Abstract
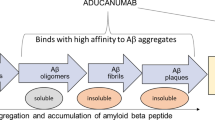
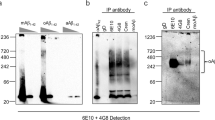
Solanezumab and Crenezumab are two humanized antibodies targeting Amyloid-β (Aβ) which are currently tested in multiple clinical trials for the prevention of Alzheimer’s disease. However, there is a scientific discussion ongoing about the target engagement of these antibodies. Here, we report the immunohistochemical staining profiles of biosimilar antibodies of Solanezumab, Crenezumab and Bapineuzumab in human formalin-fixed, paraffin-embedded tissue and human fresh frozen tissue. Furthermore, we performed a direct comparative immunohistochemistry analysis of the biosimilar versions of the humanized antibodies in different mouse models including 5XFAD, Tg4-42, TBA42, APP/PS1KI, 3xTg. The staining pattern with these humanized antibodies revealed a surprisingly similar profile. All three antibodies detected plaques, cerebral amyloid angiopathy and intraneuronal Aβ in a similar fashion. Remarkably, Solanezumab showed a strong binding affinity to plaques. We also reaffirmed that Bapineuzumab does not recognize N-truncated or modified Aβ, while Solanezumab and Crenezumab do detect N-terminally modified Aβ peptides Aβ4–42 and pyroglutamate Aβ3–42. In addition, we compared the results with the staining pattern of the mouse NT4X antibody that recognizes specifically Aβ4–42 and pyroglutamate Aβ3–42, but not full-length Aβ1–42. In contrast to the biosimilar antibodies of Solanezumab, Crenezumab and Bapineuzumab, the murine NT4X antibody shows a unique target engagement. NT4X does barely cross-react with amyloid plaques in human tissue. It does, however, detect cerebral amyloid angiopathy in human tissue. In Alzheimer mouse models, NT4X detects intraneuronal Aβ and plaques comparable to the humanized antibodies. In conclusion, the biosimilar antibodies Solanezumab, Crenezumab and Bapineuzumab strongly react with amyloid plaques, which are in contrast to the NT4X antibody that hardly recognizes plaques in human tissue. Therefore, NT4X is the first of a new class of therapeutic antibodies.









Similar content being viewed by others
Change history
25 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00401-024-02718-w
References
https://clinicaltrials.gov. Accessed 1 July 2015
Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J, Chen M, Gogineni A, Weimer RM, Mortensen DL, Friesenhahn M, Ho C, Paul R, Pfeifer A, Muhs A, Watts RJ (2012) An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci 32(28):9677–9689. doi:10.1523/JNEUROSCI.4742-11.2012
Alexandru A, Jagla W, Graubner S, Becker A, Bäuscher C, Kohlmann S, Sedlmeier R, Raber KA, Cynis H, Rönicke R, Reymann KG, Petrasch-Parwez E, Hartlage-Rübsamen M, Waniek A, Rossner S, Schilling S, Osmand AP, Demuth H, von Hörsten S (2011) Selective hippocampal neurodegeneration in transgenic mice expressing small amounts of truncated Aβ is induced by pyroglutamate-Aβ formation. J Neurosci 31(36):12790–12801. doi:10.1523/JNEUROSCI.1794-11.2011
Antonios G, Saiepour N, Bouter Y, Richard BC, Paetau A, Verkkoniemi-Ahola A, Lannfelt L, Ingelsson M, Kovacs GG, Pillot T, Wirths O, Bayer TA (2013) N-truncated Abeta starting with position four: early intraneuronal accumulation and rescue of toxicity using NT4X-167, a novel monoclonal antibody. Acta Neuropathol Commun 1(1):56. doi:10.1186/2051-5960-1-56
Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T (2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6(8):916–919. doi:10.1038/78682
Basi G, Jacobsen JS (2009) Humanized antibodies that recognize beta amyloid peptide. Google Patents. http://www.google.com/patents/US7625560
Bayer TA, Wirths O (2014) Focusing the amyloid cascade hypothesis on N-truncated Abeta peptides as drug targets against Alzheimer’s disease. Acta Neuropathol. doi:10.1007/s00401-014-1287-x
