Abstract
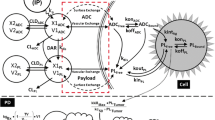
To build a multiscale mechanism based pharmacokinetic–pharmacodynamic (PK/PD) model for antibody drug conjugates (ADCs), using brentuximab-vedotin as an example, for preclinical to clinical translation of ADC efficacy. Brentuximab-vedotin experimental data, collected from diverse publications, were employed in the following steps to build and validate the model: (1) characterization of ADC and payload PK at the cellular level, (2) characterization of payload PK in plasma and tumor tissue of xenograft mouse, (3) characterization of ADC PK in mouse plasma, (4) prediction of the tumor payload concentrations in xenograft mouse by integrating parameters obtained from steps 1–3 with the novel tumor disposition model for ADC, (5) characterization of preclinical brentuximab-vedotin tumor growth inhibition data using the novel PK/PD model, (6) characterization of ADC and payload PK in cancer patients, and (7) prediction of clinical responses of brentuximab-vedotin using the PK/PD model, by integrating PK parameters obtained from step 6, and translated mouse parameters from step 5; and comparing them with clinical trial results. The tumor disposition model was able to accurately predict xenograft tumor and plasma payload concentrations. PK/PD model predicted progression free survival rates and complete response rates for brentuximab-vedotin in patients were comparable to the observed clinical results. It is essential to understand and characterize the disposition of ADC and payload, at the cellular and physiological level, to predict the clinical outcome of ADC. A first of its kind mechanistic model has been developed for ADCs, which can integrate preclinical biomeasures and PK/PD data, to predict clinical response.






Similar content being viewed by others
References
Webb S (2011) Pharma interest surges in antibody drug conjugates. Nat Biotechnol 29(4):297–298
Ducry L, Stump B (2010) Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem 21(1):5–13
Zhou Q, Gallo JM (2011) The pharmacokinetic/pharmacodynamic pipeline: translating anticancer drug pharmacology to the clinic. AAPS J 13(1):111–120
Younes A, Yasothan U, Kirkpatrick P (2012) Brentuximab-vedotin. Nat Rev Drug Discov 11(1):19–20
Sanderson RJ, Hering MA, James SF, Sun MM, Doronina SO, Siadak AW, Senter PD, Wahl AF (2005) In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res 11(2 Pt 1):843–852
Agoram BM, van der Graaf PH (2012) Biomarkers and biomeasures: key enablers for pharmacokinetic–pharmacodynamic modeling in drug discovery and development. Bioanalysis 4(10):1143–1145
Haddish-Berhane N, Shah DK, Betts A, Ma D, DiJoseph J, Leal M, Sapra P, Hans-Peter G (2012) A PK/PD modeling and simulation approach to predict clinical efficacy of antibody drug conjugates from mouse xenograft data. J Pharmacokinet Pharmacodyn (manuscript in preparation)
Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, Senter PD, Alley SC (2010) Intracellular activation of SGN-35, a potent anti-CD30 antibody-drug conjugate. Clin Cancer Res 16(3):888–897
Alley SC (2010) Antibody–Auristatin conjugates for cancer: antibody and drug pharmacokinetics and disposition. Paper presented at the AAPS national biotechnology conference, San Francisco, 16 May 2010–19 May 2010
Schmidt MM, Wittrup KD (2009) A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther 8(10):2861–2871
Thurber GM, Schmidt MM, Wittrup KD (2008) Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev 60(12):1421–1434
Thurber GM, Schmidt MM, Wittrup KD (2008) Factors determining antibody distribution in tumors. Trends Pharmacol Sci 29(2):57–61
Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, Meyer DL, Francisco JA (2004) Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res 10(20):7063–7070
Alley S, Kissler K, Morris-Tilden C, Miyamoto J, Senter P, Benjamin D (2007) Effective tumor targeting by auristatin antibody-drug conjugates. AACR meeting abstracts (annual_meeting):# 4088. AACR, Philadelphia
Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, Rejniak SX, Gordon KA, DeBlanc R, Toki BE, Law CL, Doronina SO, Siegall CB, Senter PD, Wahl AF (2003) cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102(4):1458–1465
Senter P (2009) Prospects and challenges for antibody targeting: antibody-drug conjugates (ADCs). Paper presented at the European federation for pharmaceutical sciences presentation, Stockholm
