Abstract
Alzheimer’s disease pathology is closely connected to the processing of the amyloid precursor protein (APP) resulting in the formation of a variety of amyloid-beta (Aβ) peptides. They are found as insoluble aggregates in senile plaques, the histopathological hallmark of the disease. These peptides are also found in soluble, mostly monomeric and dimeric, forms in the interstitial and cerebrospinal fluid. Due to the combination of several enzymatic activities during APP processing, Aβ peptides exist in multiple isoforms possessing different N-termini and C-termini. These peptides include, to a certain extent, part of the juxtamembrane and transmembrane domain of APP. Besides differences in size, post-translational modifications of Aβ – including oxidation, phosphorylation, nitration, racemization, isomerization, pyroglutamylation, and glycosylation – generate a plethora of peptides with different physiological and pathological properties that may modulate disease progression.
Similar content being viewed by others
Introduction
Since its identification from senile plaques, amyloid-beta (Aβ) peptide has been considered to play a central role in the pathology of Alzheimer’s disease (AD) [1]. Aβ is thought to accumulate in AD cases because of an imbalance in the production and clearance of this peptide resulting in the formation of the characteristic amyloid plaques in specific brain regions. The large majority of AD cases are of sporadic nature, showing inefficient removal of Aβ [2], whereas a minority of cases is caused by genetic mutations (familial AD) with an onset typically below age 65 years. Most of these cases are caused by autosomal dominant mutations in genes related to the processing of amyloid precursor protein (APP) leading to increased production of Aβ.
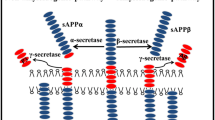
To generate Aβ from APP, the precursor has to be cleaved by a set of two proteases (Figure 1). The first cleavage occurs at a luminal, juxtamembrane position, resulting in the formation of a membrane-bound C-terminal stub that, in a subsequent step, is cleaved by an unconventional protease complex, called γ-secretase, within the transmembrane domain, thereby liberating Aβ. Both cleavages have been shown to be imprecise. β-site amyloid precursor protein cleaving enzyme 1 (BACE1), the enzyme that conducts the initial processing, step-cleaves APP at a minimum of two positions, whereas γ-secretase generates a variety of different Aβs spanning 34 to 50 amino acids in length.
Generation of different amyloid-beta domain-derived peptides from the amyloid precursor protein. The amyloid precursor protein (APP) is preferentially cleaved in the non-amyloidogenic pathway by α-secretase into a large ectodomain called sAPPα, and into a C-terminal stub called α-C-terminal fragment (α-CTF), which is further processed by γ-secretase into p3 peptides. Alternatively, APP may be cleaved in the amyloidogenic pathway by β-secretase into an ectodomain called sAPPβ and into a longer C-terminal stub called β-C-terminal fragment (β-CTF). This stub is preferentially cleaved by γ-secretase to generate amyloid-beta (Aβ) peptides, but some β-CTF precursors are cleaved by α-secretase resulting in C-terminal truncated Aβ species.
Most AD cases are sporadic, however, without changes in the production of the Aβ peptide. The propensity to form aggregates and toxic species may therefore be driven by factors other than changes in the production of certain Aβ peptides. Several post-translational modifications (PTMs) have been discovered that on the whole increase the aggregation rate of Aβ (Figure 2). Some of these modifications, such as oxidation and nitration, are obviously induced by the inflammatory milieu that is a component of AD [3].
PTMs can function as a molecular switch to evoke cellular responses, but one should consider that they may also be a result of protein aging that is random and without any physiological impact.
Truncated amyloid-beta species
Amyloid-beta species generated by α-secretase, β-secretase, and γ-secretase
Three enzymatic activities are involved in APP processing, and were named α-secretase, β-secretase, and γ-secretase at a time when their molecular identities were unknown. APP exists in several isoforms ranging from 695 to 770 amino acids in length, including the domain from which the Aβ peptide derives. In APP695 (the most abundant isoform in the brain) this domain ranges from amino acids 597 to 638. In an initial step, APP is cleaved at a juxtamembrane position at the luminal side of the membrane. This cleavage is mediated at different positions by either α-secretase or β-secretase in different compartments of the cell [4]. The majority of APP molecules in non-neuronal cells are initially cleaved by α-secretase between positions 16 and 17 of the Aβ domain. This is the so-called non-amyloidogenic pathway since the cleavage occurs within the Aβ domain, thereby preventing the production of Aβ. This event generates a stub called α-C-terminal fragment as well as a large ectodomain called sAPPα. Several members of the ADAM family of proteases are able to mediate this cleavage, but in neurons this function is likely to be exerted by the constitutively active ADAM10 protease [5].
In the amyloidogenic pathway, leading to the production of Aβ peptides, β-secretase mediates the initial rate-limiting step. The membrane-bound aspartyl-protease BACE1 has been identified as the responsible enzyme. APP is cleaved by this enzyme before position 1 of the Aβ domain [6], resulting in the release of a large ectodomain and the formation of a stub called β-C-terminal fragment. In addition, BACE1 can also cleave APP within the Aβ domain between positions 10 and 11 (β′ site) [7]. Subsequently, both N-terminally cleaved precursors are further processed by γ-secretase, a complex that consists of at least the proteins APH-1, PEN-2, nicastrin and presenilin 1 or presenilin 2 [8]. The transmembrane proteins presenilin 1 and presenilin 2 possess two critical aspartyl residues that are part of the catalytic domain of this γ-secretase subunit. The cleavage occurs within the transmembrane domain of APP, generating C-terminally truncated peptides ending with amino acids 37 to 43, due to an imprecise cleavage of these enzymes. The resulting peptides are liberated into extracellular fluids such as cerebrospinal fluid (CSF), plasma or interstitial fluid. This phenomenon is not fully understood, but endoproteolysis is thought to occur stepwise, cleaving the C-terminal stubs several times within their transmembrane domain. These cleavages are approximately three amino acids apart [9, 10]: one at amino acid 48 or 49, followed by another one at position 45 or 46, and ending with a final cleavage most often at position 38, 40 or 42. At least in the CSF of nondemented controls, about one-half of the Aβ ends at amino acid 40, 16% ends at amino acid 38, and 10% ends at amino acid 42 [11]. Aβ species ending with the alanine at position 42 have a stronger tendency to aggregate as compared with Aβ1–40. These species are thought to be the driving factor for the formation of amyloid plaques and the neurotoxic effects [12]. During this last step, other minor cleavage sites have been observed at positions 34, 37, 39, and 43 [9]. There are even shorter Aβ isoforms (Aβ1–17/18/19/20) that depend on γ-secretase [13, 14] but the precise mechanism of their generation is unknown.
