Abstract
Background
The main objective of this work aimed to investigate the impact of incorporation apricot seed kernel (ASK) in rabbit rations on their digestion coefficients, nutritive values, performance, carcass characteristics, and blood constituents (hematology and biochemistry).
Method
Forty-five New Zealand White (NZW) rabbits aged 5–6 weeks (730 ± 23 g) were divided into five groups (9 rabbit in each group) for 72 days. The first experimental ration considered the control (R1) that not contained apricot seed kernel (0% ASK). The 2nd, 3rd, 4th, and 5th experimental rations contained 0.75, 1.5, 3, and 4.5 % for R2, R3, R4, and R5, respectively.
Results
ASK had no significant effect on the nutrient digestibility except for EE and nutritive values. Incorporating ASK at different levels significantly (P < 0.05) increased average daily gain (ADG) and feed conversion ASK at different levels had no effect on both digestive tract and head weights, while giblets and carcass weights were increased (P < 0.05) compared to the control (R1). Rations contained ASK with level 0.75, 1.5, and 3% were significantly (P < 0.05) increased crude protein content of best 9th, 10th, and 11th ribs. Meanwhile, R2, R3, and R4 significantly (P < 0.05) decreased ether extract content of best 9th, 10th, and 11th ribs. Inclusion ASK had no significant difference on red blood cell count (RBCs), white blood cell count (WBCs), packed cell volume percentages (PCV), hemoglobin concentration, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). However, levels of ASK (0.75, 1.5 and 3%) were significantly (P < 0.05) decreased eosinophils. Both basophiles and lymphocytes counts were significantly (P < 0.05) increased with ASK (R4). ASK had no significant effect on total protein, albumin, ALT, total cholesterol, triglycerides, urea, and creatinine. Meanwhile, rabbits received ration contained 4.5% ASK (R5) significantly (P < 0.05) increased total globulins and AST contents.
Conclusion
From the data obtained and under the same condition for this study, it can be concluded that, apricot seed kernel can be used safety as an alternative source of protein used to rates reach up to 4.5% without adverse effects on growth rates, feed consumption, digestion coefficients, nutritive values, carcass characteristics, and blood constituents.
Similar content being viewed by others
Background
Apricot (Prunus armeniaca L.) has an important place in human nutrition and is classified as one of the Prunus species, family Rosaceae, group Rosales. It can be used as fresh, dried, or processed fruit (Yıldız 1994).
Apricot is mostly grown many Mediterranean countries including Eygpt, Pakistan, Russia, and the USA. World production of fresh apricot ranged from 2.2 to 2.7 million tons per year (Baquar 1989; Fazlin et al. 2002; Hussain et al. 2011).
In Egypt, about 3931 acres are cultivated with apricot orchards producing 15,724 tons of apricot fruits. The pit constitutes 15–16% of the whole fruit (Sarhan 1970; Hallabo et al. 1977). However, the kernel represents from 31 to 38% of the pit (Dhar and Chavhan 1963; Sarhan 1970; Filsoof et al. 1976; Hallabo et al. 1977; Abd El-Aal et al., 1986a, b).
Apricot and its kernel have many effects including antioxidant (especially β-caroten) effects, antiatherosclerosan, antianginal, anticancer, antiaging, antiparasitic, cardio/hepato/renoprotective. In addition, it has also sedative, antispasmotic, antisestradial antimicrobial, antimutagenic, antitussive, antiinflammatory, antinociceptive, and enzyme inhibitory effects and various minerals (especially K, Fe, Mg, P, and Se) and vitamins (A, C, and E). It is a rich fiber source (Raj et al. 2012; Minaiyan et al. 2014; Sharma et al. 2014).
Previous researchers have pointed out that there is an important proportion of 50% oil in Egyptian apricot kernels (Abd El-Aal et al., 1986a, b). Meanwhile, Indian varieties are containing 44% oil as found by Joshi et al. (1986). On the other hand, Salem and Salem (1973) and Hallabo et al. (1977) noted that apricot seed kernels contained oil which varied from 49.93 to 53.17%.
