223–235
Faraggiana T, Churg J, Grishman E et al (1981) Light- and electron-microscopic histochemistry of Fabry’s disease. Am J Pathol 103:247–262
Finn SL, Bernstein J (2006) Renal disease caused by familial metabolic and hematologic disease. In: Jennette JC, Olson JL, Schwartz MM, Silva FG (eds) Heptinstall’s pathology of the kidney, 6th edn. Lippincott Williams and Wilkins, Philadelphia, pp 1200–1256
Gubler MC, Heidet L, Antignac C (2006) Alport’s syndrome, thin basement membrane nephropathy, Nail–Patella syndrome, and type III collagen glomerulopathy. In: Jennette JC, Olson JL, Schwartz MM, Silva FG (eds) Heptinstall’s pathology of the kidney, 6th edn. Lippincott Williams and Wilkins, Philadelphia, pp 488–517
Olson JL (2006) The nephrotic syndrome and minimal change disease. In: Jennette JC, Olson JL, Schwartz MM, Silva FG (eds) Heptinstall’s pathology of the kidney, 6th edn. Lippincott Williams and Wilkins, Philadelphia, pp 126–155
Sessa A, Toson A, Nebuloni M et al (2002) Renal ultrastructural findings in Anderson–Fabry disease. J Nephrol 15:109–112
Savi M, Olivetti G, Neri TM et al (1977) Clinical, histopathological, and biochemical findings in Fabry’s disease. A case report and family study. Arch Pathol Lab Med 101:536–539
Kotani M, Kawashima I, Ozawa H et al (1994) Generation of one set of murine monoclonal antibodies specific for globo-series glycolipids: evidence for differential distribution of the glycolipids in rat small intestine. Arch Biochem Biophys 310:89–96
Fukushima M, Tsuchiyama Y, Nakato T et al (1995) A female heterozygous patient with Fabry’s disease with renal accumulation of trihexosylceramide detected with a monoclonal antibody. Am J Kidney Dis 26:952–955
Askari H, Kaneski CR, Semino-Mora C et al (2007) Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch 451:823–834
Valbuena C, Carvalho E, Bustorff M et al (2008) Kidney biopsy findings in heterozygous Fabry disease females with early nephropathy. Virchows Arch 453:329–338
Alroy J, Sabnis S, Kopp JB (2002) Renal pathology in Fabry disease. J Am Soc Nephrol 13(Suppl 2):S134–S138
Fischer EG, Moore MJ, Lager DJ (2006) Fabry disease: a morphologic study of 11 cases. Mod Pathol 19:1295–1301
Fogo AB, Bostad L, Svarstad E et al (2010) Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant 25:2168–2177
Sessa A, Meroni M, Battini G et al (2003) Renal involvement in Anderson–Fabry disease. J Nephrol 16:310–313
Sweeley CC, Klionsky B (1963) Fabry’s disease: classification as a sphingolipidosis and partial characterization of a novel glycolipid. J Biol Chem 238:3148–3150
Oliveira JP, Valbuena C, Baldaia Moreira A et al (2008) Splenomegaly, hypersplenism and peripheral blood cytopaenias in patients with classical Anderson–Fabry disease. Virchows Arch 453:291–300
Kanekura T, Fukushige T, Kanda A et al (2005) Immunoelectron-microscopic detection of globotriaosylceramide accumulated in the skin of patients with Fabry disease. Br J Dermatol 153:544–548
Cohen A, Hannigan GE, Williams BR et al (1987) Roles of globotriosyl- and galabiosylceramide in verotoxin binding and high affinity interferon receptor. J Biol Chem 262:17088–17091
Ergonul Z, Clayton F, Fogo AB et al (2003) Shigatoxin-1 binding and receptor expression in human kidneys do not change with age. Pediatr Nephrol 18:246–253
Hughes AK, Stricklett PK, Kohan DE (1998) Cytotoxic effect of Shiga toxin-1 on human proximal tubule cells. Kidney Int 54:426–437
Hughes AK, Stricklett PK, Schmid D et al (2000) Cytotoxic effect of Shiga toxin-1 on human glomerular epithelial cells. Kidney Int 57:2350–2359
Taguchi T, Uchida H, Kiyokawa N et al (1998) Verotoxins induce apoptosis in human renal tubular epithelium derived cells. Kidney Int 53:1681–1688
Migeon BR (2006) The role of X inactivation and cellular mosaicism in women’s health and sex-specific diseases. JAMA 295:1428–1433
Novelli M, Cossu A, Oukrif D et al (2003) X-inactivation patch size in human female tissue confounds the assessment of tumor clonality. Proc Natl Acad Sci USA 100:3311–3314
Elleder M (2008) Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch 452(705):707–708
Acknowledgements
We thank David Warnock, M.D. for critically reviewing and commenting the protocol and results of this study and Hans Ebels, M.D. for the invaluable assistance in the text editing of the manuscript.
Conflict of interest statement
Carmen Valbuena was a recipient of a non-restricted research grant from Genzyme Portugal. All authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00428-012-1196-0.
Rights and permissions
About this article
Cite this article
Valbuena, C., Leitão, D., Carneiro, F. et al. Immunohistochemical diagnosis of Fabry nephropathy and localisation of globotriaosylceramide deposits in paraffin-embedded kidney tissue sections. Virchows Arch 460, 211–221 (2012). https://doi.org/10.1007/s00428-011-1182-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-011-1182-y