Both processing pathways lead to a large variety of peptides that start either at position 1 or position 11 caused by cleavage by BACE1 or at position 17 mediated by α-secretase. The latter peptides are called p3 [15] but are not found in senile plaques, and neither do they yet appear to have any pathological or physiological role. Most of these N-terminal starting points are found in combination with the heterogeneity caused by the cleavage of γ-secretase.
To make things even more complicated, the amyloidogenic pathway and the nonamyloidogenic pathway seem not to be mutually exclusive. There are shorter isoforms of Aβ (Aβ1–14/15/16) present in the CSF that do not depend on γ-secretase cleavage but are sensitive to inhibitors of α-secretase. Interestingly, Aβ1–14/15/16 increase after γ-secretase treatment in CSF [16, 17]. This leads to the conclusion that C-terminal BACE1-cleaved stubs (C99) are not exclusive substrates for γ-secretase, and that C99 can reach compartments with α-secretase activity resulting in the liberation of Aβ1–14/15/16 along alternative pathways [13, 14, 18].
N-terminal amyloid-beta truncations independent of α-secretase or β-secretase
There are several N-terminal truncations observed in AD that cannot be explained by the action of the above-described enzymes [19]. In general, N-terminal truncations make up the majority of Aβ species in AD [20, 21] but not in the transgenic mice mouse model, which might explain the differences in the molecular mechanisms of amyloid deposition [20, 22]. In addition, the shortening of the N-terminus increases the propensity of Aβ to form aggregates in vitro[23]. Since Aβ is degraded by several secretory proteases, such as insulin-degrading enzyme and neprilysin among others [24], it is possible that truncations arise from these enzymes.
The 2-x Aβ species has been found to be increased in the brains of AD patients [25, 26] and decreased in the CSF of AD patients [27]. There was a suggestion that this species might derive from the combined action of BACE1 followed by aminopeptidase A [25]. Recently, the metalloprotease meprin-beta was reported to initially shed APP in a BACE1-independent fashion, releasing different Aβ species with several cleavage sites. These sites are reported to be identical with or proximal to the known β-secretase cleavage site [28], and overexpression of meprin-beta generates Aβ2–40 [11, 25]. However, further studies and appropriate mouse models are necessary to investigate the contribution of meprin-beta in AD.
The 3-x Aβ species has been detected in a mouse model of AD [20, 29, 30] and in senile plaques from brains of late AD cases [31]. It has been suggested that this species is generated by Cu2+-mediated amide hydrolysis or the peptide bond between amino acids 2 and 3 of Aβ [32].
One of the first Aβ peptides reported was the N-terminal truncated 4-x species [33]. In comparison with other species, Aβ4–42 was found to be relatively abundant in AD and vascular dementia [34]. In vivo, mice overexpressing Aβ4–42 suffer from a massive CA1 pyramidal neuronal loss, accompanied by memory dysfunction [35]. There is so far no candidate enzyme that mediates this cleavage.
The 5-x Aβ species was initially described in cells expressing an APP lacking the C-terminal 31 amino acids, but has also been discovered in AD patients using a 5-x Aβ neo-epitope antibody [36, 37] and in nondemented controls by mass spectometry [21]. Interestingly, using APP-overexpressing cell lines, inhibition of BACE1 resulted in the appearance of Aβ5–40 [38, 39]. This species has also been detected in the 5XFAD mouse model of AD [29] and in the CSF of dogs treated with BACE1 inhibitor [39].
The largest amino truncations, aside from that at position 11 mediated by BACE1, are so far the cleavages that occur before amino acids 7, 8 and 9 observed in the brains of AD patients [21, 22]. A candidate enzyme for the formation of the 8-x Aβ species might be angiotensin-converting enzyme [40], but so far there are no in vivo data supporting this pathway.
Amyloid-beta species modified by post-translational modification
Oxidation
The most prominent site of oxidative changes within Aβ is the methionine at position 35 (Met35). Increased oxidative stress has been described in the brains of mild cognitive impaired and AD patients. Part of this oxidative stress is mediated by the Aβ peptide itself, but other mechanisms, such as inflammatory inducers and others, may also be relevant.
Oxidation of Met35 to methionine sulfoxide in AD was first observed years ago [41]. The reaction proceeds through a radical intermediate that can be prevented by the use of radical scavengers [42]. Several studies demonstrated that oxidation of Met35 impedes the formation of Aβ protofibrils and fibrils from monomers [43, 44]. A role for Met35-oxidized Aβ in the formation of ion-channel-like structures in lipid membranes has also been reported [45].
Phosphorylation
In theory, Aβ possesses three potential phosphorylation sites at serine residues 8 and 26 and at tyrosine residue 10. There are numerous examples of phosphorylated extracellular/luminal protein suggesting the existence of extracellular kinases that facilitate this PTM. Phosphorylation of the serine at position 26 has been described in NT2 neurons and AD brains [46]. In vitro, this PTM is generated by the action of the cdc2 kinase. In turn, using a cdc2 kinase inhibitor, the neurotoxic effect of Aβ on NT-2 neurons can be reduced [46].
Phosphorylation of Aβ at serine 8 has been studied in more detail. Using phospho-serine-8-specific Aβ antibodies revealed the presence of phosphorylated Aβ in AD mouse models and AD. Under pathological conditions this species was found to be localized to amyloid plaques [47], but could also be found intracellularly [48]. Biophysically, this PTM increases the formation of oligomeric Aβ aggregates that represent nuclei for fibrillization. This species shows increased toxicity in drosophila models as compared with nonphosphorylated Aβ [47]. In addition, serine 8-phosphorylated Aβ is resistant to degradation by insulin degrading enzyme [49].