Apricot kernel flour rich in many amino acids such as arginine, aspartic acid, phenylalanine, valine, methionine, threonine, and glutamic acid (Kamel and Kakuda 1992). Its oil is rich in unsaturated fatty acids, especially oleic (31–80%) and linoleic (6.–-51%) acids, is also a good source of α-tocopherol, and in addition contains high levels of potassium and magnesium minerals and B group vitamins (Lazos 1991). Also, Gezer et al. (2011) noted that apricot kernels contained high amounts Ca, K, Na, and P.
Large amounts of fruit seeds are discarded yearly at processing plants, causing a disposal problem (Gezer et al. 2011). So both oil and meal from these fruit seeds must be utilized (Kamel and Kakuda 1992).
The main objective of this work aimed to investigate the impact of incorporating apricot seed kernel in rabbit rations on their digestion coefficients, nutritive values, performance, carcass characteristics, and blood constituents (hematology and biochemistry).
Methods
This work was carried out at the Research and Production Station located in El-Emam Malik Village, El-Bostan, West of Nubaria, and at the laboratories of Animal Production and Parasitology and Animal Diseases Departments, National Research Centre, 33 El Bohouth Street, Dokki, Cairo, Egypt.
Animals and feeds
Forty-five New Zealand White (NZW) rabbits aged 5–6 weeks with an average body weight of 730 ± 23 g were randomly divided into five equal experimental groups (9 rabbits in each group).
Three replicates of each treatments composed of three rabbits were housed together in galvanized wire cages (50 × 50 × 45 cm) and provided with stainless steel nipples for drinking and feeders allowing recording feed intake during the feeding trial that lasted for 72 days.
All experimental group rabbits were kept under the same managerial conditions and rations were offered, which were pellets with a diameter of 4 mm.
Five experimental pelleted rations were formulated to cover the nutrient requirements for rabbits according to NRC (1977).
The first ration was considered control (R1) and contained no apricot seed kernel (0% ASK). The 2nd, 3rd, 4th, and 5th experimental rations contained levels of ASK 0.75, 1.5, 3, and 4.5% respectively. Rations and water were offered ad libitum.
Digestibility trials
At the last 2 weeks of the experimental period, all rabbits were used in digestibility trials over period of 7 days to determine the nutrient digestibility and nutritive values of the tested rations. Feces were daily collected quantitatively. Feed intake of experimental rations and weight of feces were daily recorded. Representative samples were dried at 60 °C for 48 h, ground, and stored for later chemical analysis. The nutritive values expressed as total digestible nutrients (TDN) and digestible crude protein (DCP) of experimental rations that calculated using classic method as described by Abou-Raya (1967).
Carcass traits
At the end of the experimental period, three representative rabbits from each treatment were randomly chosen and fasted for 12 h before slaughtering according to Blasco et al. (1993) to determine the carcass measurements. Edible offal per gram (heart, liver, testes, kidneys, lungs, and spleen) and head were weighed and added to warm carcass weight. The 9th, 10th, and 11th ribs were frozen in polyethylene bags for later chemical analysis. The ribs of samples were dried at 60 °C for 48 h.
Analytical procedures
Chemical analysis of ASK, experimental rations, and feces were analyzed as described by AOAC (2005) methods. on the other hand, the air-dried samples of best 9th, 10th, and 11th ribs were analyzed for DM, EE, and ash according to the AOAC (2005) methods, while crude protein (CP) percentage was calculated by difference as recommended by (O’Mary et al. 1979) according to the following equation: CP content on DM basis = 100 − (Ether extract + ash).
Blood parameters
Two blood samples were collected from ear vein puncture from five rabbits from each group. The first blood sample was collected into an EDTA and was used for hematological evaluations. The second blood sample was centrifuged and the serum separation. Serum samples were stored at − 20 °C until further biochemical analyses.
Hematological investigations
Hemogram of collected blood samples was described by Weiss and Wardrop (2010). It included the red blood cell count (RBCs), packed cell volume (PCV), hemoglobin (Hb) concentration according to (Bunn 2011; Elghetany and Banki 2011), and calculated red blood indices [mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), total leukocyte counts (TLC), and differential leukocyte counts].
