Abstract
Background
Insolubility is the main requirement for ideal root end filling material to provide perfect sealing ability. Moreover, alkalinity and bioactivity provide great chance for tissues healing and remineralization. So, the aim of this work was to evaluate the chemical composition, solubility, pH change, and calcium ion release of recently introduced commercial mineral trioxide aggregate (MTA) endodontic repair cement (Harvard, Universal HandMix MTA) compared with ProRoot MTA repair material.
Methods
Solubility was evaluated after 7- and 14-day immersion time of specimens in phosphate buffer saline solution (PBS); the mean weight loss was evaluated and solubility was calculated as a percentage of the weight loss. For assessment of pH change and calcium ion release polyethylene tubes filled with the materials were soaked in distilled water for 7 and 14 days. Measurement of pH change was done by analytical pH meter. Concentrations of calcium ion release were measured using inductively coupled plasma optical emission spectroscopy. Data were statistically analyzed by independent sample t test and paired sample t test at 5% significance level.
Results
Harvard MTA endodontic cement showed significant lower solubility and higher pH values compared with that of ProRoot MTA. ProRoot MTA exhibited significant higher calcium ion release value after 14 days (P value ≤ 0.05).
Conclusion
Harvard, Universal HandMix MTA repair cement with its different chemical composition; exhibits a low solubility with enhanced alkaline pH value compared to ProRoot MTA repair material.
Similar content being viewed by others
Background
MTA is a calcium silicate-based cement, consists mainly of mixture of dicalcium silicate, tricalcium silicate, tricalcium aluminate, and a radiopacifier (Macwan and Deshpande 2014; Espir et al. 2016). Recently, efforts are directed to produce novel bioactive dental materials promoting hard tissue formation (Sarkar et al. 2005; Hamdy et al. 2019).
Nowadays, MTA has evolved as a most recommended material of choice used for several endodontic treatments such as pulp capping, root canal filling, repair of root canal perforation, and as root end filling material (Toptanci et al. 2013). This is due to its superior properties regarding biocompatibility, antibacterial activity, their ability to induce hard tissues formation through locally raising the pH to alkaline values, and the capability to release of calcium ions (Gandolfi et al. 2014; Prasad and Naik 2017). Moreover, it can set even in the presence of moisture (Nagmode et al. 2016). MTA proved to form chemical bonding with hard tooth structure forming intimate hermetic seal (Surya Raghavendra et al. 2017) and encourage healing of periapical tissues (Zaki et al. 2018).
Insolubility is a crucial feature in endodontic materials to provide intimate seal between tooth wall and restoration for subsequent successful root canal treatments (Torres et al. 2019). Despite of the previously mentioned superior properties of MTA, their high solubility rate limits the clinical success rate of endodontic MTA (Espir et al. 2016; Surya Raghavendra et al. 2017). The solubility of root canal sealers should be less than 3% according to the International Organization for Standardization (ISO) (ISO 6876:2012 2012) and American Dental Association (ADA) ( 1984).
Recently, Harvard MTA (Universal HandMix) has been introduced as repair cement based on mineral oxides and bismuth oxide. The manufacturer claims that, after mixing of powder and liquid, the set cement has a high pH value (more than 11) and minimum solubility in tissue fluid. Thus, the aim of the current study was to investigate the chemical composition of Harvard, Universal HandMix MTA repair cement and correlate it with its solubility, pH change, and calcium ion release. The obtained results were compared with that of the ProRoot MTA repair material.
Materials and methods
Two commercial MTA-based endodontic repair cements were used in this study. Harvard MTA, Universal HandMix (Harvard Dental International, Hoppegarten, Germany) and ProRoot MTA (Dentsply Tulsa Dental Products, Tulsa, USA) as a control.
XRD analysis
The chemical composition of the two tested cements was examined by X-ray powder diffraction analysis (XRD) (Bruker-AXS D8 X-ray diffractometer, Germany). XRD data were collected in the 2θ range 0–60°. The obtained XRD patterns were compared with model patterns on the Joint Committee on Powder Diffraction Standard (JCPDS) databases.
Specimen preparation
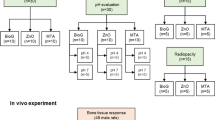
The two endodontic cements were prepared according to the manufacturer’s instructions (1 g powder was mixed with 0.33 ml distilled water). Mixing was done using stainless steel spatula to avoid inclusion of air bubble. Cement was then condensed into the specially prepared mold for each test type.
