Abstract
Background
Various inflammatory conditions may present with musculoskeletal symptoms similar to those of polymyalgia rheumatica (PMR). We investigated findings on 18F-fluorodexoxyglucose (FDG)-positron emission tomography/computed tomography (PET/CT) images that may differentiate PMR from polymyalgia-like illnesses.
Methods
We analyzed data from 25 patients with new-onset polymyalgia-like illnesses who fulfilled Bird’s diagnostic criteria for PMR and had undergone FDG–PET/CT scan. To assess the uptake by major joints and synovial bursae, particularly at PMR-specific sites (shoulder, sternoclavicular, and hip joints, interspinous bursae, ischial tuberosities, and greater trochanters), we used visual scoring system to score FDG uptake: 0, no uptake (same as bone); 1, slight uptake; 2, moderate uptake (same as the liver); 3, greater uptake than the liver; and 4, uptake as strong as in the cerebellum.
Results
The final diagnoses were PMR in 17 patients and non-PMR in eight patients (three malignancies, two infections, one cholesterol crystal embolism, one ANCA-associated vasculitis, and one undefined diagnosis). Although the serum MMP-3 levels were significantly higher in patients with PMR, C-reactive protein and erythrocyte sedimentation rate mean values did not differ between the two groups. In PMR-specific sites, FDG accumulations were observed in all cases of PMR, with a high PET-positive score of 2.00 (range, 0–3), but it was low in non-PMR cases, with a PET-positive score of 1.00 (range, 0–3).
Conclusions
The FDG accumulation patterns in polymyalgia-like illness differ from those in PMR, despite the similar clinical presentations of both conditions. An FDG–PET/CT scan is useful for differentiating PMR from other polymyalgia-like illnesses.
Similar content being viewed by others
Background
Polymyalgia rheumatica (PMR) is a common inflammatory disorder that affects the elderly. It is characterized by muscle pain and stiffness in the neck, shoulders, and pelvic girdle [1]. However, the musculoskeletal symptoms associated with other inflammatory conditions such as infections, vasculitis, arthritis, myositis, and neoplasms may be similar and sometimes fulfill the diagnostic criteria for PMR [2, 3]. Therefore, it is often difficult to distinguish PMR from “polymyalgia-like illnesses” and their long differential diagnosis list [1, 4, 5].
The 18f-fluorodexoxyglucose (FDG)–positron emission tomography/computed tomography (PET/CT) imaging technique has been useful in the evaluation of joint inflammation [6, 7]. FDG accumulations in the shoulder and hip joints, interspinous bursae, and capsules of the knee are generally seen bilaterally on PET/CT images of patients with PMR [8,9,10], and FDG accumulations in the ischial tuberosities, greater trochanters, and lumbar interspinous bursae have also been reported [11]. The presence of uptake at two or more of these sites is reported to have high sensitivity (85.7%) and specificity (88.2%) for the diagnosis of PMR [11]. While FDG–PET/CT imaging in cases of polymyalgia-like illnesses such as paraneoplastic syndrome have also been reported [12, 13], no studies have compared the imaging findings between paraneoplastic polymyalgia (or other polymyalgia-like illnesses) and PMR.
