Abstract
Background
Cardiovascular disease (CVD) is considered a major cause of death in renal insufficiency (RI). Contributing genetic factors is a recent focus of research. This study aims to elucidate apolipoprotein E (APO-E) and plasminogen activator inhibitor 1 (PAI-1) gene polymorphisms in RI children associated with CVD.
Methods
We studied 50 cases with chronic kidney disease (CKD) associated with CVD, and 30 healthy controls. Study sample was grouped as one on conservative treatment, the second on hemodialysis and the third was posttransplant. PAI-1 and APO-E gene polymorphisms were investigated using allele-specific polymerase chain reaction (AS-PCR) and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) respectively.
Results
4G4G and 4G5G were the most common PAI-1 polymorphism denoting high association of 4 G allele in renal insufficiency associated with CVD with absent link to dyslipidemia, echocardiography changes or thrombosis. E3E3 was the most common among APO-E polymorphism without relation to dyslipidemia or thrombosis. Dyslipidemia was significantly linked to thrombosis. The study confirmed the role of dyslipidemia and hemodialysis in promoting thrombosis.
Conclusion
Although PAI 4G Genotyping did not show significant association with echocardiography severity or thrombotic severity, yet genetic expression for high levels of PAI in plasma is expected in response to CRI factors known to trigger its release, in addition to those related to dialysis. APO-E3E3 genotyping showed a significant association with echocardiography severity as it enhances APO-A which contributes to CVD. The current study confirmed a significant association between dyslipidemia and CVD; however, the prevalent patterns 4G and E3E3 did not show a significant association with dyslipidemia. The genetic role for APO-A, B, O, or even other isomers for APO-E should be further studied as well.
Similar content being viewed by others
Background
Cardiovascular disease is considered a major cause of death in children with chronic renal failure. Unlike adults, atherosclerosis and coronary ischemia are questionable or subclinical in children whereas, hypertension, impaired cardiac systolic, and diastolic functions and vascular thrombosis are more frequent presentations for CVD. The role of traditional factors related to RF in promoting CVD include hypertension, anemia, dyslipidemia, hypoalbuminemia, and hyperparathyroidism. Patient gene pattern may contribute to the progress of renal damage as well as the development of accelerated CVD through altering some biochemical and biological processes that are reflected in the kidney and cardiovascular system. Plasminogen activator inhibitor-1 (PAI-1) plays a critical role in the regulation of intravascular fibrinolysis [1].
Since plasminogen activators (PA) and their inhibitors are an important role for fibrinolysis and in extracellular matrix degradation, a diminished synthesis of renal PAs and/or increased production of renal (PAI-1) may result in progression of glomerular sclerotic lesions [2]. Coronary heart disease (CHD) and atherosclerosis [1] are the leading cause of mortality and morbidity in the dialysis population [3]. Interstitial fibrosis and tubular atrophy (IFTA) is a clinicopathological entity characterized by fibrosclerosis of the different renal structures leading to the progressive decline of renal function after kidney transplantation [4]. The molecular mechanisms that underlie the pathophysiology of IFTA remain unclear [5]. The upregulation of PAI-1 has been demonstrated in human IFTA accompanied by persistent fibrin deposition in the graft [6]. PAI-1 levels are related to the rate of renal failure progression after kidney transplantation [7]. A common 4G/5G polymorphism in the promoter region of the human PAI-1 gene has been described and is associated with different levels of serum PAI-1 activity [8]. Patients with the 4G4G genotype have the highest levels of PAI-1 activity, whereas those with the 5G5G genotype have the lowest plasma PAI-1 activity [9]. The 5G variant binds the E2F transcription repressor, whereas 4G fails to do so and is associated with the higher PAI1 plasma level [10].
Apolipoprotein E (APO-E) is a lipid carrier protein mainly sensitized in the liver. It is important for uptake, transport, metabolism, and distribution of cholesterol. There are three different alleles for the APO-E gene (E2, E3, and E4) which show a variable association with different kidney and cardiovascular diseases. APO-E polymorphisms have a major role in the pathogenesis of renal diseases they influence the serum lipid profile of patients with end-stage renal disease (ESRD) and consequently the risk for atherosclerotic vascular disease and is associated with development and progress of glomerulopathesis especially with the E2 allele as a predisposing factor in most studies. E4 is associated with a more atherogenic profile in patients with ESRD and is considered a genetic marker for coronary artery disease and global atherosclerosis in posttransplant graft [11]. This study investigated the pattern for both PAI-1 4G/5G and APO-E gene polymorphisms in patients with ESRD associated with cardiovascular complications and tried to find the possible link(s) between the different allelic frequencies to the atherogenic lipid profile and cardiovascular morbidity in these patients.