Serum biochemical studies
It was earlier mentioned that the blood samples were collected through vein puncture; blood sample was placed in a plain centrifuge tube for serum separation. Serum samples were stored at − 20 °C until further biochemical analyses of total proteins (Henary et al. 1974), albumin (Doumas et al. 1971), total cholesterol (Allain et al. 1974), triglycerides (Fossati and Prencipe 1982), activities of aminotransferases (AST and ALT) (Reitman and Frankel 1957), urea according to (Patton and Crouch 1977), and creatinine according to (Husdan 1968). Serum globulins were determined by subtracting the value of serum albumin from the value of serum total proteins, also A/G ratio was calculated. Commercial diagnostic kits from Biomerieux, France, and Quimica Clinica Aplicada (QCA), Amposta, Spain, were used for assay of serum biochemical parameters.
Statistical analysis
Data on feed intake, live body weight, feed conversion, nutrient digestibility, carcass data, and blood constituents were subjected to one-way analysis of variance using to SPSS (2008). Duncan’s multiple range test (Duncan 1955) was used to separate means when the dietary treatment effect was significant according to the following model:
Ti = effect of experimental rations for i = 1–5; 1 = control ration contained 0% ASK, 2 = ration contained 0.75% ASK, 3 = ration contained 1.50% ASK, 4 = ration contained 3.00% ASK, and 5 = ration contained 4.50% ASK.
eij = the experimental error.
Results
Experimental rations
Composition and chemical analysis
Data of chemical analysis of experimental rations that illustrated in Table 1 showed that all the experimental rations were iso-nitrogenous (17.47 to 17.60% CP) but differ in EE contents that increased gradually with the increasing levels of incorporation of ASK in the rations. The corresponding value of EE ranged from 2.94 to 4.89% among the fifth tested rations and that due to increasing content of EE in ASK that reached to 50% or more.
Digestion coefficients and nutritive values
As shown in Table 2 except for EE digestibility, replacing soybean meal by ASK had no significant effect on the other nutrient digestibility and nutritive values. The best EE digestibility was realized when ASK 3% (R4), with the corresponding value of EE digestibility of (81.54%).
Growth performance
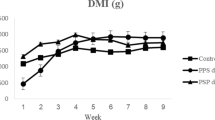
Table 3 showed that incorporating ASK at different levels increased (P < 0.05) average daily gain (ADG), by 21.94%, 25.48%, 14.98%, and 19.87% for R2, R3, R4, and R5, respectively .The best ADG was observed when rabbits received ration with 1.5% ASK (R3). On the other hand, dry matter intake (DMI) was not affected by ASK; meanwhile, R5 by ASK (4.5%) significantly (P < 0.05) decreased DMI by 2.5% compared to control (Table 3). The feed conversion significantly (P < 0.05) improved with inclusion ASK at different levels. The best feed conversion occurred when rabbits fed ration with 1.5% ASK as compared control group.
Carcass characteristics
Results presented in Table 4 Showed that ASK at different levels had no effect on both digestive tract weight that ranged from 320 to 354 g among different groups and head weight that ranged from 133 to 146 g. On the other hand, giblets and carcass weight were increased (P < 0.05). Also, results obtained mentioned that R2 and R3 in significantly increased dressing percentages comparing to control (R1); meanwhile, dressing percentages for R4 and R5 in significantly decreased compared to control (R1).
Also, as shown in Table 4 the chemical analysis of best 9th, 10th, and 11th ribs cleared that replacing 5, 10, and 20% of soybean meal by ASK significantly (P < 0.05) increased crude protein content of best 9th, 10th, and 11th ribs, however, replacing 30% caused in significant increase in crude protein. Treatments (R2, R3, and R4) significantly (P < 0.05) decreased ether extract content of best 9th, 10th, and 11th ribs.
Hematological findings
As shown in Table 5, addition of ASK in rabbit rations had insignificant increase in red blood cell count (RBCs) and hemoglobin concentration. There were no significant changes in packed cell volume percentages (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), white blood cell count (WBCs), and neutrophils count. While there was a marked insignificantly increasing in eosinophils count when rabbits fed ration R3. However, the other three levels of ASK 0.75, 3, and 4.5%, was significantly (P < 0.05) decrease eosinophils count. The present result revealed that both basophils and lymphocytes counts were significantly (P < 0.05) increased only with 3% ASK (R4), while monocytes count insignificantly increased with the increasing level of 3% replacement (R4) and decreased with 4.5% ASK (R5) comparing with the control (R1).
