Abstract
Background
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is an essential regulator of glycolysis used as a housekeeping marker for gene/protein normalisation. Given the pivotal role of GAPDH in tumour metabolism, our aim was to correlate its protein expression with tumour staging and prognosis of colorectal cancer.
Methods
GAPDH expression was immunohistochemically analysed in tumour tissues from 62 colorectal cancer (CRC) patients, and validated at mRNA level in an independent dataset comprising 98 paired stage II CRC and normal samples. Staining quantification was performed by computational image analysis, and correlations between GAPDH expression and tumour progression stage were assessed. Gene expression profiling was performed using Affymetrix microarrays. Probability of patient survival and disease-free survival were analysed by the univariate product-limit method of Kaplan-Meier. Groups were compared using Kruskal-Wallis tests.
Results
Overexpression of GAPDH is positively associated with early stage tumours without regional lymph node and distant metastases involved. These results were reinforced by those obtained at mRNA level.
Conclusion
Studying the role of GAPDH in malignant transformation can shed new light on the understanding of tumour onset and lead to the design of more efficient personalised therapies.
Similar content being viewed by others

Background
Multifactorial diseases result from the interaction between genetic susceptibility and environmental factors and do not exhibit a clear hereditary pattern, which causes difficulties in their risk evaluation, diagnosis and treatment. Cancer is a prevalent multifactorial disease characterised by loss of physiological control and malignant transformation of cells that acquire functional and genetic abnormalities, leading to tumour development and progression. In addition, cancer cells from the primary site can have the ability to invade other tissues resulting in metastasis, the major cause of death from cancer.
Colorectal cancer (CRC) is a global leading cause of cancer-related mortality being the third most common cancer in men and the second in women worldwide [1]. Fortunately, systematic risk population screening programmes facilitate early detection and treatment reducing its incidence and mortality rate. The American Joint Committee on Cancer (AJCC) TNM staging system establishes four different stages of tumour progression (I to IV) depending on the primary tumour extent (T), the regional lymph node involvement (N) and the absence or presence of distant metastasis (M) [2]. Cancer staging systems help oncologists to evaluate disease prognosis, treatment suitability and the possibilities of patients to be enrolled in specific clinical trials, contributing to fight CRC. However, further studies are required to identify new biomarkers to predict CRC outcome, help to understand its evolution and find the most suitable treatment.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH, EC 1.2.1.12) is an essential regulator of glycolysis ubiquitously overexpressed in 21 cancer classes, being one of the three glycolytic genes overexpressed in colorectal cancer, along with enolase and pyruvate kinase [3, 4]. This fact advises against its widely extended use as a housekeeping gene. GAPDH catalyses the phosphorylation and oxidation of glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate coupled with the reduction of NAD+ to NADH. GAPDH is constituted by four identical subunits of 37 kDa and has binding sequences for coenzymes NAD+ and NADH and for nucleic acids. In fact, GAPDH is one of the multifaceted glycolytic enzymes that contributes to cell survival and resistance to cell death [5], becoming a promising biomarker in cancer research. Moreover, GAPDH expression is regulated by transcription factors which are frequently altered in cancer such as HIF-1α, p53 or AP-1. In addition, GAPDH gene has insulin (IRE) and hypoxia response elements (HRE) in its promoter [6]. As a multifunctional enzyme, GAPDH is involved in endocytosis, membrane fusion, vesicle secretion, transcription coactivation, cell cycle regulation, tRNA transport, mRNA stabilisation and DNA repair and replication [6,7,8]. Paradoxically, GAPDH also plays important roles activating apoptosis by nuclear translocation upon cellular stress, and protecting from caspase-independent cell death (CICD) by autophagic removal of damaged mitochondria [7].
