Abstract
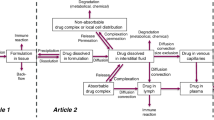
Parenteral delivery remains a compelling drug delivery route for both large- and small-molecule drugs and can bypass issues encountered with oral absorption. For injectable drug products, there is a strong patient preference for subcutaneous administration due to its convenience over intravenous infusion. However, in subcutaneous injection, in contrast to intravenous administration, the formulation is in contact with an extracellular matrix environment that behaves more like a gel than a fluid. This can impact the expected performance of a formulation. Since typical bulk fluid dissolution studies do not accurately simulate the subcutaneous environment, improved in vitro models to help better predict the behavior of the formulation are critical. Herein, we detail the development of a new model system consisting of a more physiologically relevant gel phase to simulate the rate of drug release and diffusion from a subcutaneous injection site using agarose hydrogels as a tissue mimic. This is coupled with continuous real-time data collection to accurately monitor drug diffusion. We show how this in vitro model can be used as an in vivo performance differentiator for different formulations of both large and small molecules. Thus, this model system can be used to improve optimization and understanding of new parenteral drug formulations in a rapid and convenient manner.







Similar content being viewed by others
References
Porter CJH, Charman SA. Lymphatic transport of proteins after subcutaneous administration. J Pharm Sci. 2000;89(3):297–310.
Kinnunen HM, Mrsny RJ. Improving the outcomes of biopharmaceutical delivery via the subcutaneous route by understanding the chemical, physical and physiological properties of hte subcutaneous injection site. J Control Release. 2014;182:22–32.
Medlicott NJ, Waldron NA, Foster TP. Sustained release veterinary parenteral products. Adv Drug Deliv Rev. 2004;56(10):1345–65.
Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14:559–70.
Scott JE. Extracellular matrix, supramolecular organisation and shape. J Anat. 1995;187(Pt 2):259–69.
Levick JR. Flow through interstitium and other fibrous matrices. Q J Exp Physiol. 1987;72:409–37.
Graham DT, Pomeroy AR. An in-vitro test for the duration of action of insulin suspensions. J Pharm Pharmacol. 1984;36(7):427–30.
Wu Z, Tucker EG, Razzak M, Medlicott NJ. An in vitro kinetic method for detection of precipitation of poorly soluble drugs. Int J Pharm. 2005;304(1–2):1–3.
Larsen C, Larsen SW, Jensen H, Yaghmur A, Ostergaard J. Role of in vitro release models in formulation development and quality control of parenteral depots. Expert Opin Drug Deliv. 2009;6:1283–95.
Wu Z, Hassan D, Shaw JP. In-vitro prediction of bioavailability following extravascular injection of poorly soluble drugs: an insight into clinical failure and the role of delivery systems. J Pharm Pharmacol. 2013;65:1429–39.
Marques MRC, Loebenberg R, Almukainzi M. Simulated biological fluids with possible application in dissolution testing. Dissolut Technol. 2011;18:15–28.
Gietz U, Arvinte T, Mader E, Oroszlan P, Merkle HP. Sustained release of injectable zinc-recombinant hirudin suspensions: development and validation of in vitro release model. Eur J Pharm Biopharm. 1998;45:259–64.
Klose D, Azaroual N, Siepmann F, Vermeersch G, Siepmann J. Towards more realistic in vitro release measurement techniques for biodegradable microparticles. Pharm Res. 2009;26(3):691–9.
Pernodet N, Maaloum M, Tinland B. Pore size of agarose gels by atomic force microscopy. Electrophoresis. 1997;18:55–8.
Maaloum M, Pernodet N, Tinland B. Agarose gel structure using atomic force microscopy: gel concentration and ionic strength effects. Electrophoresis. 1998;19:1606–10.
Pluen A, Netti PA, Jain RK, Berk DA. Diffusion of macromolecules in agarose gels: comparison of linear and globular configurations. Biophys J. 1999;77:542–52.
Leung DH, Lamberto DJ, Liu L, Kwong E, Nelson T, Rhodes T, et al. A new and improved method for the preparation of drug nanosuspension formulations using acoustic mixing technology. Int J Pharm. 2014;473(1–2):10–9.
Leung DH, Nelson TD, Rhodes TA, Kwong E, inventors. Merck Sharp & Dohme Corp., assignee. Nano-suspension process. USA patent US20140256818 A1. 2014 Sept 11, 2014.
Sebti I, Blanc D, Carnet-Ripoche A, Saurel R, Coma V. Experimental study and modeling of nisin diffusion in agarose gels. J Food Eng. 2004;63:185–90.
Johnson EM, Berk DA, Jain RK, Deen WM. Hindered diffusion in agarose gels: test of effective medium model. Biophys J. 1996;70:1017–26.