Methods
Patients
Fifty patients with end-stage renal disease associated with cardiovascular disease (CVD) were included in this study. The patients were followed in our university’s children hospital and were classified according to current treatment into three groups. Group I: 20 patients on conservative treatment. Group II: 20 patients on maintenance hemodialysis. Group III: 10 patients who had been transplanted. The reported signs of CVD included hypertension, echocardiographic changes, and vascular thrombosis. Thirty age and sex-matched apparently healthy controls were included in the study. Informed consents were taken from parents of the children (being incompetent patients) after full explanation of the laboratory tests done. This study protocol and the consents were approved and deemed sufficient by “The Postgraduate Clinical Research and Ethical Committee of Pediatric Department, Faculty of Medicine in our University.”
Methodology
All patients (50 cases) were subjected to good history taking for both renal and cardiovascular clinical data, hemodialysis sessions, posttransplantation follow- up, and vascular access failure due to repeated thrombosis. Laboratory investigations (serum creatinine, BUN, CBC, electrolytes, lipid profile) were done. Conventional echocardiography for assessment of cardiac indices (systolic and diastolic function). Doppler study on the renal artery at the site of anastomosis in the transplanted cases and fistula site in the hemodialysis cases for diagnosis of vascular thrombosis. Echocardiography findings were variable in severity; patients were graded in a discretionary manner into mild, moderate, and severe. The severity of thrombosis was graded in a discretionary manner into mild, moderate, and severe (Tables 1 and 2).
This study investigated the detection of plasminogen activator inhibitor gene and Apolipoprotein E gene polymorphisms by allele-specific PCR (AS-PCR) and PCR-RFLP respectively. DNA extraction was done from whole blood using QIA amp DNA Kit (QIAGEN, USA, catalog no. 51104). This single allele deletion (4G)/insertion (5G) was situated at − 675 bp of the promoter. Extracted DNA was amplified using allele-specific primers [12] and Taq PCR Master Mix (QIAGEN, catalog no. 201443) containing Taq DNA Polymerase, QIAGEN PCR Buffer (with 3 mM MgCl2) and 400 μM of each dNT.
PCR-RFLP technique was used to determine Apo-E genotyping [13]. APO-E genotypes were determined as E2/E2 with 144 bp and 96 bp bands, E4/ E4 with 72 bp and 48 bp, E3/ E4 with144 bp, 72 bp, and 48 bp, E3/E3 with 144 bp and 48 bp fragments, E2/E3 with 144 bp, 96 bp, and 48 bp fragments and E2/E4 with 144 bp, 96 bp, 72 bp, and 48 bp fragments (Fig. 1) [14].
Showing plasminogen activator inhibitor-1 (4G/5G) polymorphism genotyping, lane 1: marker-ladder 100 bp (ferments), all lanes next to lane 1: showed bands at 300 bp (representing amplification of the control primer), after lane 1: each two subsequent lanes represent insertion (4G) then deletion (5G) polymorphism genotyping for every case, lanes 2, 3 (case 1) and 6, 7 (case 3): show bands at 139 bp representing both 4G and 5G heterozygous genotype, lanes 4, 5 (case 2): show a 139 bp band only at lane 4 representing 4G genotype
Statistical methodology
The data was coded and entered using the statistical package SPSS version 15. The data was summarized using descriptive statistics. Number and percentage for qualitative values, statistical difference between groups were tested using chi-square test or Fisher’s exact test for qualitative variables. P values ≤ 0.05 were considered statistically significant.
Results
The study sample mean age was 11.5 (0.6 year − 17 years), with male/female ratio 65%/35%.
Clinical data
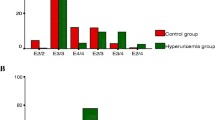
Hypertension was present in all cases of groups I and II and only two cases of group III. Echocardiography findings were classified according to our discretionary grading as shown in Table 3. Frequency for echocardiography findings in each patient group is evident in (Fig. 2).
Thrombosis was absent in group I, present at fistula site in all cases of group II and present in three cases of group III at site of renal artery anastomosis and near segment. The severity of thrombosis in groups II and III was classified according to our discretionary grading as shown in Table 4. Frequency and severity of vascular thrombosis among patients of groups II and III are evident in (Fig. 3). Triglycerides showed significant difference between studied groups (P = 0.034) (Table 5), whereas other lipid markers showed no significant difference between groups. This may be explained as TG is an early marker to be normalized after transplantation. Dyslipidemia showed high triglycerides (TG) and high low-density lipoproteins (LDL) as the most frequent pattern recording incidence of 46% and 44%, respectively, next followed by low HDL, high cholesterol, high CHO/HDL recording incidence of 28%, 20%, and 16%.
