ABSTRACT
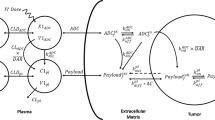
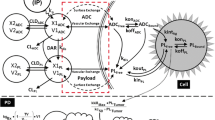
A mathematical model capable of accurately characterizing intracellular disposition of ADCs is essential for a priori predicting unconjugated drug concentrations inside the tumor. Towards this goal, the objectives of this manuscript were to: (1) evolve previously published cellular disposition model of ADC with more intracellular details to characterize the disposition of T-DM1 in different HER2 expressing cell lines, (2) integrate the improved cellular model with the ADC tumor disposition model to a priori predict DM1 concentrations in a preclinical tumor model, and (3) identify prominent pathways and sensitive parameters associated with intracellular activation of ADCs. The cellular disposition model was augmented by incorporating intracellular ADC degradation and passive diffusion of unconjugated drug across tumor cells. Different biomeasures and chemomeasures for T-DM1, quantified in the companion manuscript, were incorporated into the modified model of ADC to characterize in vitro pharmacokinetics of T-DM1 in three HER2+ cell lines. When the cellular model was integrated with the tumor disposition model, the model was able to a priori predict tumor DM1 concentrations in xenograft mice. Pathway analysis suggested different contribution of antigen-mediated and passive diffusion pathways for intracellular unconjugated drug exposure between in vitro and in vivo systems. Global and local sensitivity analyses revealed that non-specific deconjugation and passive diffusion of the drug across tumor cell membrane are key parameters for drug exposure inside a cell. Finally, a systems pharmacokinetic model for intracellular processing of ADCs has been proposed to highlight our current understanding about the determinants of ADC activation inside a cell.







Similar content being viewed by others
References
Sohayla Rostami IQ, Robert Sikorski. The clinical landscape of antibody-drug conjugates 2014. Available from: http://adcreview.com/articles/doi-10-14229jadc-2014-8-1-001/.
Singh AP, Shin YG, Shah DK. Application of pharmacokinetic-pharmacodynamic modeling and simulation for antibody-drug conjugate development. Pharm Res. 2015.
Lin K, Tibbitts J. Pharmacokinetic considerations for antibody drug conjugates. Pharm Res. 2012;29(9):2354–66.
Shah DK, Haddish-Berhane N, Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn. 2012;39(6):643–59.
Shah DK, King LE, Han X, Wentland JA, Zhang Y, Lucas J, et al. A priori prediction of tumor payload concentrations: preclinical case study with an auristatin-based anti-5T4 antibody-drug conjugate. AAPS J. 2014;16(3):453–63.
Gail D. Lewis Phillips GL, Debra L. Dugger, Lisa M. Crocker, Kathryn L. Parsons,Elaine Mai, Walter A. Bla¨ttler, John M. Lambert, Ravi V.J. Chari, Robert J. Lutz, Wai Lee T. Wong, Frederic S. Jacobson, Hartmut Koeppen, Ralph H., Schwall SRK-M, Susan D. Spencer, Mark X. Sliwkowski. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280-90.
Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006;66(8):4426–33.
Oroudjev E, Lopus M, Wilson L, Audette C, Provenzano C, Erickson H, et al. Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability. Mol Cancer Ther. 2010;9(10):2700–13.
Shen BQ, Bumbaca D, Saad O, Yue Q, Pastuskovas CV, Khojasteh SC, et al. Catabolic fate and pharmacokinetic characterization of trastuzumab emtansine (T-DM1): an emphasis on preclinical and clinical catabolism. Curr Drug Metab. 2012;13(7):901–10.
Erickson HK, Lewis Phillips GD, Leipold DD, Provenzano CA, Mai E, Johnson HA, et al. The effect of different linkers on target cell catabolism and pharmacokinetics/pharmacodynamics of trastuzumab maytansinoid conjugates. Mol Cancer Ther. 2012;11(5):1133–42.
Lopus M, Oroudjev E, Wilson L, Wilhelm S, Widdison W, Chari R, et al. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther. 2010;9(10):2689–99.
Jumbe NL, Xin Y, Leipold DD, Crocker L, Dugger D, Mai E, et al. Modeling the efficacy of trastuzumab-DM1, an antibody drug conjugate, in mice. J Pharmacokinet Pharmacodyn. 2010;37(3):221–42.
Wada R, Erickson HK, Lewis Phillips GD, Provenzano CA, Leipold DD, Mai E, et al. Mechanistic pharmacokinetic/pharmacodynamic modeling of in vivo tumor uptake, catabolism, and tumor response of trastuzumab maytansinoid conjugates. Cancer Chemother Pharmacol. 2014;74(5):969–80.
Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60(12):1421–34.
Thurber GM, Schmidt MM, Wittrup KD. Factors determining antibody distribution in tumors. Trends Pharmacol Sci. 2008;29(2):57–61.
Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther. 2009;8(10):2861–71.
Maass KF, Kulkarni C, Betts AM, Wittrup KD. Determination of cellular processing rates for a trastuzumab-maytansinoid antibody-drug conjugate (ADC) highlights key parameters for ADC design. AAPS J. 2016. doi:10.1208/s12248-016-9892-3.
D’Argenio DZ, Schumitzky A, Wang X. ADAPT 5 user’s guide: pharmacokinetic/pharmacodynamic systems analysis software. Los Angeles: Biomedical Simulations Resource; 2009.
Schmidt H, Jirstrand M. Systems Biology Toolbox for MATLAB: a computational platform for research in systems biology. Bioinformatics. 2006;22(4):514–5.
Marino S, Hogue IB, Ray CJ, Kirschner DE. A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol. 2008;254(1):178–96.
Zhang X-Y TM, Lesko LJ, Schmidt S. Sobol sensitivity analysis: a tool to guide the development and evaluation of systems pharmacology models. CPT: Pharmacometrics Syst Pharmacol. 2015;4(2):69–79.
Sukumaran S, Gadkar K, Zhang C, Bhakta S, Liu L, Xu K, et al. Mechanism-based pharmacokinetic/pharmacodynamic model for THIOMAB drug conjugates. Pharm Res. 2015;32(6):1884–93.
Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res : Off J Am Assoc Cancer Res. 2010;16(3):888–97.
Bender B, Leipold DD, Xu K, Shen BQ, Tibbitts J, Friberg LE. A mechanistic pharmacokinetic model elucidating the disposition of trastuzumab emtansine (T-DM1), an antibody-drug conjugate (ADC) for treatment of metastatic breast cancer. AAPS J. 2014;16(5):994–1008.
Bhattacharyya B, Wolff J. Maytansine binding to the vinblastine sites of tubulin. FEBS Lett. 1977;75(1):159–62.
Rock BM, Tometsko ME, Patel SK, Hamblett KJ, Fanslow WC, Rock DA. Intracellular catabolism of an antibody drug conjugate with a noncleavable linker. Drug Metab Dispos: Biol Fate Chem. 2015;43(9):1341–4.
Krippendorff BF, Oyarzun DA, Huisinga W. Predicting the F(ab)-mediated effect of monoclonal antibodies in vivo by combining cell-level kinetic and pharmacokinetic modelling. J Pharmacokinet Pharmacodyn. 2012;39(2):125–39.
Zhitomirsky B, Assaraf YG. Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance. Oncotarget. 2015;6(2):1143–56.
Hamblett KJ, Jacob AP, Gurgel JL, Tometsko ME, Rock BM, Patel SK, et al. SLC46A3 is required to transport catabolites of noncleavable antibody maytansine conjugates from the lysosome to the cytoplasm. Cancer Res. 2015;75(24):5329–40.
Acknowledgments
This work was supported by NIH grant GM114179 to DKS, and the Center for Protein Therapeutics at the State University of New York at Buffalo. K.F.M. was supported by a Hertz Foundation Fellowship and a National Science Foundation Graduate Research Fellowship. C.K. was supported by the Pfizer Worldwide Research & Development Post-Doctoral Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editor: Dhaval K. Shah
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig 1
Local sensitivity of the improved cellular disposition model with respect to unconjugated unbound (free) drug inside the cell as an output. (GIF 111 kb)
High resolution image
(TIF 35.7 kb)
Supplementary Fig 2
Local sensitivity of the improved cellular disposition model with respect to unconjugated unbound (free) drug in media as an output. (GIF 93.9 kb)
High resolution image
(TIF 33.8 kb)
Supplementary Fig 3
Local sensitivity of the improved cellular disposition model with respect to total maytansinoid in media as an output. (GIF 88.9 kb)
High resolution image
(TIF 24.2 kb)
Rights and permissions
About this article
Cite this article
Singh, A.P., Maass, K.F., Betts, A.M. et al. Evolution of Antibody-Drug Conjugate Tumor Disposition Model to Predict Preclinical Tumor Pharmacokinetics of Trastuzumab-Emtansine (T-DM1). AAPS J 18, 861–875 (2016). https://doi.org/10.1208/s12248-016-9904-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-016-9904-3