Allababidi S, Shah JC. Kinetics and mechanism of release from glyceryl monostearate-based implants: evaluation of release in a gel simulating in vivo implantation. J Pharm Sci. 1998;87:738–44.
Gillies GT, Wilhelm TD, Humphrey JAC, Fillmore HL, Holloway KL, Broaddus WC. A spinal cord surrogate with nanoscale porosity for in vitro simulations of restorative neurosurgical techniques. Nanotechnology. 2002;13:587–91.
Pomfret R, Miranpuri G, Sillay K. The substitute brain and the potential of the gel model. Ann Neurol. 2013;20(3):118–22.
Holligan DL, Gillies GT, Dailey JP. Magnetic guidance of ferrofluidic nanoparticles in an in vitro model of intraocular retinal repair. Nanotechnology. 2003;14:661–6.
Chen Z, Gillies G, Broaddus W, Prabhu S, Fillmore H, Mitchell R, et al. A realistic brain tissue phantom for intraparenchymal infusion studies. J Neurosurg. 2004;101:314–22.
Kinnunen HM, Sharma V, Contreras-Rojas LR, Yu Y, Alleman C, Sreedhara A, et al. A novel in vitro method to model the fate of subcutaneously administered biopharmaceuticals and associated formulation components. J Control Release. 2015;214:94–102.
Ye F, Larsen SW, Yaghmur A, Jensen H, Larsen C, Ostergaard J. Real-time UV imaging of piroxicam diffusion and distribution from oil solutions into gels mimicking the subcutaneous matrix. Eur J Pharm Sci. 2012;46:72–8.
Jensen SS, Jensen H, Moller EH, Cornett C, Siepmann F, Siepmann J, et al. In vitro release studies of insulin from lipid implants in solution and in a hydrogel matrix mimicking the subcutis. Eur J Pharm Sci. 2016;81:103–12.
Alvarez-Nunez FA, Yalkowsky SH. Buffer capacity and precipitation control of pH solubilized phenytoin formulations. Int J Pharm. 1999;185:45–9.
Soeborg T, Rasmussen CH, Mosekilde E, Colding-Jorgensen M. Absorption kinetics of insulin after subcutaneous administration. Eur J Pharm Sci. 2009;36:78–90.
Scholtz HE, Pretorius SG, Wessels DH, Becker RH. Pharmacokinetic and glucodynamic variability: assessment of insulin glargine, NPH insulin and insulin ultralente in healthy volunteers using a euglycaemic clamp technique. Diabetologia. 2005;48(10):1988–95.
Berenson DF, Weiss AR, Wan Z, Weiss M. Insulin analogs for the treatment of diabetes mellitus: therapeutic applications of protein engineering. Ann N Y Acad Sci. 2011;1243:E40–54.
Pandyarajan V, Weiss MA. Design of non-standard insulin analogs for the treatment of diabetes mellitus. Curr Diabetes Rep. 2012;12:697–704.
http://www.webmd.com/diabetes/guide/diabetes-types-insulin. [cited]; Available from.
Wu F, Bhansali SG, Law WC, Bergey EJ, Prasad PN, Morris ME. Fluorescence imaging of the lymph node uptake of proteins in mice after subcutaneous injection: molecular weight dependence. Pharm Res. 2012;29(7):1843–53.
Coppolino R, Coppolino S, Villari V. Study of the aggregation of insulin glargine by light scattering. J Pharm Sci. 2006;95:1029–34.
Tiong N, Elkordy AA. Effects of liquisolid formulations on dissolution of naproxen. Eur J Pharm Biopharm. 2009;73:373–84.
Tomlinson RV, Spires HR, Kent JS. Absorption, excretion and tissue residue in feedlot heifers injected with the synthetic prostaglandin, fenprostalene. J Anim Sci. 1984;59:164–9.
Kakemi K, Sezaki H, Okumura K, Kobayashi H, Furusawa S. Absorption of drugs from the skeletal muscle of the rat (3). Chem Pharm Bull. 1972;20:443–51.
Nippe S, Preube C, General S. Evaluation of the in vitro release and pharmacokinetics of parenteral injectable formulations for steroids. Eur J Pharm Biopharm. 2013;83:253–65.
Acknowledgements
We would like to thank Peter Wuelfing, Executive Director, Preclinical Development at Merck & Co., Inc. for his valuable comments and a thorough discussion on the findings presented in this manuscript, and Merck Research Laboratories for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
All studies were conducted under a protocol approved by the Merck & Co. Institutional Animal Care and Use Committees (IACUC).
Rights and permissions
About this article
Cite this article
Leung, D.H., Kapoor, Y., Alleyne, C. et al. Development of a Convenient In Vitro Gel Diffusion Model for Predicting the In Vivo Performance of Subcutaneous Parenteral Formulations of Large and Small Molecules. AAPS PharmSciTech 18, 2203–2213 (2017). https://doi.org/10.1208/s12249-016-0698-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-016-0698-5