Plasminogen activator inhibitor-1 gene polymorphism results
There was a significant difference between groups regarding frequency distribution for PAI-1gene polymorphism; 4G/4G and 4G/5G showed high frequency within each patient group (P = 0.004) (Table 6). Also, there was significant high-frequency for the presence of 4G allele (in its homozygous and heterozygous) among total patient sample versus control (P = 0.000) (Table 7) and (Fig. 4).
Apolipoprotein-E gene polymorphism results
Frequency distribution for Apo-E; E3E3 showed a significant difference between four groups (P = 0.000) (Table 8 and Fig. 5) and also significant high frequency among total patients versus control (P = 0.000) (Table 9, Fig. 5). Patient distribution according to the presence of E3E3 polymorphism showed a significant difference between groups (P = 0.000) as well as a significant difference between total patients and control (P = 0.000) (Table 7).
Correlation between prevalent gene polymorphisms and pattern of dyslipidemia in four groups
There was a non-significant association between the presence of 4G allele and hypertriglyceridemia, hypercholesterolemia, low HDL, high LDL, and high CHO/HDL in each of the four groups. There was a non-significant association between presences of E3E3 polymorphism with altered any of the lipid markers in any of the four groups (Table 10). Therefore, dyslipidemia in the studied sample was not related to the most prevalent studied gene polymorphism it could be related to other Apo isomers or to renal insufficiency.
Correlations between cardiovascular changes versus prevalent gene polymorphism (Tables 11 and 12) and dyslipidemia (Tables 13 and 14)
There was a non-significant association between echocardiography severity and presence of PAI-1 4G allele among each group and in total patients. But, there was a significant association between echocardiography severity and presence of APO-E (E3E3) polymorphism in the total patient sample but not significant in each group (Table 11). There was a non-significant association between thrombotic severity and PAI-1(4G) and APO-E (E3E3) genotyping in any group or total cases (Table 12).
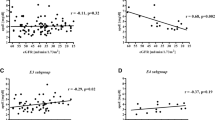
There was a significant association between echocardiography severity and dyslipidemia only in group I, such relation was non-significant in other groups and total sample (Table 13). Statistical relation between thrombotic severity with dyslipidemia shows significance in group II and total cases and non-significant in group III (Table 14).
Previous results may be summarized as echocardiography findings that were related to E3E3, and not 4G in total cases, where also dyslipidemia showed its role but only in predialytic group, possibly, reduction of TG present after HD or transplantation may explain such finding. Thrombosis was prevalent in group II, was not related to prevalent 4G or E3E3, but related to dyslipidemia and HD.
Discussion
Cardiovascular disease was recognized as a major cause of death in children with chronic kidney disease CKD. Similar to adults, children with CKD have an extremely high prevalence of traditional and uremia-related CVD risk factors as hypertension, dyslipidemia, hyperglycemia, uremia, hypoalbuminemia, anemia, and hyperparathyroidism. Early markers of cardiomyopathy include left ventricular hypertrophy, systolic and diastolic dysfunction to be followed with cardiac dilatation and valvular regurge [15]. Subclinical forms of atherosclerosis are reported in children and its early markers include increased carotid arterial intima-media thickness and coronary artery calcification. Cardiovascular disease is particularly evident in those on maintenance hemodialysis as they are very much exposed to altered hemodynamics.
Genetic factors
Contributing to CKD and/or CVD has been recently on focus. Apolipoprotein E and plasminogen activator inhibitor 1-related genes are common examples. The first one is highly involved in lipid metabolism and the latter blocks fibrinolytic pathway and thus promotes vascular thrombosis. The aim of this work is to study the two mentioned gene polymorphisms in children with CKD associated with CVD, with special consideration to those on hemodialysis, looking for special allele patterns. As dyslipidemia is a common sequence for CKD, a major risk factor for CVD and genetically controlled by the Apolipoprotein E gene; therefore, it has been also extensively studied. Links between gene results with dyslipidemia and between each of them with echocardiography findings and thrombosis will be discussed.
