Abstract
The present study was performed to assess the individual and combined potential of 3 different concentrations of Trichoderma sp. (Ts) and Bacillus thuringiensis (Bt) to control brinjal insect pests. Tested formulations were applied on larval and adult stages of the pest. The Trichoderma laboratory bioassays revealed 73% mortality of the aphid species, Aphis gossypii (Glover), and 53% mortality of the cotton leafhopper (Jassids) Amrasca bigutulla bigutulla (Ishida), while opposite results were observed in case of Bt at the highest concentration (1 × 108 cfu ml−1) used. In vivo results revealed that Ts caused a significant population reduction of the aphid (87%) than the jassid (72%), 7 days of post-treatment, at the highest concentration, while non-significant results were observed at the lowest concentrations. A. gossypii was significantly found more susceptible to the mixture of Trichoderma + Bt than the jassid (62%) and brinjal shoot and fruit borer (65%) even after 48 h of treatment application. The combined application (Trichoderma + Bt) showed maximum population reduction of jassid (88%), aphid (95%), and BSFB (96%), respectively, 7 days post-applications. The positive correlation among time and concentration was observed. The result may imply that consortium of these microbial organisms could be effective and can be incorporated in IPM programs for effective control of sucking and chewing insect pests of brinjal.
Similar content being viewed by others
Background
Brinjal (Solanum melongena L.) is cultivated throughout the world on more than 1.8 million ha with about 50.9 million tonnes production annually. In Pakistan, the cultivated area under brinjal crop is 8575 ha with annual production of 87,587 tonnes (FAO, 2018). It is grown in all cropping seasons. Aphid (Aphis gossypii), jassid (Amrasca bigutulla bigutulla Ishida), white fly (Bemisia tabaci Gennadius), and brinjal shoot and fruit borer (BSFB) (Leucinodes orbonalis Guenee) are the major pests of brinjal crop. These insect pests attack the crop at different growth stages and results in a significant decrease in yield quantity and quality. Among all the sucking and chewing insect pests and brinjal shoot and fruit borer, L. orbonalis is the major pest of brinjal crop worldwide (Chakraborty and Sarkar 2011; Dutta et al. 2011). It is the most serious pest of brinjal in Asia, especially in Pakistan, India, Nepal, Bangladesh, Sri Lanka, Thailand, the Philippines, Cambodia, Vietnam, Cambodia, Laos, Southeast Asia, Africa, and Sahara. In South Asia, brinjal shoot and fruit borer causes severe yield losses (85–90%) (Misra 2008; Jagginavar et al. 2009).
Pesticides usage for the control of the insect pests in brinjal is high (Shetty 2004). For example, it has been reported that in certain areas of the Philippines and Bangladesh, the farmers sprayed 56 and 180 times, respectively, during a single cropping season. Therefore, it is needed to switch for other environmentally safe pest control methods, such as the bioagents/biopesticides for pest management (Kabadwa et al. 2019). The entomopathogenic fungi and bacteria are the most important and auspicious candidates for pest management (Afzal et al. 2013; Xiaoman et al. 2019).
Trichoderma strains colonize and infect the outer layers of roots making a zone of chemical interaction (Mishra et al. 2018). Chemical elicitors from Trichoderma interact with putative plant receptors (Harman and Shoresh 2007). They induce systemic resistance on a wide range of plant/pathogen combinations as described in previous studies in both axenic and field soil systems (Yedidia et al. 2003). Similarly, Bacillus thuringiensis (Bt), the naturally occurring, spore-forming bacterium, is present in soil and has been utilized more efficiently for protection of food crops, forest trees, ornamentals, and stored grains (Meadows 1993). Being safe to environment and highly specified, Bt spores and crystals have been used successfully as bioinsecticide for control of different lepidopteran, coleopteran, and dipteran insect pests (Schnepf et al. 1998). Similarly, once infection with Trichoderma strains occur, a zone of chemical interaction develops at these sites. Within this zone of chemical interaction, the Trichoderma hyphae are walled off on the plant but do not kill it (Harman and Shoresh 2007). Chemical elicitors from Trichoderma produced by the walled off hyphae interact with putative plant receptors (Harman and Shoresh 2007).
The present study was conducted to assess individual and combined potential effects of Trichoderma spp. and Bt on brinjal productivity and insect pest infestation.
Materials and methods
Trichoderma and Bt formulations were obtained from the Soil and Environmental Microbiology Laboratory, Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad, Pakistan, and mass cultured in the Integrated Pest Management Laboratory, Department of Entomology, UAF, for experimentation.
