Abstract
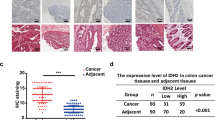
It has long been observed that many cancer cells exhibit increased aerobic glycolysis and rely more on this pathway to generate ATP and metabolic intermediates for cell proliferation. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a key enzyme in glycolysis and has been known as a housekeeping molecule. In the present study, we found that GAPDH expression was significantly up-regulated in human colorectal carcinoma tissues compared to the adjacent normal tissues, and also increased in colon cancer cell lines compared to the non-tumor colon mucosa cells in culture. The expression of GAPDH was further elevated in the liver metastatic tissues compared to the original colon cancer tissue of the same patients, suggesting that high expression of GAPDH might play an important role in colon cancer development and metastasis. Importantly, we found that 3-bromopyruvate propyl ester (3-BrOP) preferentially inhibited GAPDH and exhibited potent activity in inducing colon cancer cell death by causing severe depletion of ATP. 3-BrOP at low concentrations (1–10 μM) inhibited GAPDH and a much higher concentration (300 μM) was required to inhibit hexokinase-2. The cytotoxic effect of 3-BrOP was associated with its inhibition of GAPDH, and colon cancer cells with loss of p53 were more sensitive to this compound. Our study suggests that GAPDH may be a potential target for colon cancer therapy.
Similar content being viewed by others
References
Achanta G, Sasaki R, Feng L, Carew JS, Lu W, Pelicano H, Keating MJ, Huang P (2005) Novel role of p53 in maintaining mitochondrial genetic stability through interaction with DNA Pol gamma. EMBO J 24:3482–3492
Akers LJ, Fang W, Levy AG, Franklin AR, Huang P, Zweidler-McKay PA (2011) Targeting glycolysis in leukemia: a novel inhibitor 3-BrOP in combination with rapamycin. Leuk Res 35:814–820
Banas T, Gontero B, Drews VL, Johnson SL, Marcus F, Kemp RG (1988) Reactivity of the thiol groups of Escherichia coli phosphofructo-1-kinase. Biochim Biophys Acta 957:178–184
Borthwick EB, Connell SJ, Tudor DW, Robins DJ, Shneier A, Abell C, Coggins JR (1995) Escherichia coli dihydrodipicolinate synthase: characterization of the imine intermediate and the product of bromopyruvate treatment by electrospray mass spectrometry. Biochem J 305(Pt 2):521–524
Chen Z, Lu W, Garcia-Prieto C, Huang P (2007) The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr 39:267–274
Chen Z, Zhang H, Lu W, Huang P (2009) Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta 1787:553–560
Cini C, Foppoli C, De Marco C (1978) On the product of the reaction between cysteamine and 3-bromopyruvate. Ital J Biochem 27:233–246
Epner DE, Coffey DS (1996) There are multiple forms of glyceraldehyde-3-phosphate dehydrogenase in prostate cancer cells and normal prostate tissue. Prostate 28:372–378
Epner DE, Partin AW, Schalken JA, Isaacs JT, Coffey DS (1993) Association of glyceraldehyde-3-phosphate dehydrogenase expression with cell motility and metastatic potential of rat prostatic adenocarcinoma. Cancer Res 53:1995–1997
Ganapathy-Kanniappan S, Geschwind JF, Kunjithapatham R, Buijs M, Vossen JA, Tchernyshyov I, Cole RN, Syed LH, Rao PP, Ota S, Vali M (2009) Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res 29:4909–4918
Honda T, Tokushige M (1985) Active site-directed modification of tryptophanase by 3-bromopyruvate. J Biochem 97:851–857
Kirley JW, Day RA (1985) Irreversible inhibition of carbonic anhydrase by the carbon dioxide analog cyanogen. Biochem Biophys Res Commun 126:457–463
Ko YH, Pedersen PL, Geschwind JF (2001) Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett 173:83–91
Levy AG, Zage PE, Akers LJ, Ghisoli ML, Chen Z, Fang W, Kannan S, Graham T, Zeng L, Franklin AR, Huang P, Zweidler-McKay PA (2010) The combination of the novel glycolysis inhibitor 3-BrOP and rapamycin is effective against neuroblastoma. Invest New Drugs 2010 Oct 5 [Epub ahead of print]
Lowe PN, Perham RN (1984) Bromopyruvate as an active-site-directed inhibitor of the pyruvate dehydrogenase multienzyme complex from Escherichia coli. Biochemistry 23:91–97
Mathupala SP, Ko YH, Pedersen PL (2009) Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol 19:17–24
Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM (2006) p53 regulates mitochondrial respiration. Science 312:1650–1653
Mikuriya K, Kuramitsu Y, Ryozawa S, Fujimoto M, Mori S, Oka M, Hamano K, Okita K, Sakaida I, Nakamura K (2007) Expression of glycolytic enzymes is increased in pancreatic cancerous tissues as evidenced by proteomic profiling by two-dimensional electrophoresis and liquid chromatography-mass spectrometry/mass spectrometry. Int J Oncol 30:849–855
Nicholls C, Li H, Liu JP (2011) GAPDH: a common enzyme with uncommon functions. Clin Exp Pharmacol Physiol
Pedersen PL (2007) Warburg, me and Hexokinase 2: multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr 39:211–222
Pelicano H, Martin DS, Xu RH, Huang P (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25:4633–4646
Persons DA, Schek N, Hall BL, Finn OJ (1989) Increased expression of glycolysis-associated genes in oncogene-transformed and growth-accelerated states. Mol Carcinog 2:88–94
Rondinelli RH, Epner DE, Tricoli JV (1997) Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in late pathological stage human prostate cancer. Prostate Cancer Prostatic Dis 1:66–72
Sabates-Bellver J, Van der Flier LG, de Palo M, Cattaneo E, Maake C, Rehrauer H, Laczko E, Kurowski MA, Bujnicki JM, Menigatti M, Luz J, Ranalli TV, Gomes V, Pastorelli A, Faggiani R, Anti M, Jiricny J, Clevers H, Marra G (2007) Transcriptome profile of human colorectal adenomas. Mol Cancer Res 5:1263–1275
Schek N, Hall BL, Finn OJ (1988) Increased glyceraldehyde-3-phosphate dehydrogenase gene expression in human pancreatic adenocarcinoma. Cancer Res 48:6354–6359
Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S (1987) Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res 47:5616–5619
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Warburg O (1956a) On respiratory impairment in cancer cells. Science 124:269–270
Warburg O (1956b) On the origin of cancer cells. Science 123:309–314
Xu RH, Pelicano H, Zhou Y, Carew JS, Feng L, Bhalla KN, Keating MJ, Huang P (2005a) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 65:613–621
Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P (2005b) Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia 19:2153–2158
Yoshida H, Wood HG (1978) Crystalline pyruvate, phosphate dikinase from Bacteroides symbiosus. Modification of essential histidyl residues and bromopyruvate inactivation. J Biol Chem 253:7650–7655
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhenjie Tang and Shuqiang Yuan contribute equally to this work
Rights and permissions
About this article
Cite this article
Tang, Z., Yuan, S., Hu, Y. et al. Over-expression of GAPDH in human colorectal carcinoma as a preferred target of 3-Bromopyruvate Propyl Ester. J Bioenerg Biomembr 44, 117–125 (2012). https://doi.org/10.1007/s10863-012-9420-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-012-9420-9