Abstract
With the rising number of anterior cruciate ligament (ACL) reconstructions, revision ACL reconstructions are becoming increasingly common. A revision procedure may be performed to improved knee function, correct instability, and facilitate a return to normal activities. When performing a revision reconstruction, the surgeon decides between a single-stage or a two-stage revision. Two-stage revisions are rarely performed, but are particularly useful when addressing substantial tunnel-widening, active infection, and concomitant knee pathology (e.g., malalignment, other ligamentous injuries, meniscal or chondral lesions). Among these potential scenarios requiring a two-stage revision, tunnel-widening is the most common cause; the first stage involves graft removal, tunnel curettage, and bone grafting, followed by revision ACL reconstruction in the second stage. The purpose of this article is to review the preoperative planning, surgical considerations, rehabilitation, and outcomes of two-stage revision ACL reconstructions and summarize the recent literature outlining treatment results.
Similar content being viewed by others
Background
Anterior cruciate ligament (ACL) reconstruction rates have increased over the past 20 years to roughly 200,000 per year [1]. As this number has continued to increase, the incidence of revision ACL reconstruction (ACLR) has also grown to a rate of between 4.1 and 13.3% of all primary ACLRs performed [2]. The goal of revision ACLR is to improve knee stability and activity levels, but the outcomes are reported to be inferior to those of primary ACLR [3]. Successful revision surgery requires an understanding of the cause of failure, careful preoperative planning, meticulous surgical execution, proper postoperative rehabilitation, and appropriate patient counseling [4].
Revision ACLR surgeries can be mainly divided into one-stage and two-stage procedures. Two-stage revision ACLR typically involves an initial bone-graft procedure—to fill the widened or misplaced tunnels—and subsequent time to allow for the bone graft to heal sufficiently before the second stage is undertaken [5]. A relatively small but challenging subset of patients requires two-stage revision ACLR. Reports suggest that a two-stage procedure is performed in only 8 to 9% of revision ACLRs [6].
To date, the literature on revision ACLR surgery has primarily focused on comparing the outcomes to those of primary ACLR. While one-stage revision ACLR is well described and reported, few studies have reported the outcomes of two-stage revision ACLR. For the aforementioned reasons, in this review, we will provide an overview of two-stage revision ACLR in the following order: preoperative planning, surgical considerations, rehabilitation, outcomes, and conclusions.
Preoperative planning for two-stage ACLR
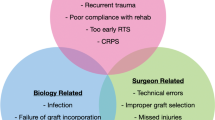
Preoperative planning for revision ACL surgery is essential for a successful outcome. The important stages in assessing a patient with failed ACL surgery include history, patient selection, physical examination and investigations, choice of graft, surgical technique, and rehabilitation [7]. Major reasons to proceed with a two-stage strategy include tunnel-widening or other loss of bone stock, tunnel malposition, arthrofibrosis, active infection, concomitant meniscal deficiency, malalignment, and focal chondral lesions and/or other ligamentous laxity that may require a staged approach [8, 9] (Table 1).
An active infection should be treated with irrigation and debridement with confirmation of eradication (e.g., normalized laboratory test results, negative cultures) before a patient has a new graft and implant put in place. Similarly, a patient with a loss of more than 5° of extension or 20° of flexion of knee motion should be considered for lysis of adhesions and manipulation under anesthesia followed by rehabilitation [4, 10].
Tunnel orientation and size are the most important causes related to the two-stage procedure, as these enlarged tunnels may complicate graft placement and fixation [11, 12]. Although there are many proposed theories for tunnel lysis, it is most accurate to state that this condition has a multifactorial origin; mechanical and biologic causes have been reported, and both contribute to enlarged graft tunnels [11, 13]. Tunnel malpositioning that will interfere with new revision reconstruction tunnel placement can reduce graft apposition within the tunnels at the time of graft fixation, thereby placing the graft stability and subsequent incorporation at greater risk of failure [11].
Currently, the “gold standard” for measuring tunnel size is the computed tomography (CT) method. Studies have shown that CT outperforms magnetic resonance imaging (MRI) and radiographs in both inter- and intra-observer reliability for evaluating tunnel-widening [14, 15]. When measuring with CT, the axial-plane image is considered incorrect because the plane of cuts is inconsistent. Therefore, the coronal and sagittal images (four-tunnel view; femur-coronal, tibia-coronal, femur-sagittal, tibia-sagittal) are primarily used (Fig. 1). Measurements are made perpendicular to the axial plane of the tunnel at the widest point [15].