Analytic test
Solubility
Specimens (n = 5) were prepared using stainless steel ring mold with an internal diameter of (10 ± 1 mm) and a height of (2 ± 0.1 mm) was prepared and weighted using analytical balance (Adam equipment 4 digits precision weighing balance, UK). The mixed MTA cements were placed in the ring mold and allowed to set at 37 °C in an incubator (CBM 2431/V, Italy), for a period more than 50% of the final setting time (24 h). Each ring mold with MTA specimen was initially weighed to determine the initial mass. Specimens were then immediately immersed in 100 ml PBS (1.7 g KH2PO4, 11.8 g Na2HPO4, 80.0 g NaCl, and 2.0 g KCl in 10 L of H2O) at 37 °C and pH = 7.2.
Rings filled with MTA were kept in PBS in the incubator for 7 and 14 days. Each ring mold filled with MTA specimen was then removed, washed with distilled water, and dried with drier paper. The weights of the mold rings with MTA specimen were measured and the weight differences were calculated. The solubility of the cement was calculated as a percentage of weight loss (Shojaee et al. 2015; Poggio et al. 2017; Torres et al. 2018).
pH change and calcium ion release
Each cement type was placed in a polyethylene tubes (2 mm in diameter) (n = 5), soaked in 10 ml distilled water, and incubated for 7 and 14 days at 37 °C. Measurement of pH change was done by pH meter (Jen-way 3510 bench pH meter, UK). The concentrations of calcium ion release were measured using inductively coupled plasma optical emission spectroscopy (ICP-OES) (Ultima 2 ICP, Horiba, USA) (Amini Ghazvini et al. 2009).
Statistical analysis
The statistical analysis was performed using Statistical Package for the Social Sciences (SPSS, IBM, USA). Independent sample t test was used to compare mean solubility values between two MTA types (Harvard and ProRoot). The significance level was set at P ≤ 0.05. Similarly, the same test was used to compare results of pH as well as calcium release between the two groups.
To assess the effect of time within each MTA type, paired sample t test was used to compare the solubility values detected in day 7 and after 1 week (detected in day 14) for the same specimens. Similarly, the pH and calcium ion release values by time were statistically analyzed using paired sample t test within each MTA type.
Results
XRD results revealed the presence of two different types of calcium silicates in Harvard MTA repair cement including 75% Ca3Mg (SiO4)2 (Merwinite) and 20% Ca3 (SiO4) O (Hatrurite). On the other hand, ProRoot MTA type formed of 90% Hatrurite. Bismite (Bi2O3) (5 and 10%) as a radiopacifier was found in both Harvard MTA and ProRoot MTA types respectively (Figs. 1 and 2).
The results of solubility, pH change, and calcium ion release values of the two tested MTA endodontic repair cements after 7 and 14 days are illustrated in Tables 1, 2 and 3. A significant lower solubility and higher pH values were revealed in Harvard MTA type compared to that of ProRoot (P < 0.05). On the other hand, no significant changes in solubility and pH values were found in Harvard MTA after 7- and 14-day immersion time. Whereas, ProRoot cement showed a significant increase in solubility after 14 than 7-day immersion time. The calcium ion release after 7-day immersion time was significantly higher in Harvard. However after 14 days, the ProRoot showed significant higher values of Ca ion release when compared to that of Harvard type which significantly decreased after 14-day immersion time.
Discussion
Recently, a diversity of MTA-based endodontic repair cements has been introduced to improve root canal treatment (Yang et al. 2018). The manufacturer of Harvard MTA Universal HandMix material claimed their low solubility and high pH value (more than 11). During this study, XRD analysis of Harvard MTA and ProRoot MTA together with solubility, pH changes, and Ca ion release evaluation was done to correlate the chemical composition with the tested properties. ProRoot MTA endodontic repair cement was used in this study as control gold standard due to its well-established superior outcomes (Schmitt et al. 2001; Torabinejad et al. 2018).
Insolubility is a crucial factor for ideal requirement of root end filling material (Shojaee et al. 2015). Following ISO 6876 for the solubility test, the amount of cements that was solubilized was determined via measurement of the difference between the initial and final weights. Such values must be near 0.1% and must not exceed 3% (ISO 6876:2012 2012). Phosphate buffer saline solution was used in the current study as a storage media to simulate the clinical conditions due to its comparable composition to dentinal fluid (Saghiri et al. 2011).
An alkaline pH environment is a significant issue that encourages the healing of dental tissue and remineralization (Amini Ghazvini et al. 2009). The bioactivity of endodontic material could be estimated from their ability to release calcium ions and maintaining a high pH value (Sarkar et al. 2005).