Interestingly, due to its glycolytic role, GAPDH may also play a part in metabolic reprogramming, one of the hallmarks of cancer [9] and a suitable target for cancer therapy as tumour cells require an increased use of the metabolic pathways to sustain their energetic and biosynthetic needs. Aerobic glycolysis with high lactate production is one of the main phenotypic characteristics of tumour metabolism, as described by Otto Warburg [10]. This phenomenon gives advantage to cancer cells by an increased production of NADH and an acidification of the microenvironment due to lactate excretion, favouring tumour invasion [11]. Glucose metabolism through anaerobic glycolysis is less efficient in ATP production than oxidative phosphorylation. However, the high rate of glucose uptake and the increased glycolytic flux in cancer cells meet the requirements for maintaining the energy status, the biosynthesis of macromolecules and the maintenance of cellular redox balance [12]. Consequently, GAPDH can be a key enzyme implicated in tumourigenesis and cancer progression.
In the current study, we have employed an objective image analysis quantification method [13] to examine the expression pattern of GAPDH throughout the stages of tumour progression in colorectal cancer. In addition, we have validated our results at mRNA level in an independent dataset comprising 98 paired stage II CRC and normal samples. Correlations between GAPDH expression and tumour progression are explored in order to clarify the role of this protein during tumour onset and development. We have identified significant differences in GAPDH expression depending on primary tumour extent, regional lymph node involvement and presence of distant metastasis.
Methods
Human tissue specimens and patients information
Immunohistochemical samples
This study included 62 non-chosen samples from 45 men and 17 women between 33 and 93 years old (average 70 ± 11 years old) with CRC which underwent surgery between November 2000 and October 2001. The Gastroenterology Department of the Hospital Clínic of Barcelona recruited all patients as part of the EPICOLON project, a prospective multicenter, nation-wide, population-based study for the establishment of the incidence and characteristics of inherited and familial colorectal cancer forms in Spain [14]. The EPICOLON project included all newly diagnosed CRC patients in any participating centre during one-year period. Pathological staging was based on the standard of the AJCC TNM classification of colon and rectal cancer, which considers transmural extension, lymph node involvement and presence of metastasis. Our study included nine tumour samples from stage I patients, 21 from stage II, 16 from stage III and 16 from stage IV.
After surgery, patients underwent standard therapeutic and follow-up measures according to recommended guidelines. Postoperative adjuvant treatment with 5-fluorouracil and leucovorin was routinely given to patients with stage II and III tumours, and radiation therapy was indicated in patients with rectal cancer. Postoperative surveillance consisted of medical history, physical examination, and laboratory studies including serum carcinoembryonic antigen (CEA) levels every three months, abdominal ultrasonography or computed tomography every six months, and chest radiograph and total colonoscopy once a year. All tumour recurrences detected during the follow-up were histologically confirmed. After a 49-month period follow-up, 26 patients had died.
Gene expression samples
An independent dataset comprising a set of 98 paired adjacent-normal and tumour tissues from CRC patients (196 samples in total) were included in this work (Colonomics project [15], CLX-: www.colonomics.org; NCBI BioProject PRJNA188510). Patients were selected to form a homogeneous clinical group of stage II, microsatellite stable (MSS) colorectal tumours. All patients had been treated with radical surgery and had not received adjuvant therapy. Adjacent normal tissue from patients was dissected with a minimum proximal distance of 10 cm from the tumour resection margins. All patients were recruited at the Bellvitge University Hospital (Spain) and the Institution’s Ethics Committee approved the protocol. Written informed consent from patients was required for inclusion in this study.