Methods
Patients
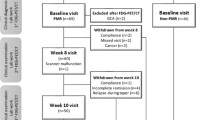
We reviewed the clinical data of 25 patients with new-onset polymyalgia-like symptoms admitted to the Division of Rheumatology of our institution between 2008 and 2013. Seventeen (9 men, 8 women; median age 77 [60–89] years) were diagnosed as having PMR, and 8 (4 men, 4 women; median age 71 [50–81] years) were diagnosed as having diseases other than PMR (non-PMR). All patients with PMR and non-PMR met the PMR criteria of Bird et al. [14], and we also evaluated their clinical symptoms and blood test results of rheumatoid factor and anti-CCP antibody using the 2012 ACR/EULAR provisional classification criteria [15]. Blood samples were obtained during the first visit. Erythrocyte sedimentation rates (ESRs), and C-reactive protein (CRP) and MMP-3 levels were measured to evaluate the degree of inflammatory activity. MMP-3 concentrations were measured using latex-enhanced immunoturbidimetric assays (Kyowa Pharmachemical, Toyama, Japan; the upper limit of the normal reference range was 59.7 ng/mL for women, and 121.0 ng/mL for men). For further MMP-3 level comparisons, we collected serum samples from 31 untreated patients with RA having had the disease for less than 6 months. Additional Table S1 shows the basic characteristics of these patients. We analyzed laboratory and imaging findings retrospectively. Individual patients cannot be identified from the materials in the manuscript, and the review board of the National Defense Medical College approved the study protocol (reference number: 2595). We posted relevant study protocol information on the web site of our division, including the announcement for the patients about their right to read the study protocol and to refuse to be included in the study (http://www.ndmc.ac.jp/hospital/section/kogenbyo/).
FDG–PET/CT imaging
The patients fasted for 6 h before the FDG–PET/CT scan. One hour before imaging, they received intravenous FDG (3.7 MBq/kg, 130–370 MBq). The scanning was performed from the vertex of the skull to the knee joints using an FDG–PET/CT scanner (Biograph LSO Duo; Siemens Medical Solutions, Knoxville, USA).
Two operators independently assigned FDG–PET/CT scores. FDG uptake was assessed in major joints and synovial bursae with particular attention to the shoulder, sternoclavicular, and hip joints, interspinous bursae, ischial tuberosities, and greater trochanters (these anatomical sites have been reported as PMR-specific FDG accumulation sites [8, 9, 11]). The scores were based on visual inspection and the maximum standardized uptake values (SUVmax). In the large joints, the synovium, perisynovium, and bursae accumulate different amounts of FDG [10]. To account for this, we set up a large volume of interest (VOI) for shoulder joints to calculate the ‘total’ SUVmax. Because of no standardized reference values for joint SUVs, we used SUVmax only for comparing the two groups. As a semi-quantitative scoring system on visual inspection, we used a modified Goerres et al. [7] scoring system to visually score FDG uptake: 0, no uptake (same as bone); 1, slight uptake; 2, moderate uptake (same as the liver); 3, greater uptake than the liver; and 4, uptake as strong as in the cerebellum. We considered FDG uptake scores > 2 as positive. The inter-reader agreement rate for the major joints and six PMR-specific sites was evaluated as excellent with a κ coefficient of 0.9031 (95% confidence interval (CI), 0.694–0.903). We calculated the PET-positive scores based on the cumulative number of positive accumulation sites within the interspinous bursae, ischial tuberosities, and greater trochanters, as proposed by Yamashita et al. [11].
Statistical analysis
We analyzed between-group differences using the non-parametric Mann–Whitney U test to compare the median values of demographic data, biomarkers, and FDG–PET/CT results, and calculated 95% CIs. We considered all p values < 0.05 as statistically significant. We analyzed the group data using the Fisher’s exact test based on the 2012 ACR/EULAR criteria. We assessed the FDG–PET/CT predictive value to diagnose PMR based on a univariate analysis and a receiver-operating characteristic (ROC) curve analysis. We used the statistical softwares GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA) and JMP Pro 14 (SAS Institute, Cary, NC, USA) to perform all calculations.
Results
Patients
The study population consisted of 25 patients. The final diagnoses of the patients in the non-PMR group were malignancy (n = 3), infection (n = 2), cholesterol crystal embolism (n = 1), ANCA-associated vasculitis (n = 1), and unknown (n = 1). In the case of the patient with the unknown diagnosis, she did not have any detectable autoantibodies, and showed no specific findings on imaging examinations (including on the FDG–PET/CT images). She recovered within a month, taking only celecoxib. Table 1 presents the clinical characteristics of all patients, and Additional Table S1 shows the detailed clinical characteristics of patients in the non-PMR group. We found no significant differences in terms of gender, age, or inflammatory variables between the PMR and non-PMR groups. Among the 17 patients in the PMR and the eight in the non-PMR groups, 16 (94%) and 6 (75%), respectively, satisfied the 2012 ACR/EULAR provisional classification criteria for PMR. Thus, we could not differentiate the two groups using those criteria. We found elevated MMP-3 levels in both of the groups, and the mean value was significantly higher in the PMR group. In comparison with patients with RA who visited our institute during the same time, the mean MMP-3 level (168.4 ng/mL; range, 26.8–478.3 ng/mL) in the 31 patients with untreated RA was the same as that in the non-PMR group, but significantly lower than that in the PMR group (Table S2).
