Abstract
Background
Magnetic resonance imaging (MRI) methods for chemoradiotherapy (CRT) response assessment of rectal cancer include posttreatment T staging (ymrT), tumor regression grading (mrTRG), volume reduction posttreatment, and modified RECIST measurement. We compared these methods in identifying good versus poor responders with the histopathological standards of T stage (ypT) and tumor regression grading (TRG).
Methods
A total of 86 patients underwent CRT in a prospective phase II trial for MRI-defined locally advanced rectal cancer. Two readers independently assessed MRIs for ymrT, mrTRG, volume change, and RECIST. Parameters for each case were categorized as good or poor response and analyzed against ypT and TRG by univariate logistic regression.
Results
A total of 83 patients had evaluable imaging, and 78 had final pathology (five did not undergo surgery). Of these, 34 patients had good response (ypT0-3a) and 44 had poor response (>ypT3a). Also, 27 patients had favorable pathologic TRG (predominant fibrosis) and 51 had unfavorable TRG (predominant tumor). Good mrTRG and ymr <T3b stage were both significantly (P = 0.001) associated with favorable pathology odds ratio [OR] = 16.11 (95 % confidence interval [95 % CI]: 3.36–77.29) and 17.50 (95 % CI: 5.38–56.89), respectively. RECIST measurements and volume reduction of >80 % showed an OR of 3.23 (95 % CI: 1.14–9.17), 4.25 (95 % CI: 0.92–15.45), respectively, for a good ypT score (P = 0.028), but there was no association for histopathological TRG.
Conclusion
Favorable and unfavorable histopathology are predicted by both ymrT and mrTRG, and we recommend these parameters for post-treatment assessment of rectal cancers treated with CRT.


Similar content being viewed by others
Reference
MERCURY. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132–9.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40.
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46.
O’Neill BD, Brown G, Heald RJ, Cunningham D, Tait DM. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol. 2007;8:625–33.
Guillem JG, Diaz-Gonzalez JA, Minsky BD, Valentini V, Jeong SY, Rodriguez-Bigas MA, et al. cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol. 2008;26:368–73.
Suarez J, Vera R, Balen E, Gomez M, Arias F, Lera JM, et al. Pathologic response assessed by Mandard grade is a better prognostic factor than down staging for disease-free survival after preoperative radiochemotherapy for advanced rectal cancer. Colorectal Dis. 2008;10:563–8.
Theodoropoulos G, Wise WE, Padmanabhan A, Kerner BA, Taylor CW, Aguilar PS, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;4:895–903.
Kuo LJ, Liu MC, Jian JJ, Horng CF, Cheng TI, Chen CM, et al. Is final TNM staging a predictor for survival in locally advanced rectal cancer after preoperative chemoradiation therapy? Ann Surg Oncol. 2007;14:2766–72.
Valentini V, Coco C, Picciocchi A, Morganti AG, Trodella L, Ciabattoni A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys. 2002;53:664–74.
Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Jr., Silva e Sousa AH, Jr., et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–7; discussion 717–8.
Chen CC, Lee RC, Lin JK, Wang LW, Yang SH. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48:722–8.
Yeo SG, Kim DY, Kim TH, Jung KH, Hong YS, Chang HJ, et al. Tumor volume reduction rate measured by magnetic resonance volumetry correlated with pathologic tumor response of preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010;78:164–71.
Patel UB, Taylor F, Blomqvist L, George CD, Evans H, Tekkis P, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753–60.
Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23.
Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol. 2005;78:245–51.
Moran B, Brown G, Cunningham D, Daniels I, Heald R, Quirke P, et al. Clarifying the TNM staging of rectal cancer in the context of modern imaging and neo-adjuvant treatment: ‘y’‘u’ and ‘p’ need ‘mr’ and ‘ct’. Colorectal Dis. 2008;10:242–3.
Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours (UICC International Union Against Cancer). Wiley, Oxford; 2009.
Hermanek P, Henson D, Hutter RVP, Sobin LH. TNM Supplement. New York: Springer-Verlag; 1993.
Willett CG, Badizadegan K, Ancukiewicz M, Shellito PC. Prognostic factors in stage T3N0 rectal cancer: do all patients require postoperative pelvic irradiation and chemotherapy? Dis Colon Rectum. 1999;42:167–73.
Salerno G, Chandler I, Wotherspoon A, Thomas K, Moran B, Brown G. Sites of surgical wasting in the abdominoperineal specimen. Br J Surg. 2008;95:1147–54.
Taylor FG, Swift RI, Blomqvist L, Brown G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. AJR Am J Roentgenol. 2008;191:1827–35.
Barbaro B, Fiorucci C, Tebala C, Valentini V, Gambacorta MA, Vecchio FM, et al. Locally advanced rectal cancer: MR imaging in prediction of response after preoperative chemotherapy and radiation therapy. Radiology. 2009;250:730–9.
Machida N, Boku N, Yoshino S, Hironaka Y, Onozawa A, Fukutomi K, et al. editors. Impact of baseline sum longest diameter of target lesions by RECIST on survival of patients with metastatic colorectal cancer (MCRC). Abstract No. 266, 2007 Gastrointestinal Cancers Symposium. American Society of Clinical Oncology: Orlando; 2007.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396.