Immunohistochemical staining
Tumours were dissected immediately after surgical resection by a pathologist, snap-frozen and stored in liquid nitrogen. Tissue specimens (2-5 μm) were placed on slides and fixed with paraformaldehyde. Immunohistochemistry was performed as previously described [13]. Briefly, following heat-induced antigen retrieval, endogenous peroxidases inhibition and unspecific staining blockage, slides were incubated with anti-GAPDH antibody (sc-47724, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Negative controls were incubated with 3% BSA instead of primary antibody. After secondary antibody incubation, samples were treated with streptavidin-peroxidase (Streptavidin-HRP, LSAB + -kit, DakoCytomation, Hamburg, Germany) and stained with 3-39-diaminobenzidine (DAB + Chromogen, Dako- Cytomation, Hamburg, Germany). Slides were viewed under a LEICA DM4000B microscope (Leica Microsystems, Germany) and a monochromatic IEEE-1394 CFW-1312 M camera (Scion Corporation, Frederik, MD, USA). GAPDH expression was quantified using ImageJ software (NIH Imaging, USA), measuring relative intensity per area and interpolating into a calibration curve plotted using a grey scale. For each sample, 4 to 15 pictures from different areas were quantified. Samples were processed in groups of eight with representation of all four stages of tumour progression each time. Values are presented as relative values in arbitrary units (a.u.). See Additional file 1: File S1 for detailed methods of immunohistochemical staining, total protein extraction and Western blot analysis.
Gene expression data
RNA extracted from an independent set of 98 paired adjacent-normal and tumour tissues from CRC patients (196 samples in total [15]) was hybridised in Affymetrix chips Human Genome U219. Gene expression profiles of these samples have been performed as described in [15] and deposited in the Gene Expression Omnibus (GEO) database [16] through accession number GSE44076.
Data analysis and statistical methods
Statistical analyses were conducted using Statgraphics statistical package (Statgraphics Centurion XVI, StatPoint Technologies Inc., Warrenton, VA). Differences between two groups were evaluated using the Student’s t-test while the analysis of variance (ANOVA) was employed when comparing more than two groups. Fisher’s least significant difference (LSD) test was used to identify which were the groups that specifically differed from the others. Outliers were identified by Dixon’s Q-test and homogeneity of variances was assessed by Levene’s test. Probability of patient survival and disease-free survival were analysed by the univariate product-limit method of Kaplan-Meier. All data are expressed as mean ± standard deviation (SD). Differences were considered to be significant at P-value < 0.05 (*), P-value < 0.01 (**) and P-value < 0.001 (***).
Results
Patients
GAPDH expression was analysed by immunohistochemistry in 62 patients with primary CRC and confirmed not to be age (P = 0.9599) or gender dependent (P = 0.8091). Table 1 lists demographic, clinical and tumour-related characteristics. After a 49-month period follow-up, 26 patients had died.
Immunohistochemical detection of GAPDH expression
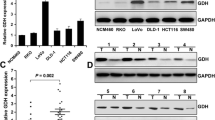
Specific and significant labelling was observed in primary tumours incubated with anti-GAPDH antibody, while control samples did not present any unspecific labelling (Fig. 1). GAPDH antibody was proved to be specific by Western blot, presenting a single band at 37 kDa (Additional file 2: Figure S1). The homoscedasticity for GAPDH expression for all the studied groups was positively assessed using Levene’s test (P = 0.7046). Having confirmed the normal distribution of our data applying the Shapiro-Wilk test, we performed parametric statistic tests to evaluate the significance of the differences observed. Clinicopathological and immunohistochemical data are summarised in Additional file 3: Table S1.
GAPDH expression in colorectal tumours in different stage of progression. a Representative colour photomicrographs presenting immunohistochemical staining (brown) for GAPDH in colorectal tumours (x200). Stained tissues at different stages of progression are displayed in the right column and homologous areas in negative controls in the left column. b Representative monochromatic photomicrographs were analysed with ImageJ software to quantify immunostaining and assess GAPDH expression (x150). Stained tissues at different stages of progression are portrayed in the right column and homologous areas in negative controls in the left column. For both (a) and (b), representative photomicrographs used were from sample 7058 for stage I, sample 7020 for stage II, sample 7112 for stage III and sample 7104 for stage IV
GAPDH expression in relation to tumour stage
GAPDH expression ranges from 127.2 to 350.2 a.u. (mean, 229.0 ± 65.0) in stage I tumours, from 17.0 to 241.3 a.u. (mean, 116.1 ± 70.4) in stage II, from 22.5 to 252.3 a.u. (mean, 125.0 ± 58.9) in stage III and 16.5 to 206.1 a.u. (mean, 92.0 ± 62.2) in stage IV (Fig. 2). These results showed a significant overexpression of GAPDH in stage I compared to the other three stages (ANOVA mean comparison, P = 0.0001). An outlier was identified (sample 7107, stage IV) using Dixon’s Q-test, presenting an abnormal high value (317.9 a.u.) compared with the rest of its group at the 95% confidence level.
