Abstract
Background
Human toxocariasis is a parasitic zoonosis caused by a parasite in the genus Toxocara and is transmitted mainly by the accidental ingestion of embryonated Toxocara canis (dog roundworm) or T. cati (cat roundworm) eggs. Several studies reported that children were the main population at risk for T. canis infection. Currently, no reports on the seroprevalence of T. canis infection in Thailand are available, and its status remains unknown among children who live in rural areas of southern Thailand.
Objective
This study aimed to investigate the seroprevalence of T. canis infection and its associated risk factors among primary schoolchildren in rural areas of Nakhon Si Thammarat Province, Thailand.
Methods
A total of 177 schoolchildren between 6 and 13 years of age were recruited between June and July 2019. Serum anti-T. canis IgG antibodies were detected with a commercial ELISA kit. A questionnaire administered by direct interviews was used to collect demographic and behavioral risk factor data.
Results
The overall seroprevalence of T. canis infection was 58.2% (103 of 177). The univariate analysis revealed that schoolchildren who did not practice handwashing before a meal (crude odds ratio (COR) = 3.67, 95% CI 1.93–6.95, P < 0.001), did not practice hand washing after animal contact (COR = 2.89, 95% CI 1.53–5.47, P = 0.001), and drank untreated water (COR = 1.87, 95% CI 1.00–3.48, P = 0.049) had an increased risk of acquiring T. canis infection. However, after adjusting for confounders, only a lack of handwashing before a meal remained a significant risk factor (adjusted odds ratio (AOR) = 2.20, 95% CI 1.11–4.34, P = 0.023). Age, sex, owning a dog, and eating fresh vegetables were not significantly associated with T. canis infection in the current study.
Conclusions
This is the first serological investigation of T. canis infection among schoolchildren in Thailand. The high rate of Toxocara seropositivity reflected high levels of exposure to T. canis among schoolchildren in rural areas of southern Thailand. The results also provide baseline data regarding modifiable risk behaviors for effective T. canis infection prevention strategies in southern Thailand, especially strengthening hand washing practices among schoolchildren.
Similar content being viewed by others
Introduction
Human toxocariasis is one of most prevalent parasitic zoonoses worldwide; it is particularly present in subtropical and tropical regions and in developing countries [1]. Toxocariasis is caused by the larvae of Toxocara canis or Toxocara cati, whose definitive hosts are dogs and cats, respectively [2]. The global prevalence of Toxocara infection in dogs is 11.1% [3]. Humans acquire Toxocara spp. via the accidental consumption of Toxocara eggs, which are present in soil contaminated by dog/cat feces, or via the ingestion of larvae in undercooked meat [2]. Clinical manifestations of human toxocariasis are mostly asymptomatic; however, severe disease can occur when larvae migrate within the body to internal organs or the eyes, causing visceral and ocular larva migrans, neurotoxocariasis, and covert/common toxocariasis [1, 4].
Humans are incidental hosts for Toxocara spp. The larvae cannot develop into adult worms in the human small intestine; thus, no Toxocara eggs are present in human stool [1, 5]. The typical diagnosis of toxocariasis usually relies on serological tests. An enzyme-linked immunosorbent assay (ELISA) using Toxocara excretory-secretory (TES) antigens has been widely used and is recommended by the Centers for Disease Control and Prevention for the detection of Toxocara-specific IgG [6, 7]. The limitations of this test are its cross-reactivity with other parasites, especially the human roundworm Ascaris lumbricoides [8], and its lack of ability to differentiate between active and past infections. However, ELISA remains useful in seroepidermiological surveys, as it is a rapid and affordable method of determining the prevalence of asymptomatic toxocariasis.
Seroprevalence studies have been performed in several countries around the world. In some parts of the world, such as Nigeria (86.1%) [9], the Republic of the Marshall Islands (86.75%), [10] and Northeast Brazil (63.6%) [11], the seroprevalence of toxocariasis was remarkably high. In Asia, the seroprevalence of toxocariasis was substantially high: 46.0% in Taiwan [12], 45.9% in Turkey [13], 49% in the Philippines [14], 51.2% in Korea, and 23.5–45.9% in Iran [15, 16]. In Western countries, the prevalence was 5.0% in the USA [17], 8.0% in Italy [18], and 16.0% in Greece [19]. A systematic review and meta-analysis revealed that the global seroprevalence of toxocariasis was 19.0%, with the highest rate in the African region (37.7%) and the lowest rate in the Eastern Mediterranean region (8.2%) [20]. Many factors have been proposed to be involved, including contact with dogs [11, 15], children’s age [21], and male sex [11, 22, 23].