Serum Biochemical Changes
Data of serum biochemical changes that presented in Table 6 showed that the levels of ASK 0.75, 1.5, 3, and 4.5% had no significant effect on total protein, albumin, alanine aminotransferase (ALT), total cholesterol, triglycerides, urea, and creatinine. Only, rabbits which received ration ASK 4.5% (R5) had significantly (P < 0.05) increased total globulins and aspartate aminotranseferase (AST) contents. For albumin, globulin ratio significantly (P < 0.05) decreased in the rabbits fed with ration 4.5% ASK (R5).
Discussion
The chemical analysis of ASK and experimental rations were in agreement with those found by Salem and Salem (1973), Abd El-Aal et al. (1986a, b), and Beyer and Melton (1990) who noted that Egyptian Amar, sweet and bitter apricot kernels contain 50.9, 53.17 and 55.96% EE, respectively. On the other hand, CF, NFE, and ash contents were in the same values for the experimental rations.
The present results of digestion coefficients and nutritive values of the experimental group, i.e., in agreement with those found by Fanimo et al. (2003) who noted that there were no differences in the nutrient digestibility of CP, EE, and NFE of the growing rabbits fed diets with 10, 20, or 30% cashew apple waste. Meanwhile, apparent digestibility of CF significantly decreased (P < 0.05) with increase in the level of CAW in the diets.
Data obtained of growth performance show in Table 3 increased (P < 0.05) average daily gain, not affected on dry matter intake (DMI), and improved their feed. These results are in agreement with those obtained by Garreau et al. (2013) who noted that ADG from day 35–49 does not differ between diets, due to the low correlation between the weight at weaning and the speed of growth. Growth at days 49, 63, and 77 was not affected by any of the incorporation rates of the apricot kernel cake in the diets and also dependent on weight at weaning, which is linked to the mothers’ diets (Ouhayoun and Dalle 1996) and the breed itself (Ouyed 2009). Also, Mennani et al. (2017) showed that daily intakes are significantly different and decrease in proportion to the incorporation rates of kernel seed meal. This could be due to the interaction of the fiber from the apricot kernel meal, as indicated by De Blas and Carabano (1996) for various agro-industrial by-products used in rabbit feed.
The results of carcass characteristics presented in Table 4 are in disagreement with those reported by Mennani et al. (2017) who noted incorporation rates of apricot kernel meal found to be higher in the New Zealand breed is inversely proportional to the lipid content of the meat of rabbits; in addition they noted that it has no effect on the carcass characteristics, which could be explained by the low substitution rate of apricot seed meal by-products, with a yield at slaughter of 66%, which remains below the range proposed by Berchiche and Lebas (1994) who found that it was recorded among 58–60%.
Data of hematological findings that obtained in Table 5 was agreed with Yılmaz (2018), who reported that sun-dried organic apricot (SDOA) consumption showed beneficial effects on red blood cells (RBC), hemoglobin (Hb), and hematocrit (HCT) and emphasized that the results may have significance for therapy, preservation, and/or eradication of some types of anemia. On the other hand, there were no significant changes in packed cell volume percentages (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and white blood cell count (WBCs). On the other hand, dietary treatments had no significant effect on neutrophil count, while there was a marked insignificant increase in eosinophil count when rabbits were fed ration R3. However, the other three levels of 0.75, 3, and 4.5% ASK were significantly (P < 0.05) decreased eosinophil count; in addition, the present result mentioned that both basophils and lymphocytes counts were significantly (P < 0.05) increased only with 3% ASK (R4), while monocytes count insignificantly increased with the increasing level of replacement up to 20% (R4) and decreased with 4.5% ASK (R5) compared with the control (R1). Although most results of hemogram were within the normal values of the results obtained by Weiss and Wardrop (2010), which indicated that rabbits fed rations containing ASK may induce slightly adverse effects on bone marrow, the fluctuations among the groups and periods are relatively close with the results of Zhang et al. (2009), Petterino and Argentino-Storino (2006), and Ismet Yılmaz (2012). Also, the variations in the hematological parameters are related to housing, breeding, age, weight, and gender of the animals. And also, it is reported by researches that an 8% protein level in the diet of laboratory animals, either for a short or a long period, does not alter some hematological parameters (Yılmaz 2012).