Previous literature has reported that if the tunnel size exceeds 10–15 mm, two-stage surgery should be performed. However, an absolute threshold for how much tunnel-widening and bone loss is acceptable to undergo a single stage with an intraoperative bone graft prior to drilling has not been established [4, 16,17,18,19]. Battaglia and Miller [12] indicated that bone grafting should be performed in cases with a tunnel diameter of 10–15 mm. Additionally, Brown and Carson [20] regarded patients with a bone tunnel of < 15 mm diameter as good candidates for grafting. They explained that because a bone tunnel of 15 mm diameter with 45° of inclination resulted in a tibial tunnel aperture of > 20 mm, a 20-mm tunnel aperture was regarded as a candidate for grafting. Yoon et al. [21] evaluated 88 patients who underwent one-stage revision ACLR. The patients were divided into two groups based on the tunnel diameter (group A, < 12 mm; group B, < 12 mm). At a mean follow-up of 7.9 years, clinical scores following revision ACLR did not differ significantly according to the tunnel size. However, the results of the postoperative side-to-side differences of the Lachman test as well as the pivot-shift test were significantly superior in group A (< 12 mm).
Surgical considerations of two-stage ACL reconstruction
Bone grafting
Autograft bone, either from the iliac crest or anterior tibial plateau, is still considered the gold standard source for grafting because of its osteoconductive, osteoinductive, and osteogenic properties. Clinically, many authors have reported good results for two-staged revision ACLR using autograft bone [4, 11]. Thomas et al. reported that the laxity measurements achieved with a two-stage revision ACLR using autograft iliac bone could be similar to those achieved after primary ACLR and clinical improvement [11]. But an iliac-crest autograft is comparatively invasive with relatively high donor-site morbidity and the potential for insufficient yield quantities [11, 22]. For an allograft, a single bone dowel approximately 1 mm larger than the diameter of the tunnel is used and placed using a bone tamp for a press-fit technique, ensuring that the entire tunnel is filled [4]. The use of allograft material negates the issue of donor-site morbidity but carries the potential risk of disease or infection transmission [23, 24]. To minimize the risk of viral and bacterial contamination, allograft bone is sterilized. However, methods used to sterilize allograft material (e.g., gamma irradiation and autoclaving), are known to adversely affect the structural and other properties of the graft material [25].
Recently, a technique for sterilizing musculoskeletal allografts using supercritical carbon dioxide (sCO2) has been developed [26]. In theory, the sCO2-sterilized graft only provides osteoconductive properties to the grafted bone tunnels. The metaphyseal location and predominantly cancellous bone surrounding the graft tissue result in high osteoinductive and osteogenic potential from the host’s bone marrow [26]. Van de pol et al. [26] reported the use of a sCO2-sterilized bone allograft to fill tunnel defects as the first stage of a two-stage revision ACLR. The mean time between the two stages was 8.8 months and in the second stage, bone-biopsy specimens were taken from the tibia. They found that a sCO2-sterilized bone allograft showed graft incorporation and remodeling through creeping substitution.
Silicate-substituted calcium phosphate (Si-CaP), which represents a synthetic, porous bone-graft substitute, may also be an appropriate bone-graft substitute [27,28,29,30]. Si-CaP appears to provide a more stable osteoconductive scaffold to support faster angiogenesis. Von recum et al. [31] used Si-CaP for a bone-graft substitute for tunnel augmentation in two-stage revision ACLR. Punch-biopsy specimens of the augmented tunnels were taken at the two-stage procedure, and histologic examination included quantitative analysis of the area of immature bone formation, lamellar bone, and bone marrow. CT analysis also included the determination of the filling rates of the tunnels. They reported that Si-CaP as a bone-graft substitute for tunnel augmentation showed favorable histologic, radiologic, and intraoperative integration comparable to the autologous iliac bone graft.