Nitric-oxide-caused modifications
Nitric oxide (NO) induces several PTMs, including the formation of S-nitrothiols at cysteine residues and nitration and dityrosine formation at tyrosine residues [50]. Increased presence of these NO-caused PTMs has been observed in AD [51, 52]. The source of NO during AD is most probably the enzyme NOS2, which is upregulated in AD [53, 54]. As a molecular target, tyrosine 10 of Aβ has been shown to increase the propensity of Aβ to aggregate and has been identified in the core of the amyloid plaques [55]. The reaction of Aβ with peroxynitrite, an intermediate NO product, in vitro has been shown to generate both nitrated Aβ and dityrosine-coupled Aβ. The latter modification could also be detected in the core of amyloid plaques [55] and may stabilize Aβ dimers [56]. Nitrated Aβ was able to initiate plaque formation in APP/PS1 mice, suggesting a central role during the early phase of AD [55]. Hippocampal long-term potentiation was suppressed more by nitrated Aβ compared with non-nitrated Aβ. This demonstrates that this PTM is involved in both the functional and structural changes in AD. In addition, formation of this Aβ species is favored by oxidative stress [56, 57].
Glycosylations
Mass spectrometry analysis of controls and AD patients revealed the presence of O-glycosylated Aβ species in CSF [58]. The glycoforms included monosialylated, disialylated, and trisialylated modifications, as well as lactone modifications. The exact molecular nature of the glycosylation has not been determined and could therefore be GlcNAc, GalNAc, or ManNAc in either α-linkage or β-linkage to the conjugated amino acid. Glycosylation occurred on Aβ1–15/16/17/18/19/20, Aβ3–15, Aβ4–15, Aβ4–17, and Aβ5–17 peptides, with Aβ1–15 and Aβ1–17 peptides being the most abundant of all Aβ1–X glycopeptides. The absolute concentration for glycosylated Aβ1–15 was calculated to be 10 to 30 pg/ml CSF, whereas that for unglycosylated Aβ1–15 ranged from 100 to 200 pg/ml. For Aβ1–15 and Aβ1–17 the glycosylations were selectively attached to tyrosine 10 of the Aβ sequence. The lack of glycosylated Aβ1-40/42 peptides in CSF led to the conclusion that tyrosine 10 O-glycosylation in APP modifies the γ-secretase cleavage, because of the proximity of this glycosylation to the transmembrane domain [58].
Pyroglutamylation
The initial attempts to identity the N-terminus of Aβ revealed a minor species beginning with glutamic acid at position 3 [59]. Development of specific antibodies to pyroglutamate Aβ demonstrated its weak solubility and presence in amyloid plaques [60]. As an initial step, formation of pyroglutamate-modified Aβ at position 3 (3pE-Aβ) requires the removal of the first two amino acids from Aβ. Aminopeptidase A has been suggested as an enzyme facilitating this processing [61], but this has yet to be proven. In addition, spontaneous amide hydrolysis by Cu2+ has been reported [32]. Further, another pyroglutamate modification at aspartate 11 was discovered (11pE-Aβ) [41, 62]. This species may arise from the alternative BACE1 cleavage side in APP [63, 64]. In a subsequent step, the terminal glutamate is converted to a pyroglutamate in a dehydration reaction. This reaction can be catalyzed by the enzyme glutaminyl cyclase [65], which is increased in AD [66]. Reduction of glutaminyl cyclase results in reduced formation of pyroglutamate Aβ in vitro[67] and in vivo[66, 68]. Further, reduced glutaminyl cyclase expression in AD mouse models is accompanied by reductions in Aβ40/42 levels, reduced plaque burden, inflammatory reaction, and improved memory and spatial learning [66, 68].
In vitro, 3pE-Aβ42 has a similar toxicological profile on neuronal cells to that of Aβ1–42 [69], which was confirmed by intracerebroventricular injections of either 3pE-Aβ42 or Aβ1–42 [70]. Like many changes in the N-terminus of Aβ, 3pE-Aβ and 11pE-Aβ show increased propensity to aggregate and to form β-sheets in vitro. This may be caused by higher hydrophobicity since two charges are lost during conversion [71]. pE-Aβ has been detected in a variety of AD mouse models, yet the time of first appearance during pathology varies strongly between different mouse models – ranging from 2 months in the APP/PS1KI model [72], to 16 months in the Tg2576 model [73], to 15 months in the APP23 model [20]. Interestingly, there has been extensive neurotoxicity described in mouse models that generate pyroglutamate-modified Aβ [74, 75].
Isomerization
Peptides are susceptible to spontaneous, non-enzymatic isomerization particularly at asparagine and aspartate residues, resulting in the formation of isoaspartate. These aspartyl-bond isomerizations affect the secondary structure of the peptide and may therefore be critical for the development of pathological processes such as aggregation and deposition [76]. In parenchymal plaque core preparations, the predominant species of Aβ at the aspartyl residues 1 and 7 is the l-isoaspartyl form [77]. Interestingly, the amount of isoaspartyl residues in Aβ preparations from vascular depositions is lower compared with preparations from senile plaques [77, 78], suggesting that Aβ from plaques are older since this PTM increases over the lifetime. Isomerization of aspartate 23 has not so far been detected by biochemical means in AD brains.
In vitro, substitutions of positions 1, 7 and 23 of Aβ by isoaspartate increased the tendency of these peptides to form β-pleated sheets [78], to form aggregates [79, 80] and to contribute to the enhanced insolubility and resistance to enzymatic degradation [81]. The presence of isoaspartate-7 Aβ detected by specific antibodies was suggested to be an indicator of plaque age since this was found mostly in the core of amyloid plaques and correlated with dementia severity [82].
Racemization
Racemization is the process of conversion of enantiomers so that both enantiomers are present. In the case of amino acids this is the conversion from the l-form to the d-form, especially at seryl and aspartyl residues. Presence of d-enantiomers of aspartyl and seryl residues in Aβ have long been described [83–85]. As for isomerized Aβ, the presence of racemized aspartyl residues in Aβ was found to be higher in amyloid plaques compared with vascular Aβ [77]. In vitro, racemization of Aβ can be induced by radicals [86]. In a recent study, the enrichment of d-Asp1 as well as of its isomer d-isoAsp1 could be demonstrated in the tissue of AD patients by mass spectometry [87]. d-Ser26-Aβ1–40 possesses a stronger tendency to form fibrils [84].