XRD analysis results revealed the presence of Bismite (bismuth oxide) as a radiopacifier in both tested material types (Bortoluzzi et al. 2009; Torabinejad et al. 2018). XRD analysis results also revealed a difference in composition between the tested materials, where Harvard type composed mainly of 75 wt% Merwinite (calcium magnesium orthosilicate) and 20 wt% Hatrurite mineral. On the other hand, Hatrurite mineral content in ProRoot constitutes 90% of the composition. Merwinite was probably used in Harvard type due to its proved excellent bioactivity, lower solubility, and appropriate mechanical properties (Ou et al. 2008; Hafezi-Ardakani et al. 2011; Hafezi et al. 2013).
The lower solubility of Harvard MTA endodontic cement than ProRoot endodontic cement may be attributed to the presence of Merwinite as a major component in Harvard endodontic cement (Hafezi-Ardakani et al. 2011). Lower solubility of Merwinite may be due to the pronounced slower dissolution tendency of magnesium compared to calcium especially in alkaline pH (Hafezi-Ardakani et al. 2011). ProRoot revealed a significant increase in solubility after 14 days than 7 days; this may be due to the high hydration reactivity of Ca3SiO5 (Hatrurite) which forms the main composition of ProRoot cement (Sokol et al. 2019).
MTA-based endodontic sealers have bioactive properties; thus, in contact with phosphate buffer saline solution, it dissociates and releases its major cationic components. Calcium ions are the most dominant ion released from MTA when stored in PBS (Espir et al. 2016). Calcium ion release depends on the composition of the mineral particles of the cement responsible for water solubility and diffusion (Gandolfi et al. 2015). The higher Ca ion release after 7 days in Harvard compared to ProRoot endodontic cement may be attributed to the higher total calcium concentration in the composition of Harvard than ProRoot endodontic cement. However, the significant decrease in Ca ion release after 14 days in both types of cements may be the result of the decrease in Ca ion dissolution by time. These findings coincide with that of Fridland and Rosado (2005) which demonstrated that the ability of MTA-based cements to partially release calcium ions into an aqueous environment was decreased over time (Fridland and Rosado 2005).
The higher alkalinity of the Harvard MTA endodontic cement than ProRoot may be due to their higher Ca ion release concentration (Poggio et al. 2017). Moreover, Harvard showed no significant changes in pH after 7- and 14-day immersion time which may be correlated to the minimum Ca ion release over time.
Conclusion
The innovative endodontic cement (Harvard, Universal HandMix MTA) exhibits a low solubility with enhanced alkaline pH value and calcium ion release due to the presence of Merwinite as a major constituent compared to ProRoot MTA repair material.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- MTA:
-
Mineral trioxide aggregate
- PBS:
-
Phosphate buffer saline solution
- ISO:
-
International Organization for Standardization
- ADA:
-
American Dental Association
- XRD:
-
X-ray powder diffraction
- JCPDS:
-
Joint Committee on Powder Diffraction Standard
- ICP-OES:
-
Inductively coupled plasma optical emission spectroscopy
- SPSS:
-
Statistical Package for the Social Sciences
References
Amini Ghazvini S, Abdo Tabrizi M, Kobarfard F, Akbarzadeh Baghban A, Asgary S (2009) Ion release and pH of a new endodontic cement, MTA and Portland cement. Iran Endod J 4(2):74–78 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23940490%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC3740134
ANSI/ADA (1984) Specification no. 57 for endodontic filling materials. J Am Dent Assoc 108(1):88
Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM (2009) The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 35(4):550–554
Espir CG, Guerreiro-Tanomaru JM, Spin-Neto R, Chávezandrade GM, Berbert FLCV, Tanomaru-Filho M (2016) Solubility and bacterial sealing ability of MTA and root-end filling materials. J Appl Oral Sci. 24(2):121–125
Fridland M, Rosado R (2005) MTA solubility: a long term study. J Endod. 31(5):376–379
Gandolfi MG, Siboni F, Botero T, Bossù M, Riccitiello F, Prati C (2015) Calcium silicate and calcium hydroxide materials for pulp capping: biointeractivity, porosity, solubility and bioactivity of current formulations. J Appl Biomater Funct Mater. 13(1):1–18