Dyslipidemia
Altered serum lipid profile (dyslipidemia) secondary to renal insufficiency is well known since early time. In this study, it was reported in each group and in the total patient sample with different patterns and frequency. Total patient sample with CKD showed that high triglycerides (TG) and high low-density lipoproteins (LDL) as the most frequent pattern recording incidence of 46% and 44% respectively, next followed by low HDL, high cholesterol, and high CHO/HDL recording incidence of (28%, 20%, 16%) (Table 3). Cases et al. reported high TG, normal to high total CHO, low HDL, normal to high LDL, and high VLDL dyslipidemic patterns to associate CRI and described it as atherogenic dyslipidemia [16]. The mean level of TG in this study showed the non-significant difference between predialytic compared to those on hemodialysis. Group III showed a lower mean of triglycerides after renal transplantation (Table 3). Aser also reported no particular effect of hemodialysis on TG as compared to predialytic patients [17]. Pattern and frequency of dyslipidemia in CKD, as well as its relation with hemodialysis and peritoneal dialysis have been extensively discussed in the literature. Normal to high total cholesterol, normal LDL, low HDL, high VLDL in predialytic, and hemodialysis patients were reported by Bregman et al. [18]. Possible mechanisms include postprandial dyslipidemia as the intestine is the site of synthesis of APO-A1 and A2, APO-B48, and APO-E [19]. Also, Charlesworth JA related the greater and prolonged postprandial rise of TG in CRI to be due to impaired clearance of chylomicron remnants [20]. Hepatic role for dyslipidemia in CRI is related to the role of the liver in the synthesis of APO-B100. AP0-C11, APO-C111, AP0–1, and AP0–11, and the role of the liver in expression of receptors for all lipoprotein classes and so its ability to synthesize HDL and VLDL [21]. Other mechanisms for dyslipidemia in CRI include insulin resistance [22], proteinuria [23], and increased oxidative stress with increase LDL [24]. LDL has a high atherogenic effect.
Relation between dyslipidemia and CVD
The relation between dyslipidemia and CVD in this study showed non-significant relation with echocardiography changes in total cases, HD, and post-transplantation. It was only significant in the predialytic group as high TG was most evident in this group (Table 10). Dyslipidemia by itself accelerates glomerular injury to mesangium, endothelium, and podocytes through lipid mediators of oxidative stress, this contributes to the development of hypertension, uremic toxins, hypervolemia, and anemia with their impact on cardiac changes [16]. In adults, dyslipidemia is a high-risk factor for atherosclerosis. Such finding is milder or lacking in children and young adults [25]. Subclinical atherosclerosis begins in childhood and is likely accelerated in children with CKD [26]. As for the association between dyslipidemia and thrombosis (Table 12), there was a significant association between dyslipidemia and the severity of thrombosis among patients on HD. High VLDL triglyceredemia, in particular, is a very important stimulant to express a high level of PAI-1 that promotes thrombosis through its antifibrinolytic effect. HD by itself is a major contributor to vascular thrombosis through vascular access trauma and the release of endothelin, thrombin, and Tpa. It may also be related to the inflammatory response that may happen on the contact of blood with the dialyzer and dialysate through activation of kallikrein-kinin system and generation of cytokines, expression of PAI-1 in high plasma levels that promotes thrombosis [27].
Plasminogen activator inhibitor-1 results
4G in its homozygous 4G/4G and its heterozygous 4G/5G forms constitute the most common within the total patient sample (Table 6 and 7, Fig. 4). The statistical comparison regarding PAI-1 gene polymorphism between the total patient sample and healthy control showed a significant difference, as 4G in its homozygous and heterozygous forms represent the most prevalent pattern (Tables 6 and 7). PAI-1 plasma level is highly determined by PAI-1 genotype in addition to other factors. Higher plasma levels are correlated with polymorphic variance in the number of guanine bases 4G/5G in the promoter at position − 675. 4G is associated with higher plasma levels. 4G/4G shows the highest level, 5G/5G shows the lowest level, and 4G/5G is intermediate [28]. Other factors that determine its level include the balance between agonists and inhibitors. Suppressors include interferon-y, nitric oxide, natriuretic factors, and lipid-lowering drugs [29]. Factors that increase its level include growth factors; coagulation factors as fibrin fragments, thrombin, and Tpa; metabolic factors as glucose, LDL, and APO-A; hormones as aldosterone, angiotensin, renin, and erythropoietin; and environmental factors as endothelial stretch, hypoxia, and endothelin [30].