Use of amyloid-beta species as diagnostic marker and as a target for treatment
Because of its disease-dependent regulation in the CSF, Aβ has been used as a biomarker for AD. In particular, the concentration of Aβ1–42 in the CSF undergoes a characteristic drop during disease progression, which is interpreted as the consequence of the ongoing parenchymal Aβ deposition in senile plaques. In addition, the CSF levels of Aβ1–40 remain constant so that the ratio of Aβ1–42/Aβ1–40 is a valuable predictor. Since the C-terminal truncated Aβ1–14/15 species are elevated after inhibition of γ-secretase, their use as a readout for trials aiming at the modulation of γ-secretase has been suggested [88]. The sensitivity can be increased by combining the markers Aβ1–42, Aβ1–38 and pTau, resulting in a 94% discrimination between nondemented patients and AD patients [89]. Beyond the diagnosis of AD, the concentration of Aβ1–38 is specifically lowered in the CSF of patients affected by fronto-temperal dementia [90].
The search for human auto-antibodies in plasma and CSF revealed that most antibodies recognized either oligomeric or post-translationally modified Aβ [91], suggesting that this species might be a relevant target for passive or active immunotherapy. Since it is unknown whether unmodified Aβ has a physiological role, there is a certain risk that preventive immunotherapy may cause undesirable effects. In addition, decreased levels of Aβ autoantibodies might even contribute to disease progression as has been shown for pyroglutamate-modified Aβ [92]. The immunotherapy directed at pathological Aβ species is therefore a road that should be followed.
Several PTMs of Aβ increase the tendency of Aβ to aggregate [46, 57, 91] and are found in the core of amyloid plaques [55, 93], suggesting that plaque formation depends on them. The use of inhibitors that interfere with their formation therefore has to be evaluated. In particular, inhibitors for glutaminyl cyclase [66], the relevant kinases for the phosphorylation of Aβ and NO-producing enzymes, especially NOS2 [55], should be tested as a therapeutic option.
Conclusion
From the plethora of Aβ species, some are generated very early during APP processing, some are modified immediately afterwards, or others are found or generated in certain cellular or extracellular compartments, and some are actually markers for the slow to non-existent turnover of these peptides in amyloid plaques. Certain regions of Aβ obviously contribute differentially to its properties, like N-terminal truncations, and certain amino acids are hotspots for PTMs. There is a possibility that some of these species might serve as excellent diagnostic markers or therapeutic targets in the future.
Abbreviations
- AD:
-
Alzheimer’s disease
- APP:
-
Amyloid precursor protein
- Aβ:
-
Amyloid-beta
- BACE1:
-
β-site amyloid precursor protein cleaving enzyme 1
- CSF:
-
Cerebrospinal fluid
- Met35:
-
Methionine at position 35
- NO:
-
Nitric oxide
- PTM:
-
Post-translational modification.
References
Querfurth HW, LaFerla FM: Alzheimer’s disease. N Engl J Med. 2010, 362: 329-344. 10.1056/NEJMra0909142.
Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ: Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science. 2010, 330: 1774-1774. 10.1126/science.1197623.
Heneka MT, O’Banion MK, Terwel D, Kummer MP: Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm Vienna Austria 1996. 2010, 117: 919-947.
Thinakaran G, Koo EH: Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008, 283: 29615-29619. 10.1074/jbc.R800019200.
Kuhn P-H, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, Rossner S, Lichtenthaler SF: ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010, 29: 3020-3032. 10.1038/emboj.2010.167.
Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M: Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999, 286: 735-741. 10.1126/science.286.5440.735.
Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C: Isolation and quantification of soluble Alzheimer’s beta-peptide from biological fluids. Nature. 1992, 359: 325-327. 10.1038/359325a0.
Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C: Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003, 5: 486-488. 10.1038/ncb960.
Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y: γ-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci Off J Soc Neurosci. 2009, 29: 13042-13052. 10.1523/JNEUROSCI.2362-09.2009.
Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, Ihara Y: Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci Off J Soc Neurosci. 2005, 25: 436-445. 10.1523/JNEUROSCI.1575-04.2005.
Bibl M, Gallus M, Welge V, Lehmann S, Sparbier K, Esselmann H, Wiltfang J: Characterization of cerebrospinal fluid aminoterminally truncated and oxidized amyloid-β peptides. Proteomics Clin Appl. 2012, 6: 163-169. 10.1002/prca.201100082.
Haass C, Selkoe DJ: Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007, 8: 101-112. 10.1038/nrm2101.
Beher D, Wrigley JDJ, Owens AP, Shearman MS: Generation of C-terminally truncated amyloid-β peptides is dependent on γ-secretase activity. J Neurochem. 2002, 82: 563-575. 10.1046/j.1471-4159.2002.00985.x.
Portelius E, Price E, Brinkmalm G, Stiteler M, Olsson M, Persson R, Westman-Brinkmalm A, Zetterberg H, Simon AJ, Blennow K: A novel pathway for amyloid precursor protein processing. Neurobiol Aging. 2011, 32: 1090-1098. 10.1016/j.neurobiolaging.2009.06.002.
Haass C, Hung AY, Schlossmacher MG, Teplow DB, Selkoe DJ: β-Amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J Biol Chem. 1993, 268: 3021-3024.
Portelius E, Van Broeck B, Andreasson U, Gustavsson MK, Mercken M, Zetterberg H, Borghys H, Blennow K: Acute effect on the Aβ isoform pattern in CSF in response to γ-secretase modulator and inhibitor treatment in dogs. J Alzheimers Dis. 2010, 21: 1005-1012.
Portelius E, Dean RA, Gustavsson MK, Andreasson U, Zetterberg H, Siemers E, Blennow K: A novel Aβ isoform pattern in CSF reflects gamma-secretase inhibition in Alzheimer disease. Alzheimers Res Ther. 2010, 2: 7-10.1186/alzrt30.
Jäger S, Leuchtenberger S, Martin A, Czirr E, Wesselowski J, Dieckmann M, Waldron E, Korth C, Koo EH, Heneka M, Weggen S, Pietrzik CU: Alpha-secretase mediated conversion of the amyloid precursor protein derived membrane stub C99 to C83 limits Aβ generation. J Neurochem. 2009, 111: 1369-1382. 10.1111/j.1471-4159.2009.06420.x.