Interestingly, GAPDH expression in stage I tumours was significantly higher than in any other stage of progression (Fig. 2). In addition, patients who presented with a more advanced disease stage (stages II, III and IV) displayed similar GAPDH expression levels without any significant differences between them.
GAPDH expression according to primary tumour extent
Considering primary tumour transmural progression extent, early stage tumours (T1 and T2) presented significantly enhanced GAPDH expression values, ranging from 125.9 to 350.2 a.u. (mean 213.1 ± 68.4; mean comparison by Student’s t-test, P = 0.00003), compared to more advanced primary tumour (T3 and T4) which ranged from 16.5 to 252.3 a.u. (mean 111.3 ± 66.3) (Fig. 3a). This statement is also applicable when only considering non-metastatic primary tumours (Fig. 3b). These data reveal a significant decrease in GAPDH expression when the depth of invasion into the wall of the intestine and the extension to adjacent structures are greater.
Correlation between GAPDH expression and TNM classifications. a GAPDH expression in relation to the primary tumour extent in all 62 patients and (b) in patients who had not developed distant metastasis. In both cases, GAPDH expression is significantly higher in tumours that have only affected the most external layers of the colonic wall (submucosa and muscularis propria) than in tumours in more invasive stages (invasion into the subserosa, non-peritonealised pericolic or perirectal tissues, other organs and/or perforation of visceral peritoneum). c The presence of metastases in regional lymph nodes is correlated with a significant decrease in primary tumour GAPDH expression. d In the absence of distant metastasis, tumours express significantly higher levels of GAPDH
GAPDH levels in primary tumours involving regional lymph nodes
GAPDH expression in patients without regional lymph node involvement (N0) (mean 147.8 ± 81.6 a.u.) is statistically superior (Student’s t-test, P = 0.0398) than in patients with metastasis in 1 to 3 (N1) and 4 or more (N2) regional lymph nodes (mean 106.7 ± 65.2 a.u.) (Fig. 3c).
Influence of distant metastasis in GAPDH expression
Samples from patients who had not developed distant metastasis (M0) displayed an average value for GAPDH expression of 141.3 ± 77.7 a.u. while the corresponding value for those patients who presented with distant metastasis (M1) was 92.0 ± 62.2 a.u. These results depicted a significant decrease in GAPDH expression (Student’s t-test, P = 0.0294) in the presence of distant metastasis (Fig. 3d).
GAPDH expression and survival in colorectal cancer
To assess the relationship between tumour GAPDH expression, patient survival and disease-free survival in colorectal cancer, we used univariate Kaplan-Meier analysis and Mantel-Cox log rank test. We found that there is no significant association between GAPDH expression levels and survival in colorectal cancer (Mantel-Cox log rank test, P = 0.609 considering all stages of tumour progression and P = 0.773 for stages I to III without distant metastasis) (Figs. 4a and 4b). On the other hand, results suggested a not statistically significant correlation between overexpression of GAPDH and increased disease-free survival (mean values 63.11 ± 3.06 and 52.77 ± 3.97 months for patients with high and lower GAPDH expression levels, respectively; Mantel-Cox log rank test, P = 0.051 considering all stages of tumour progression and P = 0.064 for stages I to III without distant metastasis) (Figs. 4c and 4d).