Benilova I, Karran E, DeStrooper B (2012) The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci 15(3):349–357. doi:10.1038/nn.3028
Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, Black R, Grundman M, Liu E (2012) Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol 69(8):1002–1010. doi:10.1001/archneurol.2012.90
Bouter Y, Dietrich K, Wittnam JL, Rezaei-Ghaleh N, Pillot T, Papot-Couturier S, Lefebvre T, Sprenger F, Wirths O, Zweckstetter M, Bayer TA (2013) N-truncated amyloid β (Aβ) 4–42 forms stable aggregates and induces acute and long-lasting behavioral deficits. Acta Neuropathol 126(2):189–205. doi:10.1007/s00401-013-1129-2
Buttini M, Masliah E, Barbour R, Grajeda H, Motter R, Johnson-Wood K, Khan K, Seubert P, Freedman S, Schenk D, Games D (2005) Beta-amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer’s disease. J Neurosci 25(40):9096–9101. doi:10.1523/JNEUROSCI.1697-05.2005
Casas C, Sergeant N, Itier J, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L (2004) Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol 165(4):1289–1300
Christensen DZ, Kraus SL, Flohr A, Cotel M, Wirths O, Bayer TA (2008) Transient intraneuronal A beta rather than extracellular plaque pathology correlates with neuron loss in the frontal cortex of APP/PS1KI mice. Acta Neuropathol 116(6):647–655. doi:10.1007/s00401-008-0451-6
Crespi Gabriela A N, Hermans SJ, Parker MW, Miles LA (2015) Molecular basis for mid-region amyloid-β capture by leading Alzheimer’s disease immunotherapies. Sci Rep 5:9649. doi:10.1038/srep09649
Das P (2001) Reduced effectiveness of AÎ21-42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging 22(5):721–727. doi:10.1016/S0197-4580(01)00245-7
DeMattos RB, Bales KR, Cummins DJ, Dodart J, Paul SM, Holtzman DM (2001) Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 98(15):8850–8855. doi:10.1073/pnas.151261398
DeMattos RB, Lu J, Tang Y, Racke MM, Delong CA, Tzaferis JA, Hole JT, Forster BM, McDonnell PC, Liu F, Kinley RD, Jordan WH, Hutton ML (2012) A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron 76(5):908–920. doi:10.1016/j.neuron.2012.10.029
Dodart J, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM (2002) Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat Neurosci 5(5):452–457. doi:10.1038/nn842
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R (2014) Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370(4):311–321. doi:10.1056/NEJMoa1312889
Feinberg H, Saldanha JW, Diep L, Goel A, Widom A, Veldman GM, Weis WI, Schenk D, Basi GS (2014) Crystal structure reveals conservation of amyloid-β conformation recognized by 3D6 following humanization to bapineuzumab. Alzheimers Res Ther 6(3):31. doi:10.1186/alzrt261
Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M (2005) Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 64(9):1563–1572. doi:10.1212/01.WNL.0000159743.08996.99
Frost JL, Le KX, Cynis H, Ekpo E, Kleinschmidt M, Palmour RM, Ervin FR, Snigdha S, Cotman CW, Saido TC, Vassar RJ, St George-Hyslop P, Ikezu T, Schilling S, Demuth H, Lemere CA (2013) Pyroglutamate-3 amyloid-beta deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am J Pathol 183(2):369–381. doi:10.1016/j.ajpath.2013.05.005
Frost JL, Liu B, Kleinschmidt M, Schilling S, Demuth H, Lemere CA (2012) Passive immunization against pyroglutamate-3 amyloid-β reduces plaque burden in Alzheimer-like transgenic mice: a pilot study. Neurodegener Dis 10(1–4):265–270. doi:10.1159/000335913
Garber K (2012) Genentech’s Alzheimer’s antibody trial to study disease prevention. Nat Biotechnol 30(8):731–732. doi:10.1038/nbt0812-731
Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR (2000) Intraneuronal Abeta42 accumulation in human brain. Am J Pathol 156(1):15–20