Data of serum biochemical changes that are presented in Table 6 agree with Firdaws et al. (2018) who reported that the apricot kernel improves the functional status of the liver by preventing an increase in the liver function marker enzyme activity induced by CCl4. Inclusion of 7% apricot kernel oil in rate diets has no significant effect on total cholesterol, triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) concentrations (Kutlu et al. 2009).
Conclusion
From the results obtained in this study, it can be mentioned that apricot seed kernel can be used safely as an alternative source of protein and used at rates reaching 4.5% without adverse effects on growth rates, feed consumption, digestion coefficients, nutritive values, carcass characteristics, blood constituents, liver function, and lipid profile. In addition, it improves the feed conversion as well as the chemical composition of the meat.
Availability of data and materials
“Not applicable” for this section.
Abbreviations
- A/G:
-
Ratio was calculated albumin/globulin
- ADG:
-
Average daily gain
- ALT:
-
Alanine aminotransferase
- AOAC:
-
Official Methods of Analysis
- ASK:
-
Apricot seed kernel
- AST:
-
Alanine
- CF:
-
Crude fiber
- CP:
-
Crude protein
- DCP:
-
Digestible crud protein
- DM:
-
Dry matter
- DMI:
-
Dry matter intake
- EE:
-
Ether extract
- FW:
-
Final weight
- Hb:
-
Hemoglobin
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- MCV:
-
Mean corpuscular volume
- NFE:
-
Nitrogen-free extract
- NRC:
-
National Research Council
- OM:
-
Organic matter
- PCV:
-
Packed cell volume
- QCA:
-
Clinica Aplicada
- R1:
-
Control that not contained apricot seed kernel (0% ASK)
- R2:
-
Replaced 5% of soybean meal by apricot seed kernel
- R3:
-
Replaced 10% of soybean meal by apricot seed kernel
- R4:
-
Replaced 20% of soybean meal by apricot seed kernel
- RBCs:
-
Red blood cell count
- SPSS:
-
Statistical package for Social Sciences
- TBWG:
-
Total body weight gain
- TDN:
-
Total digestible nutrients
- TLC:
-
Total leukocyte counts
- WBCs:
-
White blood cell count
References
Abd El-Aal MH, Hamza MA, Rahma EM (1986a) In vitro digestibility, physicochemical and functional properties of apricot kernel proteins. Food Chemistry 19:197–211
Abd El-Aal MH, Khalil MKM, Rahma EM (1986b) Apricot kernel oil: characterization, chemical composition and utilization in same baked products. Food Chemistry 19:287–298
Abou-Raya AK (1967) Animal and poultry nutrition. 1st Ed. Pub. Dar El-Maarif, Cairo (Arabic text book)
Allain CC, Poon LS, Chan CS, Richmond W, Fu PC (1974) Enzymatic determination of total serum cholesterol. Clin Chem 20:470–475
AOAC (2005) Official methods of analysis, 18th edn. Association of Official Analytical Chemists, Washington, DC, USA
Baquar SR (1989) Medicinal and poisonous plants of Pakistan. Printas, Karachi, Pakistan, p 364
Berchiche M, Lebas F (1994) Methionine supplementation of a faba bean based feed: effects on rabbit growth and carcass characteristics. World Rabbit Science 2:135–140
Beyer R, Melton LD (1990) Composition of New Zealand apricot kernels. New Zealand J Crop Horticulture Sci 18:39–42
Blasco A, Quhayaun J, Masoscro G (1993) Hormonization of criteria and terminology in rabbit meat research. World Rabbits Sci 1:3–10
Blaxter KL (1968) The energy metabolism of ruminants, 2nd edn. Charles Thomas Publisher. Spring field, Illinois, U.S.A.