Bacterial formulation
For bacteria, inoculum was prepared in TSB (tryptic soy broth) media (Paik et al. 1997) in 1000 ml Erlenmeyer flask and autoclaved at 121 °C for 20 min. After preparation of broth, a loopful of bacterial colony from the Petri plate was added into the broth under the laminar air flow chamber and kept in shaking incubator (Firstek Scientific, Tokyo, Japan) at 180 rpm for 48 h at 28 ± 1 °C. An OD of 0.5, measured with an optical density meter (Biolog® Model-21907; Biolog Inc.) at λ 600 nm, was achieved by dilution to maintain a uniform cell density (108 cfu ml−1) prior to application (Naveed et al. 2014). The Bt inoculum was further diluted to prepare formulations of different cell density (107 and 106 cfu ml−1). Inoculum and saline buffer (0.85% NaCl w/v) at ratios 1:9 and 2:18 were mixed to prepare Bt suspensions containing 107 and 106 cfu ml−1. To achieve these populations, OD0.4, 0.3 were adjusted prior to application.
Fungal formulation
For fungus inoculum, PDB (potato dextrose broth) media was used (Meyer et al. 2000) in 1000 ml Erlenmeyer flask and autoclaved at 121 °C for 20 min. After preparation of media, a 5-mm disc of fungus from the Petri plate was added into the prepared media under a laminar airflow chamber and kept at 25 ± 1 °C for 5 days. The same procedure (as described for Bt) was followed to prepare fungal formulations before application.
Leaf dip bioassays
Laboratory experiments, using leaf dip bioassay method against brinjal insect pests, were carried out. The brinjal plants were sown at the Entomological Research Farm, University of Agriculture, Faisalabad, to collect the leaves and to sample aphid, jassid, and fruit borer larvae. The collected leaves of brinjal plants were rinsed by distilled water to remove contamination (if any). A disc of 7 cm diameter was cut from the collected leaves. Then, leaf discs were dipped into the bio-pesticide formulations (Table 1) for 3 min and room dried. The leaf discs were placed in Petri dishes. The 20 specimens of aphid and jassid were fed on leaf discs, while 5 larvae of fruit borer (collected from the field plants) were fed on brinjal fruits treated with biopesticide formulations (Table 1). Mortality rates of aphid, jassid, and larvae of fruit borer were recorded 24, 48, and 72 h post-treatment of biopesticides (Mazra 2007).
Potted plant bioassay (field bioassay)
The plants were sown in earthen pots at Entomological Research Farm, Department of Entomology, University of Agriculture Faisalabad. Healthy plants were placed in open field for insect pests attack. The plants were irrigated regularly to fulfill their requirement of water and nutrients as well. When the infestation of brinjal insect pests reached near their threshold levels, i.e., aphid 5/leaf, jassid 2/leaf, and BSFB 10% damage (http://www.pestwarning.agripunjab.gov.pk/economic-thresholds), the biopesticide formulations (Table 1) were applied by foliar application method. The data of aphid and jassid were collected from upper-middle and lower leaves of each pot, and the data of the fruit borer were estimated by its damage symptoms (shoot and fruit damage). The data was recorded after 24, 48, and 72 h and 1 week post-application.
Data analysis
Population reduction percentage and percentage mortality of insects in the leaf dip bioassay were calculated by Abbott’s formula (Abbott 1925). The collected data were analyzed using a software, Statistics version 8.1, and means were compared, using Tukey’s honestly significant difference (HSD) test at 5% significant level.
Results and discussion
The results presented concentration (cfu ml−1) and time-dependent efficacy of the Trichoderma and Bt in vivo as well as in vitro experiments. The highly significant difference was observed when entomopathogens were applied both alone and in combination. The maximum population reduction percentage of the target insects was observed after 1 week of field application, while the maximum mortality was after 72 h of treatment application in laboratory bioassay.