PAI-1 contributes much to many acute and chronic kidney diseases such as acute thrombotic microangiopathy with fibrin deposition in glomeruli and arterioles as reported in hemolytic uremic syndrome (HUS), scleroderma, and antiphospholipid renal vasculitis [31]. This is also reported in crescentic glomerulonephritis, and antiglomerular basement membrane disease (antiGBM) [32]. Chronic kidney disease associated with high PAI-1 includes focal sclerosis glomerulonephritis (FSGS), diabetic nephropathy, focal necrotizing glomerulonephritis, membranous nephropathy, cyclosporine-induced glomerulosclerosis, and chronic allograft nephropathy [33]. 4G polymorphism was reported with a high incidence among CKD and also in those with high risk of posttransplant rejection [34]. PAI-1 expression is stimulated in CKD through transforming growth factor-beta (TGF-B), angiotensin II, tissue growth factors, cytokines, endothelin − 1, glucose-insulin resistance, LDL, hormones, acute phase response, oxidative stress, endotoxins. PAI-1, as it reduces plasmin activity it promotes thrombotic and necrotizing glomerular lesions that end with sclerosis and it also can directly induce migration of macrophages, trans-differentiated tubular epithelium, and myofibroblasts that end with interstitial fibrosis [34].
Association between presence of G4 and dyslipidemia (Table 10)
There was non-significant association between each form of dyslipidemia with the presence of 4G mutation within each of the patient groups and within total patient sample. The literature reported that PAI-1 plasma level correlates positively with very low-density lipoprotein triglycerides [35], such combination is well evident in CKD with renal dysfunction and is independent on the presence of 4G polymorphism as evident in this study.
Association between G4 polymorphism and severity of echocardiography changes
There was a non-significant association between severity of echocardiography changes with the presence of 4G polymorphism within each of the patient groups and within total patient sample (Table 11). CKD and renal dysfunction per se contribute to the development of CVD through many mechanisms. Primary traditional factors include arterial hypertension, hyperuricemia, diabetes, dyslipidemia particularly high TG, and low HDL-CHO. Non-traditional risk factors include hyperparathyroidism, anemia, hyper homocysteinemia, increased oxidative stress, endothelial dysfunction, apolipoprotein A, inflammatory procoagulant activity [36]. Left ventricular hypertrophy is common in the early stages of CKD greater than would be expected for the degree of hypertension. Left diastolic and systolic dysfunction, cardiac dilatation soon follow and are highly related to uremic myopathy. Some degree of coronary atherosclerosis was found in 80% of a series of autopsies for children on HD [37]. 4G/5G polymorphism in PAI-1 gene in some studies showed association with arterial ischemic strokes, and others showed a significant high level for plasma PAI-1 that was not influenced by the 4G/5G polymorphism [38]. The homozygous or heterozygous carriage of 4G allele had been associated with higher PAI-1 levels and increased risk for CVD [38].
Association between G4 polymorphism and thrombotic changes (Table 12)
There was a non-significant association between the severity of thrombosis with the presence of 4G polymorphism within each of the patient groups and within the total patient sample. Kidney dysfunction itself may generate a thrombotic milieu indirectly through electrolytes, acid-base imbalance, and uremic toxins through their actions on enzymes involved in coagulation [39]. Patients on hemodialysis show leucocyte activation and generation of cytokines that affect the fibrinolytic system. Many studies reported rise in PAI-1 in HD patients and attributed this to endothelial dysfunction and proinflammatory effect of bradykinines; however, the rise may be transient as it drops after dialysis due to bradykinine B2 receptor blockade [40].
Apolipoprotein E results
E3E3 was the most common pattern among HD and predialytic groups and among total sample reporting 75%, 70%, and 60% incidence for each. It was not reported in the control group (Tables 8 and 9 - Fig. 5). E3E3 showed a significant difference among the total patient group (50 cases) as compared to healthy control (60%, 0%) (Tables 8 and 9). APO-E polymorphism associating with renal insufficiency, in multiple studies, showed different data. Prevalence of E2 was reported among Japanese with renal insufficiency [41], E3/E4 among Sweden patients [42], and E3/4 and E4/4 in end-stage renal disease secondary to IgA nephropathy [43]. Guz et al. coincides with this study as no difference in E pattern was reported between hemodialysis and predialytic stage [44]. The study of the relation between dyslipidemia and E pattern in this work (Table 8) showed non-significant association between E3E3 pattern and hypertriglyceridemia, hypercholesterolemia, high LDL, low HDL, and high CHO/HDL among each group and among total sample. This was also reported by Guz G [50]. However, Keane W F et al., reported an association between APO-E and its different patterns with renal disease and progress of renal failure. He attributed this to the role of APO-E in altered lipids, lupoid glomerulosclerosis, dialysis-related amyloidosis, and autocrine role of APO-E as a modulator of glomerular and mesangial proliferation [45]. Eto confirmed the role of E2 in particular in renal disease and renal failure and reported high APO-E polymorphism among patients with ESRD with the secondary rise to APO-A [46]. The relation between E3E3 and echocardiography changes (Table 9) reflects a significant association between E3E3 and severity score for echocardiography changes among total cases. E3E3 pattern represents about 70% incidence in groups 1 and 2 who constitute the major part of the total sample and also that patients who show high incidence and severity of high-risk factors contributing to CVD and echocardiography changes, in other way CV changes is multifactorial and not only associated with APO-E3E3 pattern.