Correlation between GAPDH expression and patient survival. a Kaplan-Meier plot correlating GAPDH expression and survival in patients with colorectal cancer (P = 0.609). b In patients with non-metastatic colorectal cancer (P = 0.773). c Kaplan-Meier plot correlating GAPDH expression and disease-free survival in patients with colorectal cancer (P = 0.051). d In patients with non-metastatic colorectal cancer (P = 0.064). Black line represents the group of patients whose GAPDH expression is higher than the median values, and grey line represents those with GAPDH expression lower than the median values
GADPH expression validation in an independent dataset
To further validate differences in GAPDH expression, an independent dataset comprising 98 normal-tumour paired samples was used. In this series, gene expression was evaluated using Affymetrix microarrays. As a result, statistically significant differences in GAPDH expression between normal and tumour samples were found (P = 2.2 · 10-16). As expected, tumour samples overexpressed GADPH when compared with the normal ones (Fig. 5). Moreover, the variability of gene expression is greater in tumour than in normal samples (log2 expression ranging from a minimum of 9.8 and a maximum of 13.1). In addition, in order to compare the expression of GAPDH in different cancer studies, we consulted the cBioPortal database (http://www.cbioportal.org/index.do) [17, 18] and we found a significant dispersion of GAPDH expression in the cancer sites available in the database.
Discussion
In tumour cells, upregulation of aerobic glycolysis with elevated lactate production generates an acidic and hypoxic extracellular microenvironment which gives a selective growth advantage to malignant cells and facilitates invasion through destruction of adjacent normal populations, degradation of the extracellular matrix by metalloproteases and promotion of angiogenesis [19]. Hypoxia and glycolytic products such as lactate and pyruvate can stimulate hypoxia-inducible factor 1 (HIF-1α) accumulation in solid tumours leading to upregulation of survival genes, angiogenic factors and glycolytic enzymes, including GAPDH [20,21,22]. Overexpression of almost every glycolytic enzyme has been described in prostate and brain cancers [3]. However, GAPDH, enolase and pyruvate kinase are the only glycolytic genes whose expression is enhanced in colorectal cancer. According to our results, GAPDH is increased in all stages of colorectal tumour progression, reaching its maximum in stage I and with a moderate overexpression in the subsequent stages. These findings suggest that in addition to its role in glycolysis, GAPDH may be involved in non-glycolytic processes during tumour onset. Moreover, overexpression of GAPDH in CRC tumours has been validated in an independent dataset comprising 196 paired stage II CRC and normal samples, which reinforces the significant role of this gene in tumourigenesis. In fact, GAPDH is known to be a multifunctional protein implicated not only in glycolysis but also in many other highly relevant non-glycolytic processes in cells such as the cellular response to oxidative stress and DNA damage, transcriptional and post-transcriptional gene regulation, intracellular membrane trafficking, cell cycle regulation, receptor mediated cell signalling, autophagy and apoptosis [8]. All these multiple activities interconnect GAPDH expression and tumourigenesis.
In colon carcinogenesis the selective repression of mitochondrial β-F1-ATPase expression, which reduces oxidative phosphorylation, leads to an increase in GAPDH expression [23] that may explain the rise of GAPDH levels observed in the initial stages of tumourigenesis. Moreover, the reduction of β-F1-ATPase expression limits electron flux through electron transport chain, which is the main endogenous source of oxidative stress, causing the diminution of free radical generation and the risk of apoptosis. Therefore, this could be a mechanism by which cells simultaneously acquire high levels of GAPDH and protect themselves against oxidative stress. Indeed, GAPDH is a redox-sensitive enzyme whose activity is affected by oxidation of the cysteine residue located in its catalytic site [24, 25]. The oxidation of GAPDH depends on the intracellular reactive oxygen species (ROS) concentration. Interestingly, a dual modulation of GAPDH activity has been described in cells exposed to sub-apoptogenic and apoptogenic oxidative stress levels. Under mild oxidative stress conditions, such as the one that is induced by the repression of mitochondrial β-F1-ATPase, GAPDH is strongly hyperactivated, while apoptogenic stress levels inhibit GAPDH activity [26, 27]. In advanced stages of tumour progression, oxidative stress is higher than in early phases of carcinogenesis [28,29,30], which can explain the increased levels of GAPDH in stage I in comparison with later stages of progression. In addition, overexpression of GAPDH has two cooperative roles in protection towards caspase-independent cell death; a high glycolytic ATP production and the induction of autophagy-mediated clearance of damaged mitochondria [6, 7], both of which favour tumour onset.