Timing of two-stage revisional ACL reconstruction
The optimal and earliest possible timing of the two-stage procedure is still not clear. Typically, a staged procedure requires an average delay of 4 to 6 months to allow for the bone defect to heal [11, 18], likely subjecting patients to a prolonged period of knee instability and thus adding to the risk of meniscal injury, additional deterioration of muscle strength, and osteochondrosis [32]. For assessment of bone-graft incorporation, radiographs are routinely used. Some authors have described the additional use of CT scans to confirm healing at 3–5 months after bone grafting [4, 12, 33, 34]. Thomas et al. performed a CT scan at 4 months to assess healing of the bone graft in the tibial tunnel. Blurring of the tunnel margins, reactive sclerosis, and the presence of bone within the tunnel were used as signs of adequate healing. They observed that an average of 5.8 months was needed for healing of the autograft dowel to become visible on CT scans [11]. Uchida et al. [34] reported 10 consecutive patients (four female and six male patients with a mean age of 28 years) who underwent autogenous bone grafting prior to ACLR revision. CT examinations were performed at 3, 12, and 24 weeks after bone grafting. Evaluations were performed in the axial plane of the tibia using three parameters (occupying ratio, union ratio, and bone mineral density). They recommended that two-stage reconstruction could be safely performed at 24 weeks after bone grafting by the iliac-bone block-grafting technique.
Graft choice and fixation
There has been a long-standing debate as to whether an autograft or an allograft should be used for revision ACLR. A decision that will often depend on the graft used during the primary ACLR. However, many authors prefer using an autograft for revision ACLR when possible. According to the result of the multicenter ACL Revision Study (MARS) Group, the risk of graft re-rupture following revision ACLR in patients receiving an autograft is 2.78 times less likely than in those receiving an allograft [35]. Noyes et al. advocate that the allograft should not be considered as the first choice of graft for revision surgery [36]. If no autograft is available for revision surgery, they advise augmentation of the allograft with the lateral extra-articular iliotibial band procedure to reduce the high failure rate associated with the use of the allograft.
Patient age and activity level are also important factors when deciding on graft choice for revision procedures. Allografts may be well suited for recreational athletes older than 30 years of age, but autografts may be a better choice for younger athletes who wish to return to higher-level athletics [4].
Secure graft fixation is critical in ensuring a successful two-staged ACLR. Because of weak bone from bone-grafted tunnels or enlarged tunnels, the surgeons should pay careful attention to the fixation methods and consider double fixation in all revisions [37]. The insertion of an interference screw not only compresses the graft in the tunnel but also leads to an enlargement of the bone tunnel itself [13]. When aperture fixation is not possible, familiarity with, and use of, all-inside tibial and femoral sockets with cortical suspensory fixation may be necessary [4].
Additional procedure
Numerous studies have reported that additional procedures (e.g., extra-articular tenodesis, anatomical anterolateral ligament (ALL) reconstruction) could be a meaningful option in cases of revision ACLR to improved rotatory stability which is associated with re-injury.
Trojani et al. [38] have reported the outcomes of revision ACLR with and without lateral extra-articular tenodesis. They noted that although additional lateral tenodesis did not influence the International Knee Documentation Committee (IKDC) score in a multicenter study of 163 revision ACLRs, the proportion of negative pivot shifts was 80% with lateral tenodesis plus revision ACLR versus 63% without tenodesis. Louis et al. [39] have demonstrated that 349 patients who underwent revision ACLR-combined-ALL reconstructions showed improving rotational stability without increasing the risk of early and late complications and the re-rupture rate was 1.2% in their multicenter study.
Lee et al. [40] reported the results of 87 patients who underwent revision ACLR with a follow-up of more than 3 years. Patients were divided into the isolated revision ACLR group (n = 45) and the revision ACLR group in combination with ALL reconstruction (n = 42). They observed that revision ACLR in combination with ALL reconstruction significantly reduced rotational laxity and showed a higher rate of return to the same level of sports activity than revision ACLR alone, although there were no significant differences in anterior laxity or functional test results between the two groups.
Rehabilitation
In the immediate postoperative period, the weakest part of any ACLR is the fixation. After 6 to 12 weeks, failures tend to occur in mid-substance [11]. Some authors suggest that an accelerated rehabilitation program for revision ACLR is not appropriate because of weaker initial graft fixation [20]. However, Thomas et al. [11] noted that this suggestion is unnecessary, as using a two-stage technique ensures that there is good-quality bone around the tunnels, and the initial graft fixation is as secure as in the primary reconstruction.
Rehabilitation after the initial bone-grafting stage shares similarities with standard ACLR protocols [17]. The initial rehabilitation emphasis is focused on restoring tibiofemoral and patellofemoral passive range of motion, restoring quadriceps’ activation, and controlling and resolving any joint effusion. No restrictions are placed on their range of motion and patients were allowed to weightbear on the affected leg using crutches [17]. Physical therapy with muscle-strengthening and proprioceptive training can be performed. Improved muscle strength may be the decisive factor; however, changes in functional movement patterns after intensive physical therapy are also important to consider [41].