Simeoni M, Magni P, Cammia C, De Nicolao G, Croci V, Pesenti E, Germani M, Poggesi I, Rocchetti M (2004) Predictive pharmacokinetic–pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res 64(3):1094–1101
Yang J, Mager DE, Straubinger RM (2010) Comparison of two pharmacodynamic transduction models for the analysis of tumor therapeutic responses in model systems. AAPS J 12(1):1–10
Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A (2010) Brentuximab-vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 363(19):1812–1821
Bartlett NL, Forero-Torres A, Rosenblatt JD, Fanale M, Horning SJ, Thompson S, Sievers EL, Kennedy DA (2009) Complete remissions with SGN-35 weekly dosing: a phase 1 dose-escalation study in relapsed/refractory HL or systemic ALCL patients. Paper presented at the ASCO annual meeting, Orlando, 29 May 2009–6 June 2009
Fromm JR, McEarchern JA, Kennedy D, T. A, Shustov AR, Gopal AK (2010: # 1789) Preclinical and clinical binding properties, internalization kinetics, and clinicopathological activity of brentuximab-vedotin (SGN-35): a novel antibody drug conjugate for anaplastic large cell lymphoma and classical Hodgkin lymphoma. Paper presented at the American society of hematology 2010 annual meeting, Orlando, 12 April 2010–12 July 2010
Brons PP, Raemaekers JM, Bogman MJ, van Erp PE, Boezeman JB, Pennings AH, Wessels HM, Haanen C (1992) Cell cycle kinetics in malignant lymphoma studied with in vivo iododeoxyuridine administration, nuclear Ki-67 staining, and flow cytometry. Blood 80(9):2336–2343
Zhao Y, Kosorok MR, Zeng D (2009) Reinforcement learning design for cancer clinical trials. Stat Med 28(26):3294–3315
Fanale MA, Forero-Torres A, Rosenblatt JD, Advani RH, Franklin AR, Kennedy DA, Han TH, Sievers EL, Bartlett NL (2012) A phase I weekly dosing study of brentuximab-vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res 18(1):248–255
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
D’Argenio DZ, Schumitzky A (1979) A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed 9(2):115–134
Arrowsmith J (2011) Trial watch: phase II failures: 2008–2010. Nat Rev Drug Discov 10(5):328–329
Berry DA (2011) Adaptive clinical trials in oncology. Nat Rev Clin Oncol 9(4):199–207
Begley CG, Ellis LM (2012) Drug development: raise standards for preclinical cancer research. Nature 483:531–533
Ternant D, Henin E, Cartron G, Tod M, Paintaud G, Girard P (2009) Development of a drug-disease simulation model for rituximab in follicular non-Hodgkin’s lymphoma. Br J Clin Pharmacol 68(4):561–573
Lobo ED, Balthasar JP (2002) Pharmacodynamic modeling of chemotherapeutic effects: application of a transit compartment model to characterize methotrexate effects in vitro. AAPS Pharm Sci 4(4):E42
Jumbe NL, Xin Y, Leipold DD, Crocker L, Dugger D, Mai E, Sliwkowski MX, Fielder PJ, Tibbitts J (2010) Modeling the efficacy of trastuzumab-DM1, an antibody drug conjugate, in mice. J Pharmacokinet Pharmacodyn 37(3):221–242
Morgan P, Van Der Graaf PH, Arrowsmith J, Feltner DE, Drummond KS, Wegner CD, Street SD (2012) Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov Today 17(9–10):419–424
Krippendorff BF, Oyarzun DA, Huisinga W (2012) Predicting the F(ab)-mediated effect of monoclonal antibodies in vivo by combining cell-level kinetic and pharmacokinetic modelling. J Pharmacokinet Pharmacodyn 39(2):125–139
Wang X, Ma D, Olson WC, Heston WD (2011) In vitro and in vivo responses of advanced prostate tumors to PSMA ADC, an auristatin-conjugated antibody to prostate-specific membrane antigen. Mol Cancer Ther 10(9):1728–1739
Nagata S, Onda M, Numata Y, Santora K, Beers R, Kreitman RJ, Pastan I (2002) Novel anti-CD30 recombinant immunotoxins containing disulfide-stabilized Fv fragments. Clin Cancer Res 8(7):2345–2355
Sutherland MS, Sanderson RJ, Gordon KA, Andreyka J, Cerveny CG, Yu C, Lewis TS, Meyer DL, Zabinski RF, Doronina SO, Senter PD, Law CL, Wahl AF (2006) Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem 281(15):10540–10547
Acknowledgments
The author would like to thank Hugh Barton, Paolo Vicini, Joseph Boni and Jeremy Barton for critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shah, D.K., Haddish-Berhane, N. & Betts, A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn 39, 643–659 (2012). https://doi.org/10.1007/s10928-012-9276-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-012-9276-y