FDG uptake scores at each site
Table 2 summarizes the FDG uptake scores. Similar to the results in other studies on PMR patients, we found FDG accumulation (by both uptake score and SUVmax) in all the PMR-specific sites. Figure 1a shows the typical FDG–PET/CT findings in patients with PMR. Additionally, we found FDG accumulation in the sternoclavicular joints.
a Typical FDG–PET/CT findings in a patient with PMR. High FDG uptake seen in PMR-specific sites, including cervical and lumbar interspinous bursae, shoulders, sternoclavicular, and hip joints, greater trochanters, and ischial tuberosities. b FDG–PET/CT in a 50-year-old woman with ovarian cancer. Strong FDG uptake in the ovary (arrowhead). Weak FDG uptake in the shoulders but not in any other PMR-specific sites
Patients in the non-PMR group had significantly lower FDG accumulation in PMR-specific sites (Fig. 1b) and showed FDG accumulation, such as the location of malignancy (Table S1). The median semi-quantitative FDG uptake scores in patients in the non-PMR group were significantly lower than those in the patients with PMR in the shoulder, interspinous bursae, and hip and ischial tuberosities (Table 2). The median PET-positive score in patients in the non-PMR group was significantly lower than in the patients in the PMR group (Table 2).
All FDG accumulations in PMR-specific sites except sternoclavicular joint were identified as predictors of PMR in our univariate analysis (Table 3). In ROC analysis, the sensitivity and specificity of FDG accumulation in the shoulder joints were higher than the others.
Discussion
Histopathological studies have revealed that the synovium, capsule, and bursae of the shoulder of patients with PMR have inflammatory changes with infiltration of T lymphocytes and macrophages, and increased vascularity [16, 17]. Various imaging tests including US and MRI have been used to detect inflammation to diagnose PMR [8]. Adding US results increased the sensitivity and specificity (66 and 81%, respectively) over the diagnostic criteria alone; however, this increase is relatively small [15]. No US consensus for PMR diagnosis exists in terms of involved joints, range of sites to be examined, or specific findings [8, 15].
MRI is a useful technique to detect inflammatory changes in joints and adjacent tissues. Reports have described various MRI findings in PMR [8].
Similar to other published results [11] from PMR cases, we found FDG accumulation in all reported PMR-specific sites, and in sternoclavicular joints like the report using bone scintigraphy [8]. Moreover, all PMR patients in the study had PET-positive scores > 2. Three cases in the non-PMR group had FDG accumulation in one site (shoulder, ischial tuberosity, or greater trochanter); however, their PET-positive scores were < 2.
While US, MRI, and FDG–PET/CT may all detect tissue inflammation in patients with PMR, whether these three imaging examinations can discriminate between polymyalgia-like illnesses and PMR is not clear. In the 2012 ACR/EULAR provisional classification criteria for PMR, adding an US examination decreased the specificity for discriminating RA from PMR to 65% [15]. Ochi et al. reported that MRI findings in severe rotator cuff tendinopathy, periarticular soft tissue edema, and large effusions in and around the shoulder and hip joints are useful indicators for diagnosing PMR, and also for discriminating RA from PMR [18].
Takahashi et al. reported the differences in FDG–PET/CT findings between patients with PMR and those with elderly-onset RA: In the shoulders and hips, they observed specific uptake patterns in each group with circular and linear uptake patterns around the humeral head in the case of RA, and focal and non-linear uptake patterns in the case of PMR [19]. Moreover, focal uptake in front of the hip joint, indicating iliopectineal bursitis, tended to be limited to the patients with PMR [19].