Outcomes
Few studies report the outcomes of two-stage revision ACLR alone. Current studies report an average-low failure rate of 3.6% (wide range of 0–8.1%) for utilizing two-stage revision ACLR [11, 33, 34, 42, 43] (Table 2).
Thomas et al. [11] reported the results of 49 consecutive two-stage revision ACLRs in which the tibial tunnel was grafted (the bone graft was taken from the ipsilateral iliac crest) during the first stage, followed by an ACLR using various grafts and fixation methods for the second stage. The results from this group were compared to the results of a matched group of patients with primary ACLR. The two-stage group contained significantly more patients with meniscal and chondral pathology compared with the primary ACLR group. At a mean follow-up of 6 years, the laxity measurements achieved with a two-stage revision ACLR can be similar to those achieved after primary ACLR, although the IKDC rating is lower.
Franceschi et al. [33] evaluated 30 patients who underwent two-staged ACLR revision procedure after a traumatic re-rupture of the ACL. All the patients in the study underwent screw removal and filling of the tunnels with an autograft harvested from the anterior tibial metaphysis. The second stage of the revision ACLR was performed a minimum of 3 months later, after obtaining a CT demonstrating adequate filling of the tunnels using a hamstring autograft though a transtibial drilling technique. The new ligament was fixed to the tibia by a metallic screw and to the femur by a bioabsorbable screw. At a mean follow-up 6.7 years postoperatively, 66.7% of patients had returned to their preoperative sports activity level, 23.3% had changed to lower, non-impact sports, and 10% had given up any sports activity. There was also a significant improvement in the Lysholm score when comparing preoperative and postoperative values.
Uchida et al. [34] evaluated 10 consecutive patients who underwent staged revision ACLR using autogenous bone grafting and reported that all patients had a full range of motion of the knees, a negative Lachmann sign and negative pivot-shift test . An average Lysholm score at 2 years post operation was 96.6 points ± 2.1 (91–100 points).
One comparative cohort study reported that objective outcomes and subjective patient scores and satisfaction were not significantly different between one-stage and two-stage revision ACLRs and both groups had significantly improved objective outcomes and patient subjective outcomes without notable differences in failure rates [42]. They observed that the the failure rate was 10.3% in the one-stage revision group and 6.1% in the two-stage group. In additional analyses, 24% (12/49) of patients were newly found to have concomitant knee injuries (e.g., chondral defects, meniscal lesions) at the time of the second-stage operative procedure.
Diermeier et al. [43] reported the results of 54 patients who underwent bone grafting due to recurrent, symptomatic ACL deficiency following ACLR. Only 44 patients underwent a staged revision ACLR after bone grafting and 10 patients refused to undergo a revision ACLR. At a mean period of 33.9 months, there was an improvement in the Lysholm score (77.2 ± 15.5 vs 72.9 ± 18.7), IKDC score (69.0 ± 13.4 vs 69.3 ± 13.4) and Tegner activity score (4.1 ± 1.5 vs 4.6 ± 1.2) for both groups. But no significant difference was observed between the two groups. Knee-laxity measurements were elevated in the without-revision group, but the difference was not significant. Postoperatively, no complications were reported and none of the included patients had a flexion or extension deficit. However, the small number of included patients, especially in the group of patients without revision ACLR, is limited.
Conclusions
In active young patients, failed primary ACLR may require a revision ACLR. Two-stage revision ACLR should be considered in cases of tunnel lysis, infection, malalignment, meniscal deficiency, or chondral lesions. A two-stage procedure is technically more demanding than the primary or one-stage procedure and outcomes are potentially inferior, especially for active patients who make a high demand on their bodies. However, with precise indications, proper preoperative planning and operative-technique selection, two-stage revision ACLR can achieve favorable outcomes.
Availability of data and materials
Not applicable, this is a review article.