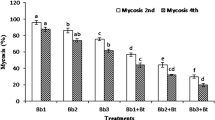
In laboratory experiments (Table 2), the results of Trichoderma alone are in accordance with Ganassi et al. (2001). The time and dose-dependent mortality was recorded. The significantly high percentage aphid mortality was observed (44, 36, and 13%) while 33, 13, and 6% jassid mortality was observed at 108, 107, and 106 (cfu ml−1) concentrations, respectively, after 24 h of post-treatment application (Pacheco 2017). The active movements of jassids may be a factor of getting less mortality than the aphids. Therefore, the jassids may be more resistant to such fungus than the aphids. Maketon et al. (2008) used Metarhizium anisopliae at the high concentration at 1013 cfu ml−1 and recorded > 70% mortality rate in Jassids, while in the obtained results, 33% mortality rate was recorded at 108 cfu ml−1. After 48 and 72 h, the percentage mortality of jassids was 46 and 53%, and 60 and 73% in the case of aphids, at the highest concentration (108 cfu ml−1) of Trichoderma sp. Similar trend was observed in percentage mortality of aphids and jassids at the lowest concentrations (107 and 106 cfu ml−1). In comparison to the field results, the population reduction trend was similar in the field pots after 48 h of post-treatment application. In case of Bt, an opposite mortality trend than Trichoderma was observed for aphids and jassids. Greater percentage mortality of jassid was observed, i.e., 40, 33, and 26%, than the aphids 36, 33, and 15% at the 3 concentrations (108, 107, and 106 cfu ml−1), respectively. Bt has known to have some genes to control the sucking pest (Kaur and Subash 2014) and may be jassid is not more resistant to those genes. In comparison to the field results, the opposite trend was observed, as more reduction was observed in aphids’ population.
In the case of brinjal shoot and fruit borer, Trichoderma alone caused 22, 44, and 66% mortality after 24, 48, and 72 h at the highest concentration (108 cfu ml−1), while the same trend was observed in the field that with the passage of time population reduction percentage increased, i.e., 27, 36, 39, and 49% after 24, 48, 72 h, and 1 week of post-applications, respectively (Table 3). Time-dependent efficacy of Trichoderma was observed in both field and laboratory experiments (Ganassi et al. 2001). At lower concentrations (107 and 106 cfu ml−1), the results were similar in laboratory bioassay when compared to the field experiment. Bt showed up to 33, 55, and 77% mortality rates of brinjal shoot and fruit borer after 24, 48, and 72 h, respectively, at the highest concentration (108 cfu ml−1) in laboratory bioassay (Table 2). Similarly, the lower concentrations (107 and 106 cfu ml−1) showed less mortality after 24 and 48 h, respectively.
The results obtained with Trichoderma alone are in accordance with Ganassi et al. (2001). The significantly higher population reduction of jassid (45%) than aphid (32%) was observed after 24 h in the pots, at the highest concentration used (108 cfu ml−1). In contrast, with the passage of time, the population reduction of aphid was significantly higher, i.e., 66, 78, and 87% than the jassid 47, 58, and 72% after 48, 72, and 168 h, respectively (Table 3). The entomopathogenic fungi are slow in action to cause an effect on target insects (Hafiza et al. 2014), and jassids movement was faster than the aphids. Therefore, the jassid may move from one place to other due to some repellent action of plant after treatment application. The population reduction of aphid increased with the passage of time because aphids remain stick to the target site and cannot change their place quickly (Ali et al. 2010). Overall, at the lowest concentrations (106 and 107 cfu ml−1), the population reduction of aphids was lower than the jassids. This may be due to the reason that aphids are resistant to Trichoderma at low concentration (Ganassi et al. 2001). Similarly, in case of Bt, the overall jassid population reduction was higher, i.e., 38, 26, and 19% than aphids 33, 26, and 26% after 24 h of all treatment application. The population reduction of aphids increased than the jassids with increase in treatment period, i.e., 48, 72, and 168 h. The reason behind that could be the same as in the case of Trichoderma. Similarly, field study also revealed time-dependent population reduction of BSFB after 24 (46%), 48 (56%), 72 (61%), and 168 (87%) h post-application. Rehab et al. (2020) also reported more than 80% population reduction of spiny bollworm Earias insulana after 1 week of post-application of five different bio-insecticides including entomopathigenic fungi and Bt. In addition, Joshi et al. (2010) and Nayak et al. (2013) reported that Bt significantly reduced the brinjal shoot and fruit borer population. The Bt was significantly more effective in vivo than in vitro conditions which is also in accordance with the previous studies (Nayak et al. 2013).
The pathogenicity of Trichoderma and Bt consortium was significantly higher in laboratory than in the field. However, a significant population reduction of aphids (73%) was recorded after 48 h at the highest concentration than jassids (62%). In accordance with individual application of entomopathogen, the aphids become significantly more susceptible to the combination of Trichoderma and Bt with the increasing time of application of the highest concentration. The population reduction of aphids was 90–95% and jassid was 79–88% after 72–168 h, respectively, at the highest concentration (108 cfu ml−1). Low concentration of Trichoderma and Bt consortium showed non-significant population reduction difference of target insects (aphids and jassids). The correlation coefficient values (r) and scatter diagram (Fig. 1) showed a positive correlation with mean percent mortality of target insect pests in laboratory as well as under field conditions.