In Thailand, previous reports revealed that Toxocara eggs were found in the stool of dogs and cats [24, 25]. Furthermore, Toxocara eggs were identified in raw vegetables from markets in southern Thailand [26]; however, their impact on humans has not been investigated. In this study, we aimed to assess the seroprevalence of Toxocara canis infection and the associated risk factors among schoolchildren in rural areas of southern Thailand.
Methods
Study design and setting
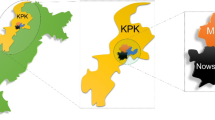
This cross-sectional study was carried out from June to July 2019 in five districts of Nakhon Si Thammarat Province, including Phrom Khiri, Tha Sala, Sichon, Khanom, and Nopphitam. Nakhon Si Thammarat Province is located in southern Thailand, approximately 780 km from the Thai capital of Bangkok (8°25′7″ N 99°57′49″ E) (Fig. 1). The average temperature is 27.1 °C, with a low temperature of 25.8 °C in January and a high temperature of 29.3 °C in May. The annual rainfall is 1454.3 mm (Climatological Center, Thai Meteorological Department). The Thailand Department of Provincial Administration estimates that the total populations of the subdistricts were 27,302 in Phrom Khiri, 113,323 in Tha Sala, 81,888 in Sichon, 20,369 in Khanom, and 33,543 in Nopphitam. The five districts are similar in terms of culture, economic status, and climate.
Map of Phrom Khiri (1), Tha Sala (2), Sichon (3), Khanom (4), and Nopphitam (5) Districts, Nakhon Si Thammarat Province, southern Thailand. (Map from Wikimedia Commons: https://commons.m.wikimedia.org/wiki/Atlas_of_Thailand#)
Study population and sample size
The study population consisted of primary schoolchildren from 7 to 13 years of age. The sample size was determined using the single proportion population formula:
where p = prevalence of intestinal parasites from a previous study, d = margin of error, and Z = standard score, which corresponds to 1.96. This formula was calculated based on a prevalence rate of 86.75% from a previous study [10], with a margin of error of 0.05 and a confidence level of 95%. The calculated sample size was 177. The exclusion criteria were immune system disorders (such as autoimmune disorders and acquired immune deficiency syndrome), steroid treatment for at least 3 months and acute illnesses on the day of blood collection. Three schools were randomly selected from each district for a total of 15 schools. Finally, the participants were selected from the school rosters using a systematic random sampling technique in which every 10th person was included in the study.
Questionnaire survey
A structured questionnaire was developed and used to collect demographic data (i.e., age, sex, and parental education and occupation) and information on possible risk factors (habits of handwashing before a meal, after playing and after animal contact; owning pets (dogs or cats); eating fresh vegetables; and drinking untreated water). Agriculturists, farm laborers, housemaids, fishing boat staff, and street vendors were considered non-skilled workers. The questionnaire was administered by two trained interviewers who conducted direct interviews with the participating schoolchildren.
Blood collection and preparation
Blood samples were collected from the antecubital vein by medical technologists and kept for 45 min at room temperature. After that, serum was separated by centrifugation at 2500 rpm for 10 min and stored at – 80 °C until the measurement of IgG class antibodies against T. canis.