We did not include RA patients in our non-PMR group because no RA patients within the study period fulfilled the Bird’s criteria, and imaging examination is an ancillary procedure for diagnosis in the routine clinical practice. When discriminating RA, physicians look for peripheral small joint arthritis, the presence of serum rheumatoid factor and anti-CCP antibody, and the diagnostic RA criteria [20]. We understand that imaging techniques like FDG–PET/CT, US, or MRI, are not the sole basis for distinguishing the different possible etiologies of inflammation in one site.
We found that FDG accumulation in the shoulder joints was a predictor with higher sensitivity and specificity for diagnosing PMR. However, a limitation of this study was that it included a small population. In addition, if we had included RA patients with shoulder joint swelling in the study, the results could have been different.
The pathogenesis of myalgia in a variety of conditions that mimic PMR is still uncertain [1]. Inflammatory changes in polymyalgia-like illnesses have not been confirmed by histopathologic examinations. In our study, patients in the non-PMR group showed significantly less FDG accumulation in PMR-specific sites than those in the PMR group. These results suggest that the PMR pathogenesis may differ from that of non-PMR illnesses, even if the clinical presentations are similar. Inflammatory changes with infiltration of lymphocytes and macrophages, and increased vascularity may not be seen in the synovium and bursae of non-PMR disorders.
In this study, the mean MMP-3 level was significantly higher in the patients in the PMR group than in those in the non-PMR group and untreated RA patients. However, we also found the level of MMP-3 to change over a wide range. Thus, the MMP-3 value may be misleading in individual cases. More data is needed to determine whether a threshold level for serum MMP-3 that can distinguish between these disorders exists or if other blood tests can be combined with MMP-3 values to diagnose PMR.
To diagnose polymyalgia-like illnesses, physical examination findings need to be carefully considered, and blood tests and imaging tests should be planned to address a wide range of diagnoses. An FDG–PET/CT may be helpful, not only for differentiating PMR from polymyalgia-like illnesses, but also for determining the correct underlying diagnosis.
Conclusions
Various patterns of FDG uptake in patients with polymyalgia-like illnesses reflect the diversity of disorders despite the similar clinical presentations. Together with the current diagnostic criteria, the FDG accumulation pattern in PMR-specific sites may increase the accuracy of PMR diagnoses.
Availability of data and materials
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rates
- PET:
-
Positron emission tomography/computed tomography
- PMR:
-
Polymyalgia rheumatica
- ROC:
-
Receiver-operating characteristic
- VOI:
-
Volume of interest
References
Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. 2013;381:63–72.
Kampitak T. Polymyalgia rheumatica as the first presentation of metastatic lymphoma. Intern Med. 2010;49:1641–3.
Keith MP, Gilliland WR. Polymyalgia rheumatica and breast cancer. J Clin Rheumatol. 2006;12:199–200.
Dasgupta B, Borg FA, Hassan N, Barraclough K, Bourke B, Fulcher J, et al. BSR and BHPR guidelines for the management of polymyalgia rheumatica. Rheumatology. 2010;49:186–90.
Ceccato F, Una C, Regidor M, Rillo O, Babini S, Paira S. Conditions mimicking polymyalgia rheumatica. Reumatol Clin. 2011;7:156–60.
Kubota K, Ito K, Morooka M, Mitsumoto T, Kurihara K, Yamashita H, et al. Whole-body FDG-PET/CT on rheumatoid arthritis of large joints. Ann Nucl Med. 2009;23:783–91.
Goerres GW, Forster A, Uebelhart D, Seifert B, Treyer V, Michel B, et al. F-18 FDG whole-body PET for the assessment of disease activity in patients with rheumatoid arthritis. Clin Nucl Med. 2006;31:386–90.
Camellino D, Cimmino MA. Imaging of polymyalgia rheumatica: indications on its pathogenesis, diagnosis and prognosis. Rheumatology. 2012;51:77–86.