Dresen RC, Beets GL, Rutten HJ, Engelen SM, Lahaye MJ, Vliegen RF, et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part I. Are we able to predict tumor confined to the rectal wall? Radiology. 2009;252:71–80.
Kang JH, Kim YC, Kim H, Kim YW, Hur H, Kim JS, et al. Tumor volume changes assessed by three-dimensional magnetic resonance volumetry in rectal cancer patients after preoperative chemoradiation: the impact of the volume reduction ratio on the prediction of pathologic complete response. Int J Radiat Oncol Biol Phys. 2010;76:1018–25.
Mayr NA, Yuh WT, Taoka T, Wang JZ, Wu DH, Montebello JF, et al. Serial therapy-induced changes in tumor shape in cervical cancer and their impact on assessing tumor volume and treatment response. AJR Am J Roentgenol. 2006;187:65–72.
Acknowledgement
GB., H. R., D. S. M., R. G. J., E. R., M. P., S. R., C. V. V., and P. Q. were paid honoraria by Sanofi-Aventis for the design of study protocol. E.V.C. received research funding at the University Hospital Leuven paid by Sanofi-Aventis.
Author information
Authors and Affiliations
Corresponding author
Appendices
APPENDIX 1. MRI TECHNIQUE
Hardware
Magnet
A 1.5T system should be used.
Coil
Surface coils are not recommended in the routine staging of rectal cancer.
Phased array coils gain the advantages of the surface coil by obtaining higher signal but with greater coverage than a single surface coil and improved homogeneity.
Coil positioning. It is important in rectal cancers that the coil is correctly centered to the whole rectum. Thus, the lower edge of the coil needs to be placed 5 cm below the symphysis pubis to ensure adequate signal is obtained from the lower rectum and anorectal junction
Suggested Sequences
The initial sequences performed are the localization images, coronal and sagittal to image the tumor and plan the high-resolution images that are performed axial to the rectum (Tables 5 and 6).
The first series is the sagittal T2W-FSE.
The second series is a large field-of-view axial section of the whole pelvis from the iliac crest to the symphasis pubis.
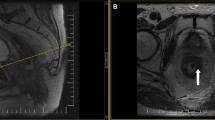
Sequence 3: While the second series is being acquired, the high-resolution images can be planned. The sagittal T2 weighted images obtained are used to planT2-weighted thin-section axial images through the rectal cancer and adjacent perirectal tissues. It is critical that these images are performed perpendicular to the long axis of the rectum. The images are obtained by using a 16-cm field of view, a 3-mm section thickness, and no intersection gap.
APPENDIX 2. EXAMPLES OF ymrT3a-T3d
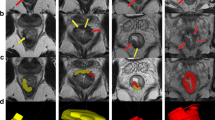
Stage T3a rectal cancer. a Thin-section T2 weighted axial MR images shows a T3a rectal cancer (curved arrow), with extension of less than 1 mm beyond the muscularis propria. The invasive margin is at the 11 o’clock position. b Section of the explanted rectum shows tumor invasion at the 11 o’clock position (curved arrow)
Stage T3b rectal cancer. a and b Thin-section T2 weighted axial MR images show baseline a and post-CRT b images of a T3b rectal cancer, with extension of 3 mm beyond the muscularis propria on both images. The invasive margin is at the 4 o’clock position (curved arrow). c Micrograph (original magnification ×0.4, hematoxylin–eosin [H–E] stain) shows neoplastic glands (curved arrow) extending beyond the muscularis propria (arrowheads)
Stage T3c rectal cancer. a Thin-section T2 weighted axial MR images show baseline a images of a T3c rectal cancer with tumor invasion at the 6 o’clock and 10 o’clock positions (curved arrows). There is 10-mm and of 8-mm invasion from the muscularis propria, respectively. b and c Post-CRT thin-section T2 weighted axial MR images b, with corresponding micrograph c (original magnification ×0.1, hematoxylin–eosin [H–E] stain) shows tumor (curved arrow) with extension of 8 mm beyond the muscularis propria (arrowheads) on both images
Stage T3d rectal cancer. a A T2 weighted sagittal MR shows a baseline T3d rectal cancer, with extension (curved arrow) of greater than 15 mm beyond the muscularis propria. b Post-CRT thin-section T2 weighted axial images show the invasive margin (curved arrow) at the 7 o’clock position. The arrowhead indicates the muscularis propria. c Micrograph (original magnification ×0.4, hematoxylin–eosin (H–E) stain) shows neoplastic glands (arrow) extending beyond the muscularis propria (arrowheads)
APPENDIX 3. RAW DATA FOR INTEROBSERVER AGREEMENT
Rights and permissions
About this article
Cite this article
Patel, U.B., Brown, G., Rutten, H. et al. Comparison of Magnetic Resonance Imaging and Histopathological Response to Chemoradiotherapy in Locally Advanced Rectal Cancer. Ann Surg Oncol 19, 2842–2852 (2012). https://doi.org/10.1245/s10434-012-2309-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-012-2309-3