Bunn HF (2011) Approach to the anemias. In: Goldman L, Schafer AI (eds) Goldman's Cecil Medicine, 24th edn. Elsevier Saunders, Philadelphia, Pa, p 2011 Chap 161
De Blas JC, Carabano R (1996) A review on the energy value of sugar beet pulp for rabbits. World Rabbit Sci 4:33–36
Dhar KL, Chavhan RSN (1963) Oil from seed kernels of Prunus armemiaca. Agric. Univ. J. Res 12:9
Doumas BL, Watson T, Biggs WA (1971) Albumin standards and measurement of serum with bromocresol green. Clin. Chem. Acta 31:87–96
Duncan DB (1955) Multiple rang and multiple F–test biometrics 11: 1- 42
Elghetany MT, Banki K (2011) Erythrocytic disorders. In: McPherson RA, Pincus MR, eds. Henry's Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, Pa: Elsevier Saunders 2011: Chap 32
Fanimo AO, Oduguwa OO, Alade AA, TO O, Adesehinwa AK (2003) Growth performance, nutrient digestibility and carcass characteristic of growing rabbits fed cashew apple waste. Livestock Res Rural Dev 15(8):15–23
Fazlin ASM, Ahmad Z, Lim HH (2002) Compendium of medicinal plants used in Malaysia. Herbal Medicine Research Centre (HMRC), Institute for Medical Research 2: 260
Filsoof M, Mehran M, Farrohi F (1976) Determination and comparison of oil characteristics in Iranian almond, apricot and peach nuts. Fette, Seifen Abstr 78:150
Firdaws A, Abbas B, Nabee N (2018) Effect of apricot kernel on some hematological, histological and biochemical parameters in CCl4-induced liver injury in rats. Al-Kitab J Pure Sci 1(2):217–228
Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clinical Chemistry 28(10):2077–2080
Garreau H, Hurtaud J, Drouilhet L (2013) Estimation des paramètres génétiques de la croissance et de l’efficacité alimentaire dans deux lignées commerciales. 15èmes Journées de la Recherche Cunicole, 19-20 Novembre. Le Mans, France P15-18
Gezer I, Hacisefero Ğullar H, Özcan MM, Arslan D, Asma BM, Unver A (2011) Physico-chemical proparties of apricot (Prunus armeniaca L.) kernels. South Western J Horticulture Biol Environ 2(1):1–13
Hallabo SAS, El-Wakeil FA, Morsi MKS (1977) Chemical and physical properties of apricot kernel, apricot kernel oil and almond kernel oil. Egypt J Food Sc 3:1–6
Henary RJ, Cannon DC, Winkleman JW (1974) Clinical chemistry historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp. Toxicol Pathol 2006(57):213–219
Husdan H (1968) Chemical determination of creatinine with deproteinization. Clin. Chem 14:222
Hussain IS, Gulzar S, Shakir I (2011) Physio chemical properties of bitter and sweet apricot kernel flour and oil from North of Pakistan. Internet J Food Safety 13:11–15
Joshi S, Srivastava RK, Dhar DN (1986) The chemistry of Prunusarmeniaca. British Food J 88:74–78
Kamel BS, Kakuda Y (1992) Characterization of the seed oil and meal from apricot, cherry, nectarine, peach and plum. J Am Oil Chem Soc 69:492–494
Kutlu T, Gökhan D, Burhan A, Ali E (2009) Protective effect of dietary apricot kernel oil supplementation on cholesterol levels and antioxidant status of liver in hypercholesteremic rats. J Food Agric Environ 7(3&4):61–65
Lazos ES (1991) Composition and oil characteristics of apricot, peach and cherry kernel. Grasasy Aceites 42:127–131
Mennani A, Rafik A, Arbouche asmine Y, Etienne M, Fodil A, Saâdia AH (2017) Effects of incorporating agro-industrial by-products into diet of New Zealand rabbits: case of rebus of date and apricot kernel meal. Veterinary World, EISSN, pp 2231–0916 www.veterinaryworld.org/Vol.10/December-2017/7.pdf
Minaiyan M, Ghannadi A, Asadi M, Etemad M, Mahzouni P (2014) Anti-inflammatory effect of Prunus armeniaca L. (Apricot) extracts ameliorates TNBS-induced ulcerative colitis in rats. Res Pharm Sci 9(4):225–231