Gouras GK, Willén K, Tampellini D (2012) Critical role of intraneuronal Aβ in Alzheimer’s disease: technical challenges in studying intracellular Aβ. Life Sci 91(23–24):1153–1158. doi:10.1016/j.lfs.2012.06.004
Grundke-Iqbal I, Iqbal K, George L, Tung YC, Kim KS, Wisniewski HM (1989) Amyloid protein and neurofibrillary tangles coexist in the same neuron in Alzheimer disease. Proc Natl Acad Sci USA 86(8):2853–2857
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8(2):101–112. doi:10.1038/nrm2101
Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12(10):383–388
Imbimbo BP, Ottonello S, Frisardi V, Solfrizzi V, Greco A, Seripa D, Pilotto A, Panza F (2012) Solanezumab for the treatment of mild-to-moderate Alzheimer’s disease. Expert Rev Clin Immunol 8(2):135–149. doi:10.1586/eci.11.93
Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D (2000) A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature 408(6815):979–982. doi:10.1038/35050110
Jawhar S, Trawicka A, Jenneckens C, Bayer TA, Wirths O (2010) Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2010.05.027
Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH (2002) Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci 22(15):6331–6335
Kummer MP, Heneka MT (2014) Truncated and modified amyloid-beta species. Alzheimers Res Ther 6(3):28. doi:10.1186/alzrt258
Kuo YM, Kokjohn TA, Beach TG, Sue LI, Brune D, Lopez JC, Kalback WM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Roher AE (2001) Comparative analysis of amyloid-beta chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer’s disease brains. J Biol Chem 276(16):12991–12998. doi:10.1074/jbc.M007859200
Lannfelt L, Möller C, Basun H, Osswald G, Sehlin D, Satlin A, Logovinsky V, Gellerfors P (2014) Perspectives on future Alzheimer therapies: amyloid-β protofibrils—a new target for immunotherapy with BAN2401 in Alzheimer’s disease. Alzheimers Res Ther 6(2):16. doi:10.1186/alzrt246
Legleiter J, Czilli DL, Gitter B, DeMattos RB, Holtzman DM, Kowalewski T (2004) Effect of different anti-Abeta antibodies on Abeta fibrillogenesis as assessed by atomic force microscopy. J Mol Biol 335(4):997–1006
Lemere CA (2013) Immunotherapy for Alzheimer’s disease: hoops and hurdles. Mol Neurodegener 8:36. doi:10.1186/1750-1326-8-36
Lesné SE, Sherman MA, Grant M, Kuskowski M, Schneider JA, Bennett DA, Ashe KH (2013) Brain amyloid-β oligomers in ageing and Alzheimer’s disease. Brain 136(Pt 5):1383–1398. doi:10.1093/brain/awt062
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82(12):4245–4249
McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MHL, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HTJ, Przybylski M, St George-Hyslop P (2002) Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med 8(11):1263–1269. doi:10.1038/nm790
McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL (1999) Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann. Neurol 46(6):860–866
Meissner JN, Bouter Y, Bayer TA (2014) Neuron loss and behavioral deficits in the TBA42 mouse model expressing N-truncated pyroglutamate amyloid-beta3–42. J Alzheimers Dis. doi:10.3233/JAD-142868
Miles LA, Crespi Gabriela A N, Doughty L, Parker MW (2013) Bapineuzumab captures the N-terminus of the Alzheimer’s disease amyloid-beta peptide in a helical conformation. Sci Rep 3:1302. doi:10.1038/srep01302
Moechars D, Dewachter I, Lorent K, Reversé D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, van Leuven F (1999) Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem 274(10):6483–6492
Moreth J, Mavoungou C, Schindowski K (2013) Passive anti-amyloid immunotherapy in Alzheimer’s disease: what are the most promising targets? Immun Ageing 10(1):18. doi:10.1186/1742-4933-10-18
Näslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283(12):1571–1577
Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, van Eldik L, Berry R, Vassar R (2006) Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J Neurosci 26(40):10129–10140. doi:10.1523/JNEUROSCI.1202-06.2006
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39(3):409–421
Orgogozo J, Gilman S, Dartigues J, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C (2003) Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 61(1):46–54
Panza F, Logroscino G, Imbimbo BP, Solfrizzi V (2014) Is there still any hope for amyloid-based immunotherapy for Alzheimer’s disease? Curr Opin Psychiatry 27(2):128–137. doi:10.1097/YCO.0000000000000041
Pike CJ, Overman MJ, Cotman CW (1995) Amino-terminal deletions enhance aggregation of beta-amyloid peptides in vitro. J Biol Chem 270(41):23895–23898
Pimplikar SW (2009) Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int J Biochem Cell Biol 41(6):1261–1268. doi:10.1016/j.biocel.2008.12.015
Price JL, Morris JC (1999) Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 45(3):358–368
Prins ND, Scheltens P (2013) Treating Alzheimer’s disease with monoclonal antibodies: current status and outlook for the future. Alzheimers Res Ther 5(6):56. doi:10.1186/alzrt220
Ravid R, Swaab DF (1993) The Netherlands brain bank—a clinico-pathological link in aging and dementia research. J Neural Transm Suppl 39:143–153
Reisberg B, Ferris SH, de Leon MJ, Crook T (1982) The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 139(9):1136–1139
Richard BC, Kurdakova A, Baches S, Bayer TA, Weggen S, Wirths O (2015) Gene dosage dependent aggravation of the neurological phenotype in the 5XFAD Mouse Model of Alzheimer’s disease. J Alzheimers Dis. doi:10.3233/JAD-143120
Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, Mathis CA, Blennow K, Barakos J, Okello AA, Rodriguez Martinez de Liano S, Liu E, Koller M, Gregg KM, Schenk D, Black R, Grundman M (2010) 11C-PiB PET assessment of change in fibrillar amyloid-β load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 9(4):363–372. doi:10.1016/S1474-4422(10)70043-0
Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR (2014) Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370(4):322–333. doi:10.1056/NEJMoa1304839
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400(6740):173–177. doi:10.1038/22124
Schmitz C, Rutten BPF, Pielen A, Schäfer S, Wirths O, Tremp G, Czech C, Blanchard V, Multhaup G, Rezaie P, Korr H, Steinbusch HWM, Pradier L, Bayer TA (2004) Hippocampal neuron loss exceeds amyloid plaque load in a transgenic mouse model of Alzheimer’s disease. Am J Pathol 164(4):1495–1502. doi:10.1016/S0002-9440(10)63235-X
Selkoe DJ (2011) Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med 17(9):1060–1065. doi:10.1038/nm.2460
Sergeant N, Bombois S, Ghestem A, Drobecq H, Kostanjevecki V, Missiaen C, Wattez A, David J, Vanmechelen E, Sergheraert C, Delacourte A (2003) Truncated beta-amyloid peptide species in pre-clinical Alzheimer’s disease as new targets for the vaccination approach. J Neurochem 85(6):1581–1591
Seubert P, Barbour R, Khan K, Motter R, Tang P, Kholodenko D, Kling K, Schenk D, Johnson-Wood K, Schroeter S, Gill D, Jacobsen JS, Pangalos M, Basi G, Games D (2008) Antibody capture of soluble Abeta does not reduce cortical Abeta amyloidosis in the PDAPP mouse. Neurodegener Dis 5(2):65–71. doi:10.1159/000112834
Solomon B, Koppel R, Frankel D, Hanan-Aharon E (1997) Disaggregation of Alzheimer beta-amyloid by site-directed mAb. Proc Natl Acad Sci USA 94(8):4109–4112
Solomon B, Koppel R, Hanan E, Katzav T (1996) Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer beta-amyloid peptide. Proc Natl Acad Sci USA 93(1):452–455
Spencer B, Masliah E (2014) Immunotherapy for Alzheimer’s disease: past, present and future. Front Aging Neurosci 6. doi:10.3389/fnagi.2014.00114
Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Lieberburg I, Arrighi HM, Morris KA, Lu Y, Liu E, Gregg KM, Brashear HR, Kinney GG, Black R, Grundman M (2012) Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 11(3):241–249. doi:10.1016/S1474-4422(12)70015-7
Takahashi RH, Milner TA, Li F, Nam EE, Edgar MA, Yamaguchi H, Beal MF, Xu H, Greengard P, Gouras GK (2002) Intraneuronal Alzheimer Abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am J Pathol 161(5):1869–1879
Watt AD, Crespi Gabriela A N, Down RA, Ascher DB, Gunn A, Perez KA, McLean CA, Villemagne VL, Parker MW, Barnham KJ, Miles LA (2014) Do current therapeutic anti-Aβ antibodies for Alzheimer’s disease engage the target? Acta Neuropathol. doi:10.1007/s00401-014-1290-2
Wilcock DM, Gordon MN, Ugen KE, Gottschall PE, DiCarlo G, Dickey C, Boyett KW, Jantzen PT, Connor KE, Melachrino J, Hardy J, Morgan D (2001) Number of Abeta inoculations in APP + PS1 transgenic mice influences antibody titers, microglial activation, and congophilic plaque levels. DNA Cell Biol 20(11):731–736. doi:10.1089/10445490152717596
Wirths O, Bethge T, Marcello A, Harmeier A, Jawhar S, Lucassen PJ, Multhaup G, Brody DL, Esparza T, Ingelsson M, Kalimo H, Lannfelt L, Bayer TA (2010) Pyroglutamate Abeta pathology in APP/PS1KI mice, sporadic and familial Alzheimer’s disease cases. J Neural Transm 117(1):85–96. doi:10.1007/s00702-009-0314-x
Wirths O, Breyhan H, Cynis H, Schilling S, Demuth H, Bayer TA (2009) Intraneuronal pyroglutamate-Abeta 3–42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol 118(4):487–496. doi:10.1007/s00401-009-0557-5
Wirths O, Erck C, Martens H, Harmeier A, Geumann C, Jawhar S, Kumar S, Multhaup G, Walter J, Ingelsson M, Degerman-Gunnarsson M, Kalimo H, Huitinga I, Lannfelt L, Bayer TA (2010) Identification of low molecular weight pyroglutamate A{beta} oligomers in Alzheimer disease: a novel tool for therapy and diagnosis. J Biol Chem 285(53):41517–41524. doi:10.1074/jbc.M110.178707
Wisniewski T, Goñi F (2014) Immunotherapy for Alzheimer’s disease. Biochem Pharmacol 88(4):499–507. doi:10.1016/j.bcp.2013.12.020
Wittnam JL, Portelius E, Zetterberg H, Gustavsson MK, Schilling S, Koch B, Demuth H, Blennow K, Wirths O, Bayer TA (2012) Pyroglutamate amyloid β (Aβ) aggravates behavioral deficits in transgenic amyloid mouse model for Alzheimer disease. J Biol Chem 287(11):8154–8162. doi:10.1074/jbc.M111.308601
Zago W, Buttini M, Comery TA, Nishioka C, Gardai SJ, Seubert P, Games D, Bard F, Schenk D, Kinney GG (2012) Neutralization of soluble, synaptotoxic amyloid beta species by antibodies is epitope specific. J Neurosci 32(8):2696–2702. doi:10.1523/JNEUROSCI.1676-11.2012
Acknowledgments
This work was supported by funding from the National Health and Medical Research Council of Australia (NHMRC) to LAM and MWP (APP1021935) and from the Victorian Government Operational Infrastructure Support Scheme to St. Vincent’s Institute. MWP is a NHMRC Research Fellow. JSLN received a Ph.D. stipend from the Mexican Ministry of Education (SEP) for the Improvement of Faculty (PROMEP). We greatly acknowledge the financial support by the Alzheimer Forschungs Initiative to TAB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A patent application for the antibody NT4X was filed by the University Medicine of Goettingen and Thomas A. Bayer.
Rights and permissions
About this article
Cite this article
Bouter, Y., Noguerola, J.S.L., Tucholla, P. et al. Abeta targets of the biosimilar antibodies of Bapineuzumab, Crenezumab, Solanezumab in comparison to an antibody against N-truncated Abeta in sporadic Alzheimer disease cases and mouse models. Acta Neuropathol 130, 713–729 (2015). https://doi.org/10.1007/s00401-015-1489-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1489-x