Ancolio K, Dumanchin C, Barelli H, Warter JM, Brice A, Campion D, Frébourg T, Checler F: Unusual phenotypic alteration of beta amyloid precursor protein (βAPP) maturation by a new Val-715 → Met βAPP-770 mutation responsible for probable early-onset Alzheimer’s disease. Proc Natl Acad Sci USA. 1999, 96: 4119-4124. 10.1073/pnas.96.7.4119.
Schieb H, Kratzin H, Jahn O, Möbius W, Rabe S, Staufenbiel M, Wiltfang J, Klafki HW: Beta-amyloid peptide variants in brains and cerebrospinal fluid from amyloid precursor protein (APP) transgenic mice: comparison with human Alzheimer amyloid. J Biol Chem. 2011, 286: 33747-33758. 10.1074/jbc.M111.246561.
Sergeant N, Bombois S, Ghestem A, Drobecq H, Kostanjevecki V, Missiaen C, Wattez A, David J-P, Vanmechelen E, Sergheraert C, Delacourte A: Truncated beta-amyloid peptide species in pre-clinical Alzheimer’s disease as new targets for the vaccination approach. J Neurochem. 2003, 85: 1581-1591. 10.1046/j.1471-4159.2003.01818.x.
Kalback W, Watson MD, Kokjohn TA, Kuo Y-M, Weiss N, Luehrs DC, Lopez J, Brune D, Sisodia SS, Staufenbiel M, Emmerling M, Roher AE: APP transgenic mice Tg2576 accumulate Aβ peptides that are distinct from the chemically modified and insoluble peptides deposited in Alzheimer’s disease senile plaques. Biochemistry (Mosc). 2002, 41: 922-928. 10.1021/bi015685+.
Pike CJ, Overman MJ, Cotman CW: Amino-terminal deletions enhance aggregation of β-amyloid peptides in vitro. J Biol Chem. 1995, 270: 23895-23898. 10.1074/jbc.270.41.23895.
Wang DS, Dickson DW, Malter JS: β-Amyloid degradation and Alzheimer’s disease. J Biomed Biotechnol. 2006, 2006: 58406-
Wiltfang J, Esselmann H, Cupers P, Neumann M, Kretzschmar H, Beyermann M, Schleuder D, Jahn H, Rüther E, Kornhuber J, Annaert W, De Strooper B, Saftig P: Elevation of beta-amyloid peptide 2–42 in sporadic and familial Alzheimer’s disease and its generation in PS1 knockout cells. J Biol Chem. 2001, 276: 42645-42657. 10.1074/jbc.M102790200.
Arai T, Akiyama H, Ikeda K, Kondo H, Mori H: Immunohistochemical localization of amyloid beta-protein with amino-terminal aspartate in the cerebral cortex of patients with Alzheimer’s disease. Brain Res. 1999, 823: 202-206. 10.1016/S0006-8993(98)01366-3.
Bibl M, Gallus M, Welge V, Esselmann H, Wolf S, Rüther E, Wiltfang J: Cerebrospinal fluid amyloid-β 2–42 is decreased in Alzheimer’s, but not in frontotemporal dementia. J Neural Transm Vienna Austria 1996. 2012, 119: 805-813.
Bien J, Jefferson T, Causević M, Jumpertz T, Munter L, Multhaup G, Weggen S, Becker-Pauly C, Pietrzik CU: The metalloprotease meprin β generates amino terminal-truncated amyloid β peptide species. J Biol Chem. 2012, 287: 33304-33313. 10.1074/jbc.M112.395608.
Wittnam JL, Portelius E, Zetterberg H, Gustavsson MK, Schilling S, Koch B, Demuth H-U, Blennow K, Wirths O, Bayer TA: Pyroglutamate amyloid β (Aβ) aggravates behavioral deficits in transgenic amyloid mouse model for Alzheimer disease. J Biol Chem. 2012, 287: 8154-8162. 10.1074/jbc.M111.308601.
Casas C, Sergeant N, Itier J-M, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L: Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Aβ42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004, 165: 1289-1300. 10.1016/S0002-9440(10)63388-3.
Güntert A, Döbeli H, Bohrmann B: High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neuroscience. 2006, 143: 461-475. 10.1016/j.neuroscience.2006.08.027.
Drew SC, Masters CL, Barnham KJ: Alanine-2 carbonyl is an oxygen ligand in Cu2+ coordination of Alzheimer’s disease amyloid-beta peptide – relevance to N-terminally truncated forms. J Am Chem Soc. 2009, 131: 8760-8761. 10.1021/ja903669a.
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K: Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985, 82: 4245-4249. 10.1073/pnas.82.12.4245.
Lewis H, Beher D, Cookson N, Oakley A, Piggott M, Morris CM, Jaros E, Perry R, Ince P, Kenny RA, Ballard CG, Shearman MS, Kalaria RN: Quantification of Alzheimer pathology in ageing and dementia: age-related accumulation of amyloid-beta(42) peptide in vascular dementia. Neuropathol Appl Neurobiol. 2006, 32: 103-118. 10.1111/j.1365-2990.2006.00696.x.
Bouter Y, Dietrich K, Wittnam JL, Rezaei-Ghaleh N, Pillot T, Papot-Couturier S, Lefebvre T, Sprenger F, Wirths O, Zweckstetter M, Bayer TA: N-truncated amyloid β (Aβ) 4–42 forms stable aggregates and induces acute and long-lasting behavioral deficits. Acta Neuropathol (Berl). 2013, 126: 189-205. 10.1007/s00401-013-1129-2.
Takeda K, Araki W, Akiyama H, Tabira T: Amino-truncated amyloid beta-peptide (Aβ5-40/42) produced from caspase-cleaved amyloid precursor protein is deposited in Alzheimer’s disease brain. FASEB J Off Publ Fed Am Soc Exp Biol. 2004, 18: 1755-1757.
Murayama KS, Kametani F, Tabira T, Araki W: A novel monoclonal antibody specific for the amino-truncated beta-amyloid Aβ5–40/42 produced from caspase-cleaved amyloid precursor protein. J Neurosci Methods. 2007, 161: 244-249. 10.1016/j.jneumeth.2006.11.010.