NRC (1977). National Research Council. Nutrient requirements of rabbits, National Academy of Science Washington, D.C
O'Mary CC, Everett LM, Graig AD (1979) Production and carcass characteristics of Angus and Charolais x Angus steers. J Anim Sci 48:239
Ouhayoun J, Dalle ZA (1996) Harmonization of muscle and meat criteria in rabbit meat research. World Rabbit Sci 4:211–218
Ouyed A (2009) Évaluation du rendement en carcasse, en muscle et du poids des différentes parties des lapins de lignées pures et hybrides. Rapport final déposé au Conseil Pour le développement de l’agriculture du Québec et au Ministère de L’Agriculture, des Pêcheries et de l’Alimentation du Québec 1996
Patton CJ, Crouch SR (1977) Spectrophotomattic and kinetics investigation of the Berthelot reaction for the determination of ammonia. Anal Chem 49:464
Petterino C, Argentino-Storino A (2006) Clinical chemistry and hematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol 57:213–219
Raj V, Jain A, Chaudhary J (2012) Prunus Armeniaca (Apricot): An overview. J Pharm Res 5(8):3964–3966
Reitman SMD, Frankel S (1957) A colorimeter method for determination of serum glutamic oxaloacetic acid and glutamic pyruvic acid transferases. Am J Clin Pathol 28:56–63
Salem SA, Salem FMA (1973) Egyptian apricot kernels seeds. Deutsche Lebensmittel- in Turkey. J Food Eng 53:111–114
Sarhan MAI (1970) Studies on the production of apricot juice. MSc Thesis Faculty of Agric Cairo University Cairo
Sharma S, Satpathy G, Gupta RK (2014) Nutritional, phytochemical, antioxidant and antimicrobial activity of Prunus armenicus. J Pharmacog Phytochem 3(3):23–28
SPSS (2008) Statistical package for social sciences, Statistics for Windows, Version 17.0. Released 2008. SPSS Inc, Chicago, U.S.A
Weiss DJ, Wardrop KJ (2010) Schalm's veterinary hematology, 6th edn. Blackwell Publishing Ltd, Ames, Iowa, USA
Yıldız F (1994) New technologies in apricot processing. Journal of Standard, Apricot Special Issue p: 67-69 Ankara
Yılmaz I (2012) Effects of sun dried organic apricot on some hematological parameters in rats. J Pharm Res 1(3):18–22
Yılmaz I (2018) The biological and pharmacological importance of apricot. SOJ Pharm Pharm Sci 5(1):1–4
Zhang WZ, Cui WM, Zhang X, Wang W, Jia XD, Zhang XP, Li N (2009) Subchronic toxicity study on soy ısoflavones in rats. Biomed Environ Sci 22:259–264
Acknowledgements
Our deep thanks for the Rabbits Experiment Unit, Research and Production Station, National Research Centre, for saving facilities that make this work possible.
Funding
All authors equally shared in financing the cost of the research paper.
Author information
Authors and Affiliations
Contributions
HAAO cooperated in plan of work and field work, collected samples, prepared data and statistical analysis, and wrote the manuscript. SMA cooperated in plan of work and revised the manuscript. AAA cooperated in plan of work, field work and manufacture of tested rations and collected samples. YAAE cooperated in plan of work and field work, sample collection, laboratory analysis, and follow up of the publication with the journal (corresponding author). SMN cooperated in collected blood samples and laboratory analysis of blood. SAN cooperated in collected blood samples, laboratory analysis of blood, and writing and revision the section of blood parameters. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
“Not applicable” for this study.
Consent for publication
“Not applicable” for this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omer, H.A.A., Ahmed, S.M., Abedo, A.A. et al. Incorporation apricot seed kernel as untraditional source of protein in rabbit rations. Bull Natl Res Cent 44, 37 (2020). https://doi.org/10.1186/s42269-020-00292-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-020-00292-1