The literature reported the prevalence of APO-A among those with coronary heart disease as they show atherogenic lipid profile and even independently on dyslipidemia. AP0-E enhances APO-A which contributes to CVD and directly contributes to the development of atherosclerosis [47]. Association between apolipoprotein E3E3 and thrombotic grading as evident in (Table 10) shows that thrombotic grade severity was not associated with APO-E3E3 pattern. As thrombosis showed association with dyslipidemia, whereas dyslipidemia did not show significant association with APO-E3E3 presence, we can say that renal insufficiency (RI)-induced dyslipidemia is more influential in promoting thrombosis than APO-E role. The role of dyslipidemia in promoting thrombosis is well known.
Conclusion
Although PAI 4G Genotyping (the most frequent in this study) did not show significant association with echocardiography severity or thrombosis severity, yet genetic expression for high levels of PAI in plasma is expected in response to chronic renal insufficiency (CRI) factors known to trigger its release, in addition to those related to dialysis as previously mentioned. APO-E3E3 Genotyping (the most frequent in this study) showed a significant association with echocardiography severity score as it enhances APO-A which contributes to CVD. The study confirmed the significant association between dyslipidemia and CVD (echocardiography and vascular); however, the two prevalent patterns 4G and E3E3 did not show a significant association with dyslipidemia. The genetic role for other APO-A, B, O, or even other isomers for APO-E should be studied as well. Therefore, the presence of dyslipidemia in such patients, irrespective of being secondary to CRI and or genetic predisposition, should be early managed to slow decline of renal function, to prevent posttransplant interstitial fibrosis and graft loss and to prevent the development of accelerated CVD. Extended study to allocate other patterns of gene polymorphism of high-risk value will be of great help.
Availability of data and materials
Not applicable
Abbreviations
- Anti-GBM:
-
Antiglomerular basement membrane disease
- APO-E:
-
Apolipoprotein E
- AS-PCR:
-
Allele-specific polymerase chain reaction
- BUN:
-
Blood urea nitrogen
- CBC:
-
Complete blood picture
- CHD:
-
Coronary heart disease
- CHO/HDL:
-
Cholesterol/high-density lipoprotein
- CKD:
-
Chronic kidney disease
- CRI:
-
Chronic renal insufficiency
- CVD:
-
Cardiovascular disease
- ESRD:
-
End-stage renal disease
- FSGN:
-
Focal sclerosis glomerulonephritis
- HDL:
-
High-density lipoprotein
- HUS:
-
Hemolytic uremic syndrome
- IFTA:
-
Interstitial fibrosis and tubular atrophy
- LDL:
-
Low-density lipoproteins HD
- PA:
-
Plasminogen activators
- PAI-1:
-
Plasminogen activator inhibitor 1
- PCR-RFLP:
-
Polymerase chain reaction-restriction fragment length polymorphism
References
Hamsten A, Wiman B, de Faire U et al (1985) Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. N Engl J Med 313:1557–1563
Yamamoto K, Loskutoff DJ, Saito H (1998) Renal expression of fibrinolytic genes and tissue factor in a murine model of renal disease as a function of age. Semin Thromb Headmost 24:261–268
London GM, Marchais SJ, Metivier F et al (2000) Cardiovascular risk in end-stage renal disease: vascular aspects. Nephrol Dial Transplant 15(5):97–10
Solez K, Colvin RB, Racusen LC et al (2007) Banff 2005 meeting report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy. Am J Transplant 7(3):518–526
Massy ZA, Guijarro C, Wiederkehr MR et al (1996) Chronic renal allograft rejection: immunologic and non-immunologic risk factors. Kidney Int 49:518–524
Wang Y, Pratt JR, Hartley B et al (1997) Expression of tissue type plasminogen activator and type 1 plasminogen activator inhibitor, and persistent fibrin deposition in chronic allograft failure. Kidney Int 52:371–377
Lahlou A, Peraldi MN, Thervet E et al (2002) Chronic graft dysfunction in renal transplant patients: potential role of plasminogen activator inhibitor type 1. Transplantation 73:1290–1295
Rérolle JP, Munteanu E, Drouet M et al (2008) PAI-1 donor polymorphism influences long-term kidney graft survival. Nephrol Dial Transplant 23(10):3325–3332
Eriksson P, van Kallin BT, Hooft FM et al (1995) Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc Natl Acad Sci U S A 92:1851–1855