Portelius E, Olsson M, Brinkmalm G, Rüetschi U, Mattsson N, Andreasson U, Gobom J, Brinkmalm A, Hölttä M, Blennow K, Zetterberg H: Mass spectrometric characterization of amyloid-β species in the 7PA2 cell model of Alzheimer’s disease. J Alzheimers Dis. 2013, 33: 85-93.
Mattsson N, Rajendran L, Zetterberg H, Gustavsson M, Andreasson U, Olsson M, Brinkmalm G, Lundkvist J, Jacobson LH, Perrot L, Neumann U, Borghys H, Mercken M, Dhuyvetter D, Jeppsson F, Blennow K, Portelius E: BACE1 inhibition induces a specific cerebrospinal fluid β-amyloid pattern that identifies drug effects in the central nervous system. PloS One. 2012, 7: e31084-10.1371/journal.pone.0031084.
Hu J, Igarashi A, Kamata M, Nakagawa H: Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (Aβ); retards Aβ aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem. 2001, 276: 47863-47868.
Näslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, Silberring J, Gandy SE, Winblad B, Greengard P: Relative abundance of Alzheimer Aβ amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci USA. 1994, 91: 8378-8382. 10.1073/pnas.91.18.8378.
Hensley K, Aksenova M, Carney JM, Harris M, Butterfield DA: Amyloid beta-peptide spin trapping. I: peptide enzyme toxicity is related to free radical spin trap reactivity. Neuroreport. 1995, 6: 489-492. 10.1097/00001756-199502000-00021.
Palmblad M, Westlind-Danielsson A, Bergquist J: Oxidation of methionine 35 attenuates formation of amyloid beta-peptide 1–40 oligomers. J Biol Chem. 2002, 277: 19506-19510. 10.1074/jbc.M112218200.
Hou L, Kang I, Marchant RE, Zagorski MG: Methionine 35 oxidation reduces fibril assembly of the amyloid Aβ-(1–42) peptide of Alzheimer’s disease. J Biol Chem. 2002, 277: 40173-40176. 10.1074/jbc.C200338200.
Barnham KJ, Ciccotosto GD, Tickler AK, Ali FE, Smith DG, Williamson NA, Lam Y-H, Carrington D, Tew D, Kocak G, Volitakis I, Separovic F, Barrow CJ, Wade JD, Masters CL, Cherny RA, Curtain CC, Bush AI, Cappai R: Neurotoxic, redox-competent Alzheimer’s beta-amyloid is released from lipid membrane by methionine oxidation. J Biol Chem. 2003, 278: 42959-42965. 10.1074/jbc.M305494200.
Milton NG: Phosphorylation of amyloid-beta at the serine 26 residue by human cdc2 kinase. Neuroreport. 2001, 12: 3839-3844. 10.1097/00001756-200112040-00047.
Kumar S, Rezaei-Ghaleh N, Terwel D, Thal DR, Richard M, Hoch M, Mc Donald JM, Wüllner U, Glebov K, Heneka MT, Walsh DM, Zweckstetter M, Walter J: Extracellular phosphorylation of the amyloid β-peptide promotes formation of toxic aggregates during the pathogenesis of Alzheimer’s disease. EMBO J. 2011, 30: 2255-2265. 10.1038/emboj.2011.138.
Kumar S, Wirths O, Theil S, Gerth J, Bayer TA, Walter J: Early intraneuronal accumulation and increased aggregation of phosphorylated Aβ in a mouse model of Alzheimer’s disease. Acta Neuropathol (Berl). 2013, 125: 699-709. 10.1007/s00401-013-1107-8.
Kumar S, Singh S, Hinze D, Josten M, Sahl H-G, Siepmann M, Walter J: Phosphorylation of amyloid-β peptide at serine 8 attenuates its clearance via insulin-degrading and angiotensin-converting enzymes. J Biol Chem. 2012, 287: 8641-8651. 10.1074/jbc.M111.279133.
Radi R, Cassina A, Hodara R, Quijano C, Castro L: Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002, 33: 1451-1464. 10.1016/S0891-5849(02)01111-5.
Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, Keller JN, Markesbery WR, Sultana R: Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer’s disease. Brain Res. 2007, 1148: 243-248.
Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA: Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem. 2003, 85: 1394-1401. 10.1046/j.1471-4159.2003.01786.x.
Heneka MT, Wiesinger H, Dumitrescu-Ozimek L, Riederer P, Feinstein DL, Klockgether T: Neuronal and glial coexpression of argininosuccinate synthetase and inducible nitric oxide synthase in Alzheimer disease. J Neuropathol Exp Neurol. 2001, 60: 906-916.
Vodovotz Y, Lucia MS, Flanders KC, Chesler L, Xie QW, Smith TW, Weidner J, Mumford R, Webber R, Nathan C, Roberts AB, Lippa CF, Sporn MB: Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer’s disease. J Exp Med. 1996, 184: 1425-1433. 10.1084/jem.184.4.1425.
Kummer MP, Hermes M, Delekarte A, Hammerschmidt T, Kumar S, Terwel D, Walter J, Pape H-C, König S, Roeber S, Jessen F, Klockgether T, Korte M, Heneka MT: Nitration of tyrosine 10 critically enhances amyloid β aggregation and plaque formation. Neuron. 2011, 71: 833-844. 10.1016/j.neuron.2011.07.001.
Al-Hilaly YK, Williams TL, Stewart-Parker M, Ford L, Skaria E, Cole M, Bucher WG, Morris KL, Sada AA, Thorpe JR, Serpell LC: A central role for dityrosine crosslinking of amyloid-β in Alzheimer’s disease. Acta Neuropathol Commun. 2013, 1: 83-10.1186/2051-5960-1-83.
Thiabaud G, Pizzocaro S, Garcia-Serres R, Latour J-M, Monzani E, Casella L: Heme binding induces dimerization and nitration of truncated β-amyloid peptide Aβ16 under oxidative stress. Angew Chem Int Ed Engl. 2013, 52: 8041-8044. 10.1002/anie.201302989.