Detection of anti-T. canis IgG antibodies
Serum anti-T. canis IgG antibodies were detected with a commercial ELISA kit (ab108775, Abcam, UK), which has > 95% sensitivity and specificity according to the manufacturer’s instructions. In brief, all samples were diluted to 1:100 with IgG sample diluent, and all controls (T. canis IgG-positive, T. canis IgG-negative, and T. canis IgG cutoff (CO)) were prepared. The absorbance values were 0.150–1.300 for CO, < 0.200 and < cutoff for negative controls and > cutoff for positive controls. A precoated plate was incubated (100 μL/well) with the control or diluted samples for 1 h at 37 °C. After incubation with the serum samples, the plates were washed 3 times with ×1 washing solution and incubated (100 μL/well) with protein A HRP conjugate for 30 minutes at room temperature. The plates were washed 3 times and incubated (100 μL/well) with TMB substrate solution for exactly 15 min at room temperature in the dark. The reaction was stopped (100 μL/well) with stop solution for 15 min at room temperature. The assay included negative and positive serum samples in addition to a blank (no serum sample). Absorbance at 492 nm was measured using an automatic microplate reader. For interpretation of the results, samples with an absorbance value of more than 10% above the CO were considered positive. If the absorbance value was between 10% above and 10% below the CO, it was considered inconclusive (in the gray zone), and a fresh sample was run again. If the results of the second test were less than 10% above or below the CO control value, the sample was considered negative.
Data analysis
Data were entered, cleaned, and analyzed using IBM SPSS Statistics for Windows, Version 23. Quantitative variables are described as the medians and interquartile ranges (IQRs), and qualitative variables are described as the frequencies (percentages). A chi-square test was used to compare the proportions of T. canis infection among subgroups stratified by sex, age group, level of education, district, parental occupation, and parental education. A univariate logistic regression model was constructed to investigate risk factors associated with T. canis infection. The variables with P values less than 0.2 in the univariate logistic regression model were included in a multiple logistic regression model that was adjusted for confounding factors. Differences were considered to be statistically significant when the P value obtained was less than 0.05.
Results
Sociodemographic characteristics
A total of 177 schoolchildren (91 boys and 86 girls) from 5 districts were included in this study. The median age of the study population was 9.82 years (IQR 9–11 years). The enrolled schoolchildren resided in 5 districts of Nakhon Si Thammarat, including Khanom (n = 35), Sichon (n = 34), Phrom Kiri (n = 34), Nopphitam (n = 36), and Thasala (n = 38). Their parents’ occupation and education level were mostly non-skilled workers and high school or less, respectively (Table 1).
Seroprevalence of Toxocara canis infection
The overall seroprevalence of T. canis infection among the participants was 58.2% (95% CI 50.9–65.5%). Boys had a higher seropositive rate (64.8%) than girls (51.2%). The rates of seropositivity (54.3% in Khanom, 52.9% in Sichon, 58.8% in Phrom Kiri, 55.6% in Nopphitam, and 68.4% in Thasala) were not significantly different among the 5 districts (P = 0.672). The highest seropositivity was observed among third-grade students (68.3%), followed by sixth-grade students (65.0%), while the lowest rate was observed among fourth-grade students (43.8%). Chi-square tests showed that there were no significant associations between T. canis infection and sex, age group, level of education, district, parents’ occupation and parents’ education (Table 1).
Associated risk factors for Toxocara canis infections
The univariate analysis revealed that handwashing before a meal, handwashing after animal contact, and drinking untreated water were significantly associated with seropositivity for T. canis. However, the multivariate analysis indicated that handwashing before a meal was the only factor associated with T. canis seropositivity. Children who did not practice handwashing before a meal were more likely to be infected with T. canis than those who did (AOR = 2.20, 95% CI 1.11–4.34) (Table 2).
When stratified by age, the rates of seropositivity for T. canis were approximately equal among age groups (56.3%, 58.5%, and 58.7% for children 6–8 years of age, 9–10 years of age and 11–13 years of age, respectively). Boys were more likely to be infected with T. canis than girls. Children who did not practice handwashing after playing, children who owned dogs and cats and children who ate fresh vegetables were more likely to be infected with T. canis than their counterparts. However, there was no statistical significance among these factors in either the univariate or multivariate analyses (Table 2).