Abbreviations
- ACL:
-
Anterior cruciate ligament
- ACLR:
-
Anterior cruciate ligament reconstruction
- ALL:
-
Anterolateral ligament
- sCO2:
-
Supercritical carbon dioxide
- Si-CaP:
-
Silicate-substituted calcium phosphate
References
Ohly NE, Murray IR, Keating JF (2007) Revision anterior cruciate ligament reconstruction: timing of surgery and the incidence of meniscal tears and degenerative change. J Bone Joint Surg Br 89:1051–1054
van Eck CF, Schkrohowsky JG, Working ZM, Irrgang JJ, Fu FH (2012) Prospective analysis of failure rate and predictors of failure after anatomic anterior cruciate ligament reconstruction with allograft. Am J Sports Med 40:800–807
Group M, Ding DY, Zhang AL, Allen CR, Anderson AF, Cooper DE et al (2017) Subsequent surgery after revision anterior cruciate ligament reconstruction: rates and risk factors from a multicenter cohort. Am J Sports Med 45:2068–2076
Erickson BJ, Cvetanovich G, Waliullah K, Khair M, Smith P, Bach B Jr et al (2016) Two-stage revision anterior cruciate ligament reconstruction. Orthopedics 39:e456–e464
Noyes FR, Barber-Westin SD (2006) Anterior cruciate ligament revision reconstruction: results using a quadriceps tendon-patellar bone autograft. Am J Sports Med 34:553–564
MARS Group, Wright RW, Huston LJ, Spindler KP, Dunn WR, Haas AK et al (2010) Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med 38:1979–1986
Dye SF (1996) The future of anterior cruciate ligament restoration. Clin Orthop Relat Res 325:130–139
Andernord D, Desai N, Bjornsson H, Ylander M, Karlsson J, Samuelsson K (2015) Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med 43:121–127
Carson EW, Anisko EM, Restrepo C, Panariello RA, O'Brien SJ, Warren RF (2004) Revision anterior cruciate ligament reconstruction: etiology of failures and clinical results. J Knee Surg 17:127–132
Mayr R, Rosenberger R, Agraharam D, Smekal V, El Attal R (2012) Revision anterior cruciate ligament reconstruction: an update. Arch Orthop Trauma Surg 132:1299–1313
Thomas NP, Kankate R, Wandless F, Pandit H (2005) Revision anterior cruciate ligament reconstruction using a 2-stage technique with bone grafting of the tibial tunnel. Am J Sports Med 33:1701–1709
Battaglia TC, Miller MD (2005) Management of bony deficiency in revision anterior cruciate ligament reconstruction using allograft bone dowels: surgical technique. Arthroscopy 21:767
Wilson TC, Kantaras A, Atay A, Johnson DL (2004) Tunnel enlargement after anterior cruciate ligament surgery. Am J Sports Med 32:543–549
Groves C, Chandramohan M, Chew C, Subedi N (2013) Use of CT in the management of anterior cruciate ligament revision surgery. Clin Radiol 68:e552–e559
Marchant MH Jr, Willimon SC, Vinson E, Pietrobon R, Garrett WE, Higgins LD (2010) Comparison of plain radiography, computed tomography, and magnetic resonance imaging in the evaluation of bone tunnel widening after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 18:1059–1064
Bhatia S, Korth K, Van Thiel GS, Frank RM, Gupta D, Cole BJ et al (2016) Effect of tibial tunnel diameter on femoral tunnel placement in transtibial single bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 24:51–57
Chahla J, Dean CS, Cram TR, Civitarese D, O’Brien L, Moulton SG et al (2016) Two-stage revision anterior cruciate ligament reconstruction: bone grafting technique using an allograft bone matrix. Arthrosc Tech 5:e189–e195
Hofbauer M, Muller B, Murawski CD, Baraga M, van Eck CF, Fu FH (2013) Strategies for revision surgery after primary double-bundle anterior cruciate ligament (ACL) reconstruction. Knee Surg Sports Traumatol Arthrosc 21:2072–2080
Magnussen RA, Debieux P, Benjamin B, Lustig S, Demey G, Servien E et al (2012) A CT-based classification of prior ACL femoral tunnel location for planning revision ACL surgery. Knee Surg Sports Traumatol Arthrosc 20:1298–1306
Brown CH Jr, Carson EW (1999) Revision anterior cruciate ligament surgery. Clin Sports Med 18:109–171
Yoon KH, Kim JS, Park SY, Park SE (2018) One-stage revision anterior cruciate ligament reconstruction: results according to preoperative bone tunnel diameter: five to fifteen-year follow-up. J Bone Joint Surg Am 100:993–1000
Banwart JC, Asher MA, Hassanein RS (1995) Iliac crest bone graft harvest donor site morbidity. a statistical evaluation. Spine (Phila Pa 1976) 20:1055–1060