Halim A, Brinkmalm G, Rüetschi U, Westman-Brinkmalm A, Portelius E, Zetterberg H, Blennow K, Larson G, Nilsson J: Site-specific characterization of threonine, serine, and tyrosine glycosylations of amyloid precursor protein/amyloid beta-peptides in human cerebrospinal fluid. Proc Natl Acad Sci USA. 2011, 108: 11848-11853. 10.1073/pnas.1102664108.
Mori H, Takio K, Ogawara M, Selkoe DJ: Mass spectrometry of purified amyloid beta protein in Alzheimer’s disease. J Biol Chem. 1992, 267: 17082-17086.
Saido TC, Iwatsubo T, Mann DM, Shimada H, Ihara Y, Kawashima S: Dominant and differential deposition of distinct beta-amyloid peptide species, AβN3(pE), in senile plaques. Neuron. 1995, 14: 457-466. 10.1016/0896-6273(95)90301-1.
Sevalle J, Amoyel A, Robert P, Fournié-Zaluski M-C, Roques B, Checler F: Aminopeptidase A contributes to the N-terminal truncation of amyloid beta-peptide. J Neurochem. 2009, 109: 248-256. 10.1111/j.1471-4159.2009.05950.x.
Liu K, Solano I, Mann D, Lemere C, Mercken M, Trojanowski JQ, Lee VM-Y: Characterization of Aβ11–40/42 peptide deposition in Alzheimer’s disease and young Down’s syndrome brains: implication of N-terminally truncated Aβ species in the pathogenesis of Alzheimer’s disease. Acta Neuropathol (Berl). 2006, 112: 163-174. 10.1007/s00401-006-0077-5.
Huse JT, Liu K, Pijak DS, Carlin D, Lee VM-Y, Doms RW: Beta-secretase processing in the trans-Golgi network preferentially generates truncated amyloid species that accumulate in Alzheimer’s disease brain. J Biol Chem. 2002, 277: 16278-16284. 10.1074/jbc.M111141200.
Liu K, Doms RW, Lee VM-Y: Glu11 site cleavage and N-terminally truncated Aβ production upon BACE overexpression. Biochemistry (Mosc). 2002, 41: 3128-3136. 10.1021/bi015800g.
Schilling S, Hoffmann T, Manhart S, Hoffmann M, Demuth H-U: Glutaminyl cyclases unfold glutamyl cyclase activity under mild acid conditions. FEBS Lett. 2004, 563: 191-196. 10.1016/S0014-5793(04)00300-X.
Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, Holzer M, Hutter-Paier B, Prokesch M, Windisch M, Jagla W, Schlenzig D, Lindner C, Rudolph T, Reuter G, Cynis H, Montag D, Demuth H-U, Rossner S: Glutaminyl cyclase inhibition attenuates pyroglutamate Aβ and Alzheimer’s disease-like pathology. Nat Med. 2008, 14: 1106-1111. 10.1038/nm.1872.
Cynis H, Scheel E, Saido TC, Schilling S, Demuth H-U: Amyloidogenic processing of amyloid precursor protein: evidence of a pivotal role of glutaminyl cyclase in generation of pyroglutamate-modified amyloid-beta. Biochemistry (Mosc). 2008, 47: 7405-7413. 10.1021/bi800250p.
Jawhar S, Wirths O, Schilling S, Graubner S, Demuth H-U, Bayer TA: Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate Aβ formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice. J Biol Chem. 2011, 286: 4454-4460. 10.1074/jbc.M110.185819.
Tekirian TL, Yang AY, Glabe C, Geddes JW: Toxicity of pyroglutaminated amyloid beta-peptides 3(pE)-40 and -42 is similar to that of Aβ1–40 and -42. J Neurochem. 1999, 73: 1584-1589.
Youssef I, Florent-Béchard S, Malaplate-Armand C, Koziel V, Bihain B, Olivier J-L, Leininger-Muller B, Kriem B, Oster T, Pillot T: N-truncated amyloid-β oligomers induce learning impairment and neuronal apoptosis. Neurobiol Aging. 2008, 29: 1319-1333. 10.1016/j.neurobiolaging.2007.03.005.
He W, Barrow CJ: The Aβ3-pyroglutamyl and 11-pyroglutamyl peptides found in senile plaque have greater beta-sheet forming and aggregation propensities in vitro than full-length Aβ. Biochemistry (Mosc). 1999, 38: 10871-10877. 10.1021/bi990563r.
Christensen DZ, Kraus SL, Flohr A, Cotel M-C, Wirths O, Bayer TA: Transient intraneuronal Aβ rather than extracellular plaque pathology correlates with neuron loss in the frontal cortex of APP/PS1KI mice. Acta Neuropathol (Berl). 2008, 116: 647-655. 10.1007/s00401-008-0451-6.
Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG: Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001, 21: 372-381.
Wirths O, Breyhan H, Cynis H, Schilling S, Demuth H-U, Bayer TA: Intraneuronal pyroglutamate-Aβ 3–42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol (Berl). 2009, 118: 487-496. 10.1007/s00401-009-0557-5.
Alexandru A, Jagla W, Graubner S, Becker A, Bäuscher C, Kohlmann S, Sedlmeier R, Raber KA, Cynis H, Rönicke R, Reymann KG, Petrasch-Parwez E, Hartlage-Rübsamen M, Waniek A, Rossner S, Schilling S, Osmand AP, Demuth H-U, von Hörsten S: Selective hippocampal neurodegeneration in transgenic mice expressing small amounts of truncated Aβ is induced by pyroglutamate-Aβ formation. J Neurosci Off J Soc Neurosci. 2011, 31: 12790-12801. 10.1523/JNEUROSCI.1794-11.2011.
Szendrei GI, Fabian H, Mantsch HH, Lovas S, Nyéki O, Schön I, Otvos L: Aspartate-bond isomerization affects the major conformations of synthetic peptides. Eur J Biochem FEBS. 1994, 226: 917-924. 10.1111/j.1432-1033.1994.t01-1-00917.x.
Roher AE, Lowenson JD, Clarke S, Wolkow C, Wang R, Cotter RJ, Reardon IM, Zürcher-Neely HA, Heinrikson RL, Ball MJ: Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer’s disease. J Biol Chem. 1993, 268: 3072-3083.