On the other hand, under high oxidative stress conditions, chaperone-mediated autophagy (CMA) is upregulated in order to selectively remove altered or damaged proteins. As a CMA putative substrate, GAPDH contains a KFERQ-like motif that is recognised by chaperone heat shock cognate 70 (Hsc70) for lysosomal degradation [31]. The abovementioned increase of oxidative stress in advanced stages of tumour progression can trigger GAPDH inactivation and subsequent CMA, resulting in the observed reduction of protein expression compared to early tumour stages.
The capability to sustain proliferative signalling is one of the hallmarks required in tumourigenesis [9]. GAPDH can enhance cell proliferation during tumour onset by binding to the SET protein at the same site of cyclin B and reversing the inhibitory effects of SET on cyclin B-CDK1 complex [32]. As we mentioned before, metabolic reprogramming is another hallmark of cancer that allows tumour cells to fulfil the increased demands for energy and macromolecules required to sustain the accelerated proliferation rates [9]. In fact, glycolysis is a key pathway in the tumour metabolic reprogramming and a potential target for cancer therapy [33]. Non-tumour cells are expected to be less sensitive to glycolysis inhibition than malignant cells, which are more dependent on this pathway to generate ATP and proliferate [34]. Therefore, GAPDH emerges as a promising therapeutic target against cancer since it is overexpressed in 21 cancer classes [3] and involved not only in cell proliferation and glycolysis but also in several other mechanisms which are upregulated in tumour cells [35, 36].
Previous studies have correlated GAPDH expression with an overall poor prognosis. However, in these reports tumour samples were obtained from patients with advanced stages of tumour progression (II–IV) [4, 23, 37, 38], without tumour staging classification [39] or where GAPDH gene but not protein expression was quantified [40, 41]. In the present study, we have observed a significant overexpression of GAPDH protein during the tumour onset which is associated with patients in the early stages of disease (stage I) and a better outcome.
Finally, the use of GAPDH as a housekeeping gene or internal standard for gene/protein level normalisation is still widespread even though it is well documented that this use is inappropriate [42]. Our results further support this conclusion as we have observed significant differences in GAPDH expression between stages of colorectal tumour progression as well as heterogeneity in GAPDH gene expression levels between samples.
Conclusion
In this study, we found out that GAPDH expression in colorectal cancer is significantly upregulated during tumour onset. These results suggest that GAPDH plays an important role in colorectal tumourigenesis and could be used as an early detection biomarker. The monitoring of GAPDH levels in high-risk population may enable early diagnosis and better recovering rates. The study of the expression of GAPDH-related proteins can further contribute to the knowledge of the mechanisms underlying tumour onset and to the design of more efficient and personalised anti-oncogenic therapies.
Abbreviations
- CRC:
-
Colorectal cancer
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- NAD+:
-
Nicotinamide adenine dinucleotide oxidised form
- NADH:
-
Nicotinamide adenine dinucleotide reduced form
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–386.
Edge SBBD, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010.
Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–20.
Tang Z, Yuan S, Hu Y, Zhang H, Wu W, et al. Over-expression of GAPDH in human colorectal carcinoma as a preferred target of 3-bromopyruvate propyl ester. J Bioenerg Biomembr. 2012;44:117–25.
Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142–50.
Colell A, Green DR, Ricci JE. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009;16:1573–81.
Colell A, Ricci JE, Tait S, Milasta S, Maurer U, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–97.
Sirover MA. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: biochemical mechanisms and regulatory control. Biochim Biophys Acta. 2011;1810:741–51.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Warburg O, Posener K, Negelein E. Über den Stoffwechsel der Karzinomzellen. Biochem Z. 1924;152:309–44.
Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–82.
Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95.
Diaz-Moralli S, Tarrado-Castellarnau M, Alenda C, Castells A, Cascante M. Transketolase-like 1 expression is modulated during colorectal cancer progression and metastasis formation. PLoS One. 2011;6:e25323.
Castellvi-Bel S, Ruiz-Ponte C, Fernandez-Rozadilla C, Abuli A, Munoz J, et al. Seeking genetic susceptibility variants for colorectal cancer: the EPICOLON consortium experience. Mutagenesis. 2012;27:153–9.
Sanz-Pamplona R, Berenguer A, Cordero D, Mollevi DG, Crous-Bou M, et al. Aberrant gene expression in mucosa adjacent to tumor reveals a molecular crosstalk in colon cancer. Mol Cancer. 2014;13:46.
Barrett T, Edgar R. Gene expression omnibus: microarray data storage, submission, retrieval, and analysis. Methods Enzymol. 2006;411:352–69.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9.
Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72.
Graven KK, Troxler RF, Kornfeld H, Panchenko MV, Farber HW. Regulation of endothelial cell glyceraldehyde-3-phosphate dehydrogenase expression by hypoxia. J Biol Chem. 1994;269:24446–53.
Graven KK, Bellur D, Klahn BD, Lowrey SL, Amberger E. HIF-2alpha regulates glyceraldehyde-3-phosphate dehydrogenase expression in endothelial cells. Biochim Biophys Acta. 2003;1626:10–8.
Cuezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaria G, et al. The bioenergetic signature of cancer: a marker of tumor progression. Cancer Res. 2002;62:6674–81.
Hwang NR, Yim SH, Kim YM, Jeong J, Song EJ, et al. Oxidative modifications of glyceraldehyde-3-phosphate dehydrogenase play a key role in its multiple cellular functions. Biochem J. 2009;423:253–64.
Sirover MA. Structural analysis of glyceraldehyde-3-phosphate dehydrogenase functional diversity. Int J Biochem Cell Biol. 2014;57C:20–6.
Cerella C, D’Alessio M, Cristofanon S, De Nicola M, Radogna F, et al. Subapoptogenic oxidative stress strongly increases the activity of the glycolytic key enzyme glyceraldehyde 3-phosphate dehydrogenase. Ann N Y Acad Sci. 2009;1171:583–90.
Cerella C, Dicato M, Diederich M. Modulatory roles of glycolytic enzymes in cell death. Biochem Pharmacol. 2014;92:22–30.
Panis C, Victorino VJ, Herrera AC, Freitas LF, De Rossi T, et al. Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Res Treat. 2012;133:881–8.
Acquaviva R, Iauk L, Sorrenti V, Lanteri R, Santangelo R, et al. Oxidative profile in patients with colon cancer: effects of Ruta chalepensis L. Eur Rev Med Pharmacol Sci. 2011;15:181–91.
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16.
Cuervo AM, Terlecky SR, Dice JF, Knecht E. Selective binding and uptake of ribonuclease A and glyceraldehyde-3-phosphate dehydrogenase by isolated rat liver lysosomes. J Biol Chem. 1994;269:26374–80.
Carujo S, Estanyol JM, Ejarque A, Agell N, Bachs O, et al. Glyceraldehyde 3-phosphate dehydrogenase is a SET-binding protein and regulates cyclin B-cdk1 activity. Oncogene. 2006;25:4033–42.
Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152.
Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–46.
Ganapathy-Kanniappan S, Kunjithapatham R, Geschwind JF. Glyceraldehyde-3-phosphate dehydrogenase: a promising target for molecular therapy in hepatocellular carcinoma. Oncotarget. 2012;3:940–53.
Krasnov GS, Dmitriev AA, Snezhkina AV, Kudryavtseva AV. Deregulation of glycolysis in cancer: glyceraldehyde-3-phosphate dehydrogenase as a therapeutic target. Expert Opin Ther Targets. 2013;17:681–93.