Blockmans D, De Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18-fluorodeoxyglucose positron emission tomography in isolated polymyalgia rheumatica: a prospective study in 35 patients. Rheumatology. 2007;46:672–7.
Cimmino MA, Camellino D, Paparo F, Morbelli S, Massollo M, Cutolo M, et al. High frequency of capsular knee involvement in polymyalgia rheumatica/giant cell arteritis patients studied by positron emission tomography. Rheumatology. 2013;52:1865–72.
Yamashita H, Kubota K, Takahashi Y, Minaminoto R, Morooka M, Ito K, et al. Whole-body fluorodeoxyglucose positron emission tomography/computed tomography in patients with active polymyalgia rheumatica: evidence for distinctive bursitis and large-vessel vasculitis. Mod Rheumatol. 2012;22:705–11.
Durant C, Hervier B, Ansquer C, Masseau A, Hamidou M. Occult Hodgkin lymphoma presenting as polymyalgia rheumatica: value of [18F]-FDG positron emission tomography. Ann Hematol. 2010;89:111–2.
Suzuki S, Ikusaka M, Miyahara M, Shikino K. Positron emission tomography findings in a patient with multiple myeloma of polymyalgia rheumatica-like symptoms caused by paraneoplastic syndrome. BMJ Case Rep. 2014;2014:bcr2013203326.
Bird HA, Esselinckx W, Dixon AS, Mowat AG, Wood PH. An evaluation of criteria for polymyalgia rheumatica. Ann Rheum Dis. 1979;38:434–9.
Dasgupta B, Cimmino MA, Kremers HM, Schmidt WA, Schirmer M, Salvarani C, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European league against rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum. 2012;64:943–54.
Gordon I, Rennie AM, Branwood AW. Polymyalgia rheumatica: biopsy studies. Ann Rheum Dis. 1964;23:447–55.
Meliconi R, Pulsatelli L, Uguccioni M, Salvarani C, Macchioni P, Melchiorri C, et al. Leukocyte infiltration in synovial tissue from the shoulder of patients with polymyalgia rheumatica. Quantitative analysis and influence of corticosteroid treatment. Arthritis Rheum. 1996;39:1199–207.
Ochi J, Nozaki T, Okada M, Suyama Y, Kishimoto M, Akaike G, et al. MRI findings of the shoulder and hip joint in patients with polymyalgia rheumatica. Mod Rheumatol. 2015;25:1–7.
Takahashi H, Yamashita H, Kubota K, Miyata Y, Okasaki M, Morooka M, et al. Differences in fluorodeoxyglucose positron emission tomography/computed tomography findings between elderly onset rheumatoid arthritis and polymyalgia rheumatica. Mod Rheumatol. 2015;25:546–51.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81.
Acknowledgments
Authors thank Dr. Jiro Ishida (Tokorozawa PET Diagnostic Imaging Clinic) and Dr. Yoko Satoh (PET center, Kofu Neurosurgical Hospital) for their valuable assistance in interpretations for PET/CT findings.
Funding
This work was supported by The Foundation for Promotion of Defense Medicine (grant number 33026). This Foundation had no role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
HH had full access to the data in the study and takes responsibility for data integrity and accurate data analysis. HH and KI contributed to the conception and design of the study. TN contributed to the data acquisition. HH, KT, and KI contributed to data analysis and interpretation. HH and KI drafted the manuscript. HH, FK and KI contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The review board of the National Defense Medical College approved the study protocol (reference number: 2595).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1
. Description of data: Clinical characteristics of eight patients in the non-PMR group who fulfilled Bird’s diagnostic criteria for PMR
Additional file 2: Table S2.
Description of data: Clinical characteristics of patients with PMR and 31 untreated patients with RA having presented the disease for less than 6 months
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Horikoshi, H., Nakanishi, T., Tamura, K. et al. 18F-fluorodeoxyglucose–positron emission tomography/computed tomography for the diagnosis of polymyalgia-like illnesses: a retrospective study. BMC Rheumatol 4, 21 (2020). https://doi.org/10.1186/s41927-020-00121-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41927-020-00121-y