Fabian H, Szendrei GI, Mantsch HH, Greenberg BD, Otvös L: Synthetic post-translationally modified human Aβ peptide exhibits a markedly increased tendency to form beta-pleated sheets in vitro. Eur J Biochem FEBS. 1994, 221: 959-964. 10.1111/j.1432-1033.1994.tb18811.x.
Shimizu T, Fukuda H, Murayama S, Izumiyama N, Shirasawa T: Isoaspartate formation at position 23 of amyloid beta peptide enhanced fibril formation and deposited onto senile plaques and vascular amyloids in Alzheimer’s disease. J Neurosci Res. 2002, 70: 451-461. 10.1002/jnr.10350.
Fukuda H, Shimizu T, Nakajima M, Mori H, Shirasawa T: Synthesis, aggregation, and neurotoxicity of the Alzheimer’s Aβ1-42 amyloid peptide and its isoaspartyl isomers. Bioorg Med Chem Lett. 1999, 9: 953-956. 10.1016/S0960-894X(99)00121-3.
Kuo YM, Webster S, Emmerling MR, De Lima N, Roher AE: Irreversible dimerization/tetramerization and post-translational modifications inhibit proteolytic degradation of Aβ peptides of Alzheimer’s disease. Biochim Biophys Acta. 1998, 1406: 291-298. 10.1016/S0925-4439(98)00014-3.
Fonseca MI, Head E, Velazquez P, Cotman CW, Tenner AJ: The presence of isoaspartic acid in β-amyloid plaques indicates plaque age. Exp Neurol. 1999, 157: 277-288. 10.1006/exnr.1999.7058.
Iwatsubo T, Saido TC, Mann DM, Lee VM, Trojanowski JQ: Full-length amyloid-beta (1-42(43)) and amino-terminally modified and truncated amyloid-beta 42(43) deposit in diffuse plaques. Am J Pathol. 1996, 149: 1823-1830.
Kubo T, Kumagae Y, Miller CA, Kaneko I: Beta-amyloid racemized at the Ser26 residue in the brains of patients with Alzheimer disease: implications in the pathogenesis of Alzheimer disease. J Neuropathol Exp Neurol. 2003, 62: 248-259.
Tomiyama T, Asano S, Furiya Y, Shirasawa T, Endo N, Mori H: Racemization of Asp23 residue affects the aggregation properties of Alzheimer amyloid beta protein analogues. J Biol Chem. 1994, 269: 10205-10208.
Tambo K, Yamaguchi T, Kobayashi K, Terauchi E, Ichi I, Kojo S: Racemization of the aspartic acid residue of amyloid-β peptide by a radical reaction. Biosci Biotechnol Biochem. 2013, 77: 416-418.
Inoue K, Hosaka D, Mochiuki N, Akatsu H, Tsutsumiuchi K, Hashizume Y, Matsukawa N, Yamamoto T, Toyo’oka T: Simultaneous determination of post-translational racemization and isomerization of N-terminal amyloid-beta in Alzheimer’s brain tissues by covalent chiral derivatized ultra-performance liquid chromatography tandem mass spectrometry. Anal Chem. 2013, 86: 797-804.
Portelius E, Gustavsson MK, Zetterberg H, Andreasson U, Blennow K: Evaluation of the performance of novel Aβ isoforms as theragnostic markers in Alzheimer’s disease: from the cell to the patient. Neurodegener Dis. 2012, 10: 138-140. 10.1159/000334537.
Welge V, Fiege O, Lewczuk P, Mollenhauer B, Esselmann H, Klafki H-W, Wolf S, Trenkwalder C, Otto M, Kornhuber J, Wiltfang J, Bibl M: Combined CSF tau, p-tau181 and amyloid-beta 38/40/42 for diagnosing Alzheimer’s disease. J Neural Transm Vienna Austria 1996. 2009, 116: 203-212.
Bibl M, Mollenhauer B, Lewczuk P, Esselmann H, Wolf S, Otto M, Kornhuber J, Rüther E, Wiltfang J: Cerebrospinal fluid tau, p-tau 181 and amyloid-β38/40/42 in frontotemporal dementias and primary progressive aphasias. Dement Geriatr Cogn Disord. 2011, 31: 37-44. 10.1159/000322370.
Britschgi M, Olin CE, Johns HT, Takeda-Uchimura Y, LeMieux MC, Rufibach K, Rajadas J, Zhang H, Tomooka B, Robinson WH, Clark CM, Fagan AM, Galasko DR, Holtzman DM, Jutel M, Kaye JA, Lemere CA, Leszek J, Li G, Peskind ER, Quinn JF, Yesavage JA, Ghiso JA, Wyss-Coray T: Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer’s disease. Proc Natl Acad Sci USA. 2009, 106: 12145-12150. 10.1073/pnas.0904866106.
Marcello A, Wirths O, Schneider-Axmann T, Degerman-Gunnarsson M, Lannfelt L, Bayer TA: Reduced levels of IgM autoantibodies against N-truncated pyroglutamate Aβ in plasma of patients with Alzheimer’s disease. Neurobiol Aging. 2011, 32: 1379-1387. 10.1016/j.neurobiolaging.2009.08.011.
Sullivan CP, Berg EA, Elliott-Bryant R, Fishman JB, McKee AC, Morin PJ, Shia MA, Fine RE: Pyroglutamate-Aβ 3 and 11 colocalize in amyloid plaques in Alzheimer’s disease cerebral cortex with pyroglutamate-Aβ 11 forming the central core. Neurosci Lett. 2011, 505: 109-112. 10.1016/j.neulet.2011.09.071.
Acknowledgements
The authors would like to thank Catherine Widmann, Dr Sascha Weggen and Dr Claus Pietrzik for discussion. This study was supported by the Deutsche Forschungsgemeinschaft (KFO177, TP4) to MTH and by grants of the INMiND project of the European Union to MTH. MTH is member of the DFG Cluster of Excellence ImmunoSensation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they applied for a patent for the use of nitrated Aβ.
Authors’ contributions
MPK and MTH wrote the manuscript. Both authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kummer, M.P., Heneka, M.T. Truncated and modified amyloid-beta species. Alz Res Therapy 6, 28 (2014). https://doi.org/10.1186/alzrt258
Published:
DOI: https://doi.org/10.1186/alzrt258