Gandolfi MG, Siboni F, Primus CM, Prati C (2014) Ion release, porosity, solubility, and bioactivity of MTA plus tricalcium silicate. J Endod. 40(10):1632–1637
Hafezi M, Abbasi-Shahni M, Zamanian A, Hesaraki S (2013) Preparation and characterization of whitlockite-merwinite nanocomposite. J Ceram Process Res. 14(1):96–99
Hafezi-Ardakani M, Moztarzadeh F, Rabiee M, Talebi AR (2011) Synthesis and characterization of nanocrystalline merwinite (Ca 3 Mg(SiO4)2) via sol-gel method. Ceram Int. 37(1):175–180
Hamdy TM, Mousa SMA, Sherief MA (2019) Effect of incorporation of lanthanum and cerium-doped hydroxyapatite on acrylic bone cement produced from phosphogypsum waste. Egypt J Chem Available from: https://ejchem.journals.ekb.eg/article_56188.html
ISO 6876:2012 (2012) Dentistry -- Root canal sealing materials. Int Organ Stand 9
Macwan C, Deshpande A (2014) Mineral trioxide aggregate (MTA) in dentistry: a review of literature. J Oral Res Rev. 6(2):71
Nagmode PS, Satpute AB, Patel AV, Ladhe PL (2016) The effect of mineral trioxide aggregate on the periapical tissues after unintentional extrusion beyond the apical foramen. Case Rep Dent 2016
Ou J, Kang Y, Huang Z, Chen X, Wu J, Xiao R et al (2008) Preparation and in vitro bioactivity of novel merwinite ceramic. Biomed Mater 3(1)
Poggio C, Dagna A, Ceci M, Meravini MV, Colombo M, Pietrocola G (2017) Solubility and pH of bioceramic root canal sealers: a comparative study. J Clin Exp Dent. 9(10):e1189–e1194
Prasad BSK, Naik CT (2017) Mineral trioxide aggregate in endodontics. Int J Appl Dent Sci. 3(1):71–75
Saghiri MA, Ricci J, Joupari MD, Aeinehchi M, Ahmadi K, Bahramian N (2011) A comparative study of MTA solubility in various media. Iran Endod J. 6(1):21–24
Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I (2005) Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 31(2):97–100
Schmitt D, Lee J, Bogen G (2001) Multifaceted use of ProRootTM MTA root canal repair material. Pediatr Dent. 23(4):326–330
Shojaee NS, Sahebi S, Karami E, Sobhnamayan F (2015) Solubility of two root-end filling materials over different time periods in synthetic tissue fluid: a comparative study. J Dent (Shiraz, Iran) 16(3):189–18994
Sokol EV, Kokh SN, Sharygin VV, Danilovsky VA, Seryotkin YV, Liferovich R et al (2019) Mineralogical diversity of ca2 sio4-bearing combustion metamorphic rocks in the hatrurim basin: implications for storage and partitioning of elements in oil shale clinkering. Minerals 9(8)
Surya Raghavendra S, Jadhav GR, Gathani KM, Kotadia P (2017) Bioceramics in endodontics – a review. J Istanbul Univ Fac Dent 51(0)
Toptanci IR, Dalli M, Colak H (2013) The composition and biologic actions of mineral trioxide aggregate: a review. Konuralp Tip Derg. 5(2):70–74
Torabinejad M, Parirokh M, Dummer PMH (2018) Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview – part II: other clinical applications and complications. Int Endodontic J 51:284–317
Torres FFE, Guerreiro-Tanomaru JM, Bosso-Martelo R, Chavez-Andrade GM, Tanomaru FM (2018) Solubility, porosity and fluid uptake of calcium silicate-based cements. J Appl Oral Sci. 26:e20170465
Torres FFE, Guerreiro-Tanomaru JM, Bosso-Martelo R, Espir CG, Camilleri J, Tanomaru-Filho M (2019) Solubility, porosity, dimensional and volumetric change of endodontic sealers. Braz Dent J. 30(4):368–373
Yang DK, Kim S, Park JW, Kim E, Shin SJ (2018) Different setting conditions affect surface characteristics and microhardness of calcium silicate-based sealers. Scanning. 2018
Zaki DY, Zaazou MH, Khallaf ME, Hamdy TM (2018) In vivo comparative evaluation of periapical healing in response to a calcium silicate and calcium hydroxide based endodontic sealers. Open Access Maced J Med Sci. 6(8):1475–1479
Acknowledgements
Thanks are due to the National Research Centre for allowing the use of the Dental materials laboratory facilities.
Funding
The work was self-funded by the authors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Manar Gala and Tamer M. Hamdy performed the experimental work. Tamer M. Hamdy interpreted the analyzed data, wrote, and corresponded the manuscript. Dalia Y. Zaki revised and reviewed the draft manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galal, M., Zaki, D.Y., Rabie, M.I. et al. Solubility, pH change, and calcium ion release of low solubility endodontic mineral trioxide aggregate. Bull Natl Res Cent 44, 42 (2020). https://doi.org/10.1186/s42269-020-00303-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-020-00303-1