Campbell DG, Li P (1999) Sterilization of HIV with irradiation: relevance to infected bone allografts. Aust N Z J Surg 69:517–521
Eagan MJ, McAllister DR (2009) Biology of allograft incorporation. Clin Sports Med 28:203–214 vii
Islam A, Chapin K, Moore E, Ford J, Rimnac C, Akkus O (2016) Gamma radiation sterilization reduces the high-cycle fatigue life of allograft bone. Clin Orthop Relat Res 474:827–835
Van de Pol GJ, Bonar F, Salmon LJ, Roe JP, Pinczewski LA (2018) Supercritical carbon dioxide-sterilized bone allograft in the treatment of tunnel defects in 2-stage revision anterior cruciate ligament reconstruction: a histologic evaluation. Arthroscopy 34:706–713
Hing KA, Revell PA, Smith N, Buckland T (2006) Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 27:5014–5026
Hing KA, Wilson LF, Buckland T (2007) Comparative performance of three ceramic bone graft substitutes. Spine J 7:475–490
Jenis LG, Banco RJ (2010) Efficacy of silicate-substituted calcium phosphate ceramic in posterolateral instrumented lumbar fusion. Spine (Phila Pa 1976) 35:E1058–E1063
Lerner T, Liljenqvist U (2013) Silicate-substituted calcium phosphate as a bone graft substitute in surgery for adolescent idiopathic scoliosis. Eur Spine J 22(Suppl 2):S185–S194
von Recum J, Schwaab J, Guehring T, Grutzner PA, Schnetzke M (2017) Bone incorporation of silicate-substituted calcium phosphate in 2-stage revision anterior cruciate ligament reconstruction: a histologic and radiographic study. Arthroscopy 33:819–827
Diamantopoulos AP, Lorbach O, Paessler HH (2008) Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med 36:851–860
Franceschi F, Papalia R, Del Buono A, Zampogna B, Diaz Balzani L, Maffulli N et al (2013) Two-stage procedure in anterior cruciate ligament revision surgery: a five-year follow-up prospective study. Int Orthop 37:1369–1374
Uchida R, Toritsuka Y, Mae T, Kusano M, Ohzono K (2016) Healing of tibial bone tunnels after bone grafting for staged revision anterior cruciate ligament surgery: a prospective computed tomography analysis. Knee 23:830–836
MARS Group (2014) Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med 42:2301–2310
Noyes FR, Barber-Westin SD, Roberts CS (1994) Use of allografts after failed treatment of rupture of the anterior cruciate ligament. J Bone Joint Surg Am 76:1019–1031
Richter DL, Werner BC, Miller MD (2017) Surgical pearls in revision anterior cruciate ligament surgery: when must I stage? Clin Sports Med 36:173–187
Trojani C, Beaufils P, Burdin G, Bussiere C, Chassaing V, Djian P et al (2012) Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc 20:1565–1570
Louis ML, D'Ingrado P, Ehkirch FP, Bertiaux S, Colombet P, Sonnery-Cottet B et al (2017) Combined intra- and extra-articular grafting for revision ACL reconstruction: a multicentre study by the French Arthroscopy Society (SFA). Orthop Traumatol Surg Res 103:S223–S2S9
Lee DW, Kim JG, Cho SI, Kim DH (2019) Clinical outcomes of isolated revision anterior cruciate ligament reconstruction or in combination with anatomic anterolateral ligament reconstruction. Am J Sports Med 47:324–333
Chmielewski TL, Hurd WJ, Rudolph KS, Axe MJ, Snyder-Mackler L (2005) Perturbation training improves knee kinematics and reduces muscle co-contraction after complete unilateral anterior cruciate ligament rupture. Phys Ther 85:740–749
Mitchell JJ, Chahla J, Dean CS, Cinque M, Matheny LM, LaPrade RF (2017) Outcomes after 1-stage versus 2-stage revision anterior cruciate ligament reconstruction. Am J Sports Med 45:1790–1798
Diermeier T, Herbst E, Braun S, Saracuz E, Voss A, Imhoff AB et al (2018) Outcomes after bone grafting in patients with and without ACL revision surgery: a retrospective study. BMC Musculoskelet Disord 19:246
Acknowledgements
We thank Eun-Ji Jeon and Min-Ji Kim for their support.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to all of the following: (1): the conception and design of the study, (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kim, DH., Bae, KC., Kim, DW. et al. Two-stage revision anterior cruciate ligament reconstruction. Knee Surg & Relat Res 31, 10 (2019). https://doi.org/10.1186/s43019-019-0010-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43019-019-0010-6