The correlation coefficient values and scatter diagram showed a positive correlation with mean percent mortality of target insect pests in laboratory as well as under field conditions (R2: coefficient of determination), (Ŷ: dose-dependent percentage mortality of target pests) (X: densities of aphid, jassid, and brinjal shoot and fruit borer)
Generally, the BSFB is more susceptible to Bt than entomopathogenic fungi (Joshi et al. 2010 and Nayak et al. 2013) because Bt is more effective against borer than sucking insect pests (Krishna et al. 2002). The 51, 38, and 30% population reduction while 66, 55 and 44% mortality rate was observed after 24 h of Bt and Trichoderma consortium application at all concentrations (108, 107, and 106 cfu ml−1). After 48 h of treatment application, similar trend of population reduction (65, 51, and 46%) and mortality (88, 77, and 66%) was recorded in field and laboratory conditions, respectively. The 96, 80, and 72% population reduction at all concentrations (108, 107, and 106 cfu ml−1) was observed after 168 h respectively. These findings are also in accordance with recent findings of Rehab et al. (2020). The maximum mortality (88%) of BSFB was recorded after 72 h treatment application in laboratory by combined application of Trichoderma and Bt (Table 2). The maximum population reduction (96%) was observed after 168 h of treatment application at highest concentration used in the field (Table 3). These results revealed that combined application of entomopathogens can lead to a sustainable reduction in both chewing and sucking insect pests. The laboratory conditions always showed high mortality than pots in open field conditions, which may be the result of pest migration to other host plants and vice versa.
Conclusion
It may be concluded that the combination of Trichoderma spp. and B. thuringiensis showed a synergistic/additive effect and gave better control of brinjal insect pests. Single formulation provided also significant population reductions of brinjal insect pests. So, this microbial consortium could be used as safe potential biocontrol agent against insect pests of brinjal. However, field trial evaluation with this microbial consortium is required.
Availability of data and materials
The data used and analyzed during this project are available from the corresponding author on reasonable request.
Abbreviations
- IPM:
-
Integrated pest management
- Bt:
-
Bacilllus thuringiensis
- Ts:
-
Trichoderma species
- BSFB:
-
Brinjal shoot and fruit borer
- TSP:
-
Tryptic soya broth
- PDA:
-
Potato dextrose broth
- HSD:
-
Honestly significant difference
- CFU:
-
Colony-forming unit
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18(2):265–267
Afzal S, Tariq S, Sultana V, Ara J, Ehteshamul-Haque S (2013) Managing the root diseases of okra with endo-root plant growth promoting Pseudomonas and Trichoderma viride associated with healthy okra roots. Pak J Bot 45(4):1455–1460
Ali H, Shah RA, Zeb Q, Badshah H, Rehman M (2010) Evaluation of some chemicals against the aphids, jassids and white flies in potato. Sci Int 22(4):289–291
Chakraborty S, Sarkar P (2011) Management of Leucinodes orbonalis Guenee on eggplants during the rainy season in India. J Plant Protec Res 51(4):325–328.
Dutta P, Singha AK, Das P, Kalita S (2011) Management of brinjal fruit and shoot borer, Leucinodes orbanalis Guenee in agro-ecological condition of West Tripura. Schol J Agric Sci 1:16–19
FAO 2018. FAOSTAT. http://www.fao.org/faostat/en/#data/QC (Retrieved on 03-04-2020)
Ganassi S, Moretti A, Stornelli C, Fratello B, Pagliai AB, Logrieco A, Sabatini MA (2001) Effect of Fusarium, Paecilomyces and Trichoderma formulations against aphid Schizaphis graminum. Mycopathologia 151(3):131–138
Hafiza TG, Saeed S, Khan FZA (2014) Entomopathogenic fungi as effective insect pest management tactic: a review. Appl Sci Bus Econ 1(1):10–18
Harman GE, Shoresh M (2007) The mechanisms and applications of symbiotic opportunistic plant symbionts. In: Vurro M, Gressel J (eds) Novel biotechnologies for Biocontrol Agent Enhancement and Management. Springer, Dordrecht. Pp, pp 131–155
Jagginavar SB, Sunitha ND, Biradar AP (2009) Bioefficacy of flubendiamide 480 SC against brinjal fruit and shoot borer, Leucinodes orbonalis Guen. Karnataka J Agric Sci 22(3):712–713
Joshi N, Virk JS, Brar KS (2010) Efficacy of a Bacillus thuringiensis var. kurstaki Berliner formulation against shoot and fruit borer, Leucinodes orbonalis Guenee on brinjal. Insect Sci Ludhiana 23(2):224–227
Kabadwa BC, Roopali S, Jatinder K (2019) Present status and future prospects of bio-agents in agriculture. Int J Curr Microbiol App Sci 8(4):2138–2153
Kaur S, Singh S (2014) Field efficacy of systemic insecticides and microbial pesticides against aphid and fruit borer on tomato in Punjab. Veg Sci 41(2):171–176
Krishna TM, Lal OP, Srivastava YNS, Handa SK (2002) Field efficacy of different insecticides, Bacillus thuringiensis var. kurstaki (Bt), neem and diflubenzuron for the control of shoot and fruit borer, Leucinodes orbonalis Guen. on eggplant. Entomol Res 26(1):43–49
Maketon M, N., Orosz-Coghlan P, Hotaga D (2008) Field evaluation of metschnikoff (Metarhizium anisopliae) Sorokin in controlling cotton jassid (Amrasca biguttula biguttula) in aborigine (Solanum aculeatissimum). Int Agri Biol 10(1):47-51.