Hjerpe E, Egyhazi Brage S, Carlson J, Frostvik Stolt M, Schedvins K, et al. Metabolic markers GAPDH, PKM2, ATP5B and BEC-index in advanced serous ovarian cancer. BMC Clin Pathol. 2013;13:30.
Barbazan J, Muinelo-Romay L, Vieito M, Candamio S, Diaz-Lopez A, et al. A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer. Int J Cancer. 2014;135:2633–43.
Revillion F, Pawlowski V, Hornez L, Peyrat JP. Glyceraldehyde-3-phosphate dehydrogenase gene expression in human breast cancer. Eur J Cancer. 2000;36:1038–42.
Puzone R, Savarino G, Salvi S, Dal Bello MG, Barletta G, et al. Glyceraldehyde-3-phosphate dehydrogenase gene over expression correlates with poor prognosis in non small cell lung cancer patients. Mol Cancer. 2013;12:97.
Wang D, Moothart DR, Lowy DR, Qian X. The expression of glyceraldehyde-3-phosphate dehydrogenase associated cell cycle (GACC) genes correlates with cancer stage and poor survival in patients with solid tumors. PLoS One. 2013;8:e61262.
Rubie C, Kempf K, Hans J, Su T, Tilton B, et al. Housekeeping gene variability in normal and cancerous colorectal, pancreatic, esophageal, gastric and hepatic tissues. Mol Cell Probes. 2005;19:101–9.
Acknowledgements
The authors thank to Mrs Ursula Valls for technical assistance in the experiments.
Funding
This study received financial support from the Ministerio de Ciencia e Innovación, Spain (SAF2011-25726), the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR)-Generalitat de Catalunya (2014SGR1017), the Ministerio de Economía y Competitividad from the Spanish Government and FEDER funds from the European Union “una manera de hacer Europa” (SAF2014-56059-R, SAF2015-70270-REDT), and the Instituto de Salud Carlos III supported by The European Regional Development Fund (ERDF) grants PI11-01439 and Catalan Government DURSI 2014SGR647. MC acknowledges the support received through the prize ICREA Academia for excellence in research, funded by ICREA Foundation-Generalitat de Catalunya.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
MTC, SDM, IHP and RSP carried out the experiments. MTC, SDM, AC, RSP and MC analysed the data, performed the statistical analysis, interpreted the results and drafted the manuscript. MTC, SDM and MC conceived and designed the study. MTC, SDM, IHP, RSP, CA, VM, AC and MC contributed reagents/materials/analysis tools. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not Applicable
Ethics approval and consent to participate
This study obtained the ethics approval from the Hospital Clínic of Barcelona and the Bellvitge University Hospital ethics committees and the written informed consent from all patients. All clinical investigation has been conducted according to the principles expressed in the Declaration of Helsinki.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: File S1.
Supporting Materials and Methods. Detailed immunohistochemical staining, total protein extraction and Western blot analysis materials and methods. (PDF 312 kb)
Additional file 2: Figure S1.
Mouse monoclonal anti-GAPDH antibody specificity. Anti-GAPDH antibody (sc-47724, Santa Cruz Biotechnology, Santa Cruz, CA, USA) specificity was proven by Western blot, staining a single band at 37 kDa. (PDF 249 kb)
Additional file 3: Table S1.
Clinicopathological and immunohistochemical data. Id, arbitrary number used to preserve the privacy of patients; a.u., arbitrary units; M, male; F, female; TNM staging system: T, primary tumour extent; N, regional lymph node involvement; M, absence or presence of distant metastasis. (PDF 547 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Tarrado-Castellarnau, M., Diaz-Moralli, S., Polat, I.H. et al. Glyceraldehyde-3-phosphate dehydrogenase is overexpressed in colorectal cancer onset. transl med commun 2, 6 (2017). https://doi.org/10.1186/s41231-017-0015-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41231-017-0015-7