Dawson S, Hamsten A, Wiman B et al (1991) Genetic variation at the plasminogen activator inhibitor-1 locus is associated with altered levels of plasma plasminogen activator inhibitor-1 activity. Arterioscler Thromb 11:183–190
Rodrigo E, Gonzalez-Lamuno D, Ruiz JC et al (2002) Apolipoprotein C III and E polymorphisms and cardiovascular syndrome, hyperlipidemia, and insulin resistance in renal transplantation. Am J Transplant 2:343–348
Mitchell Lewis S, Barbara J (2006) Imelda bates. Dacie and Lewis practical hematolog, 10th edn, pp 217–219 chapter 10. https://trove.nla.gov.au/version/46536306
Tagu D, Moussard C (2006) In: Tagu D, Moussard C (eds) Polymorphism of a genome (RFLP) techniques for molecular biology, 1st edn, p 201 ISBN: 9780429078606
Al-Dabbagh NM, Al-Dohayan N, Arfin M et al (2009) Apolipoprotein E polymorphisms and primary glaucoma in Saudis. Mol Vis 15:912–919
Furth SL, Abraham AG, Jerry-Fluker J et al (2011) Metabolic abnormalities, CVD risk factors and GFR decline in children with CKD. Clin J Am Soc Nephrol 6:2132–2140
Cases A, Coll E (2005) Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl 99:S87–S93. https://doi.org/10.1111/j.1523-1755.2005.09916.x
Kimak E, Ksiazek A, Solski J et al (2006) Disturbed Lipoprotien composition in non-dialyzed, hemodialysis, continuous ambulatory peritoneal dialysis and posttransplant patients with chronic renal failure. Clin Chem Lab Med 44(1):64–69. https://doi.org/10.1515/CCLM.2006.013
Bregman H, Daugirdas JT, Ing TS (1994) Complications during hemodialysis. In: Daugirdas JT, Ing TS (eds) Handbook of dialysis. Little, Brown, New York, p 149
Fisher EA, Ginsberg HN (2002) Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B containing lipoproteins. J Biol Chem 277(20):17377–17380PMID: 12006608. https://doi.org/10.1074/jbc.R100068200
Charlesworth JA, Kriketos AD, Jones JE et al (2005) Insulin resistance and postprandial triglyceride levels in primary renal disease. Metabolism. 54:821–828
Ginsberg HN (2002) New perspectives on atherogenesis: role of abnormal triglyceride-rich lipoprotein metabolism. Circulation. 106:2137–2142
Ginsberg HN, Zhang YL, Hernandez-Ono A (2006) Metabolic syndrome: focus on dyslipidemia. Obesity (Silver Spring) 14(1):41S–49S
Klausen KP, Scharling H, Jensen JS (2006) Very low level of micro albuminuria is associated with increased risk of death in subjects with cardiovascular or cerebrovascular diseases. J Intern Med 260:231–237
Massy ZA, Nguyen-Khoa T (2002) Oxidative stress and chronic renal failure: markers and management. J Nephrol 15:336–341
Mitsnefes MM, Kimball TR, Witt SA et al (2004) Abnormal carotid artery structure and function in children and adolescents with successful renal transplantation. Circulation. 110:97–101
Chavers B, Schnaper HW (2001) Risk factors for cardiovascular disease in children on maintenance dialysis. Adv Ren Replace Ther 8:180–190
Segarra A, Chacon P, Martinez-Eyarre C et al (2001) Circulating levels of plasminogen activator inhibitor type-1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: biochemical correlations and role as independent predictors of coronary artery stenosis. J Am Soc Nephrol 12:1255–1263
Shaoyong S, Chen S, Zhao J et al (2006) Plasminogen activator Inhibitor-1 gene: selection of tagging single nucleotide polymorphisms and association with coronary heart disease. Arterioscler Thromb Vasc Biol 26:948–954
Gallicchio M, Hufnagl P, Wojta J et al (1996) IFNgamma inhibits thrombin- and endotoxininduced plasminogen activator inhibitor type 1 in human endothelial cells. J Immunol 157:2610–2617
Kohler HP, Grant PJ (2000) Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med 342:1792–1801
Chandler W, Jelacic S, Boster D et al (2002) Prothrombotic coagulation abnormalities during Escherichia coli 0157 :H 7 infections. N Engl J Med 346:23–32
Lee HS, Park SY, Moon KC et al (2001) mRNA expression of urokinase and plasminogen activator inhibitor-1 in human crescentic glomerulonephritis. Histopathology. 39:203–209
Eddy AA, Fogo AB (2006) Plasminogen activator Inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol 17:2999–3012
Reis K, Arinsoy T, Derici U et al (2005) Angiotensinogen and plasminogen activator inhibitor-1 gene polymorphism in relation to chronic allograft dysfunction. Clin Transpl 19:10–14