Mazra MSA (2007) Interaction effects between Beauveria bassiana and imidacloprid against Thrips tabaci (Thysanoptera: Thripidae). Agr App Biol Sci 72(3):549–555
Meyer SL, Massoud SI, Chitwood DJ, Roberts DP (2000) Evaluation of Trichoderma virens and Burkholderia cepacia for antagonistic activity against root-knot nematode, Meloidogyne incognita. Nematol 2(8):871–879
Mishra RK, Abhishek B, Naimuddin K, Krishna K, Kiran G, Sujayanand GK, Saabale PR, Satheesh NSJ, Birinchi KS, Dharmendra K, Monika M, Dhirendra KS, Narendra PS (2018) Utilization of biopesticides as sustainable solutions for management of pests in legume crops: achievements and prospects. Egypt J Biol Pest Cont 28(3):1–11
Misra HP (2008) New promising insecticides for the management of brinjal shoot and fruit borer, Lecinodes orbonalis Guenee. Pest Manag Hort Ecosys 14:140–147
Naveed M, Mitter B, Yousaf S, Pastar M, Afzal M, Sessitsch A (2014) The endophyte Enterobacter sp. FD17: a maize growth enhancer selected based on rigorous testing of plant beneficial traits and colonization characteristics. Biol Fertil Soils 50(2):249–262
Nayak US, Baral K, Rath LK, Mondal P (2013) Comparative efficacy of some bio-pesticides and botanicals against brinjal shoot and fruit borer, Leucinodes orbonalis Guenee. J Interacad 17(1):39–43
Pacheco JC, Poltronieri AS, Porsani MV, Zawadneak MAC, Pimentel IC (2017) Entomopathogenic potential of fungi isolated from intertidal environments against the cabbage aphid Brevicoryne brassicae (Hemiptera: aphididae). Bio Sci Technol 27(4):496–509
Paik HD, Bae SS, Park SH, Pan JG (1997) Identification and partial characterization of tochicin, a bacteriocin produced by Bacillus thuringiensis subsp tochigiensis. J Ind Microbiol Biotechnol 19(4):294–298
Rehab AAD, Dalia EL, Hemat ZM (2020) Field application of bio-insecticides on spiny bollworm, Earias insulana (Bosid.) on cotton by using recent low volume ground spraying equipment. Egypt Acad Biol Sci 13(1):47–57
Schnepf E, Crickmore NV, Van RJ, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev 62(3):775–806
Shetty PK (2004) Socio-ecological implications of pesticide use in India. Eco Polit Weekly 39:5261–5267
Xiaoman L, Aocheng C, Dongdong Y, Canbin O, Qiuxia W, Yuan L (2019) Overview of mechanisms and uses of biopesticides. Int J Pest manag:1–8
Yedidia I, Shoresh M, Kerem Z, Benhamou N, Kapulnik Y, Chet I (2003) Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl Environ Microbiol 69(12):7343–7353
Acknowledgements
The authors highly acknowledged Dr. Manuel Pplantegenest from Agro-campus Ouest (France) for the review and valuable comments for this article.
Funding
N/A
Author information
Authors and Affiliations
Contributions
AN designed the experiment and completed the write up. MDG and MS helped to design the study, and MN and MB helped SI to conduct the experiment. MW and MJA reviewed the manuscript and made valuable changes, and HA helped in statistical analysis. All authors approved the final article after reading.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A
Consent for publication
N/A
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nawaz, A., Gogi, M.D., Naveed, M. et al. In vivo and in vitro assessment of Trichoderma species and Bacillus thuringiensis integration to mitigate insect pests of brinjal (Solanum melongena L.). Egypt J Biol Pest Control 30, 60 (2020). https://doi.org/10.1186/s41938-020-00258-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00258-5