Kaysen GA (2001) The microinflamatory state in uremia: causes and potencial consequences. J Am Soc Nephrol 12:1549–1557
Martin LC, Franco RJ, Gavras I (2004) Association between hypervolemia and ventricular hypertrophy in hemodialysis patients. Am J Hypertens 17:1163–1169
Portman RJ, Hawkins E, Verani R (1991) Premature atherosclerosis in pediatric renal patients: report of the southwest pediatric nephrology study group. Pediatr Res 29:349A Presented to the Society for Pediatric Research, New Orleans, April 30, 1991. https://www.fda.gov/media/112288/download
Assawamakin A, Sriratanaviriyakul N, Lalerd Y (2012) Meta-analysis of the plasminogen activator inhihitor 1 (PAI-1) gene with insertion/deletion 4G/5G polymorphism and its susceptibility to ischemic stroke in Thai population. Asian Biomedicine 6(2):203–217 Asian Biomedicine Research, Reviews and News www.asianbiomed.org. ISSN 19057415
Dubin R, Cushman M, Folsom AR et al (2011) Kidney function and multiple hemostatic markers: cross sectional associations in the multi-ethnic study of atherosclerosis. BMC Nephrol 12:3
Marney AM, Luther JM et al (2009) Endogenous bradykinin contributes to increased plasminogen activator inhibitor 1 antigen following hemodialysis. J Am Soc Nephrol 20:2246–2252
Oda H, Yorioka N, Ueda C, Kushihata S et al (1999) Apolipoprotein E polymorphism and renal disease. Kidney Int Suppl 71:S25–S27
Roussos L, Floren CH, Carlson J et al (1999) Increased prevalence of apolipoprotein E3/E4 genotype among Swedish renal transplant recipients. Nephron. 83:25–30
Yorioka N, Nishida Y, Oda H, Watanabe T, Yamakido M (1999) Apo-E polymorphism in IgA nephropathy. Nephron. 83(3):246–249. https://doi.org/10.1159/000045517
Guz G, Ozdemir FN, Sezer S et al (2000) Effect of apolipoprotein E polymorphism on serum lipid, lipoproteins, and atherosclerosis in hemodialysis patients. Am J Kidney Dis 36:826–836
Keane WF, Kasiske BL, O’Donnell MP (1988) Lipids and progressive glomerulosclerosis. Am J Nephrol 8:261–271
Eto M, Saito M, Okada M et al (2002) Apolipoprotein E genetic polymorphism, remnant lipoproteins, and nephropathy in type 2 diabetic patients. Am J Kidney Dis 40:243–251
Curtiss LK, Boisvert WA (2000) Apolipoprotein E and atherosclerosis. Curr Opin Lipidol 11:243–251
Acknowledgements
We would like to thank all patients and their family members for their valuable contributions to the study.
Funding
The authors declare that they did not receive any financial support from agencies or others.
Author information
Authors and Affiliations
Contributions
BM and AB contributed to the conception and design of study. NG, BM, RS, and AS acquired the data. BM, AB, and RS contributed to the analysis and interpretation of the data. BM, AB, RS, and NG drafted the manuscript. BM and AS revised the manuscript. All authors read and approved the final manuscript.
Authors’ information
Professor Bahia Hassan Moustafa is a Professor and Consultant of Pediatric Nephrology, President of African Pediatric Nephrology Association (AFPNA) (2000–2006), Counselor of International Pediatric Nephrology Association (IPNA) (2000–2006), Head of Pediatric Nephrology and Dialysis Unit, Cairo university children hospitals since 2006–2008, and Head of Pediatrics Unit, Cairo University since 2005–2008.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study protocol and the consents were approved and deemed sufficient by
“The Postgraduate Clinical Research and Ethical Committee of Pediatric Department, Faculty of Medicine, Cairo University.” Ethics committee’s reference number (352011)
and informed written consent was obtained in every case from their legal guardians.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests. They declare that the research was conducted in the absence of any commercial or financial relationships.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Moustafa, B.H., Badr, A., Selim, A. et al. Apolipoprotein E and plasminogen activator inhibitor 1 gene polymorphism in children with chronic renal insufficiency associated with cardiovascular disease. Egypt Pediatric Association Gaz 67, 10 (2019). https://doi.org/10.1186/s43054-019-0011-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43054-019-0011-9