Discussion
Toxocariasis is a prevalent parasitic zoonosis worldwide, especially in the tropics and subtropics. This study was the first serological investigation of T. canis infection among schoolchildren in southern Thailand, with a prevalence rate of 58.2%, which was higher than the global seroprevalence (19.0%) and the pooled seroprevalence in Southeast Asia (34.1%) [20]. The rate of T. canis infection in this study was slightly higher than the rates in other countries in Asia, including the Philippines (49.0%) [14], Taiwan (57.5%) [27], Turkey (45.9%) [13], Korea (51.2%) [28], and Isfahan, Iran (45.9%) [16], whereas the rate was lower than those in some other parts of the world, including Nigeria (86.1%) [9] and the Republic of the Marshall Islands (86.8%) [10]. In general, multiple factors, including environmental, geographic, cultural, and socioeconomic factors, contribute to the prevalence of T. canis infection. Our study areas included five districts of Nakhon Si Thammarat Province, all of which were considered rural. Most of the countryside in these districts contains rubber plantations and farmlands, and dogs are commonly owned as pets; moreover, there is a large number of stray dogs in these rural districts. Nakhon Si Thammarat has a tropical rainforest climate; the temperature ranges from 24 to 34 °C, which is suitable for the development of Toxocara eggs to the infective larval stage [29]. We hypothesized that climate, soil and the number of dogs in the areas influenced the rate of seropositivity in this study.
Among the five districts, Thasala showed the highest rate of Toxocara seropositivity (68.4%); however, there was no statistically significant difference among the five districts. This might be explained by the similarities in climate, geography, and culture among the districts. Children whose parents were non-skilled workers and had low education levels had a relatively higher rate of Toxocara seropositivity. This trend was also observed in a previous study in the Republic of the Marshall Islands [10]. However, parents’ education levels and occupations did not significantly affect the seroprevalence rate in this study. This might be due to a small number of children whose parents were skilled workers and had obtained college degrees.
Among the age groups, there was no significant difference in the rate of Toxocara seropositivity in the current study. The rates of Toxocara seropositivity were approximately similar across all age groups: 56.3% in children 6–8 years of age, 58.5% in children 9–10 years of age, and 58.7% in children 11–13 years of age. Similar findings were observed in the study from the Republic of the Marshall Islands [10]. However, this result was in contrast to the results of several studies among schoolchildren that showed that older age was a significant risk factor for T. canis infection [9, 15, 22, 30, 31]. The detection of Toxocara via serology increases over time because of the persistence of serum IgG. The possible explanation for the similar seropositivity rates among the different age groups might be that the children might have been in contact with Toxocara eggs since a young age, and the IgG persists over a long period of time. Our study revealed that boys tended to be infected with Toxocara spp. more often than girls (64.8% in boys and 51.2% in girls). This trend was also observed in previous studies [11, 22, 23]; it has been hypothesized that boys engage in more outdoor activities than girls; thus, they are more likely to be in contact with soil and dogs. However, sex was not significantly associated with Toxocara seropositivity in either the univariate or multivariate analysis in the current study.
Regarding modifiable risk behaviors, handwashing before a meal appeared to be the only significant risk factor associated with Toxocara infection in both the univariate and multivariate analyses. Schoolchildren who did not practice handwashing before a meal were 2.2 times more likely to be infected with T. canis than those who did. This effect was also observed in a previous study among Brazilian schoolchildren [32]. Furthermore, the univariate analysis in this study revealed that handwashing after animal contact and drinking untreated water were significantly associated with T. canis infection; however, these associations were not statistically significant in the multivariate analysis. Humans acquire T. canis by ingesting infective eggs, which can be found in public places worldwide, including Thailand [33]. This study emphasizes the importance of hand hygiene to prevent parasite eggs from entering the body. Despite the report of Toxocara egg contamination in raw vegetables in Nakhon Si Thammarat Province in a previous study [26], eating fresh vegetables was not significantly associated with T. canis infection in the current study. This may be explained by the small number of schoolchildren who ate fresh vegetables (36, 20.3%); thus, contamination from vegetables was not a likely route of transmission among these children. Schoolchildren who engaged in risky behaviors, such as owning dogs and owning cats, also tended to have a higher rate of T. canis infection than those who did not; however, no significant associations were observed among these factors and T. canis infection.
This study had the following limitations. First, the method for the detection of anti-T. canis IgG antibodies was an ELISA, which is not the gold standard test, Toxocara spp. larval excretory-secretory Western blot, and could yield false positive results due to cross-reactivity with other helminths, especially A. lumbricoides [8, 34]. However, our study sites were not endemic areas for A. lumbricoides [35, 36]; hence, the chance of false positivity was likely low. Second, the study design was cross-sectional, and seroprevalence and risk factors were evaluated simultaneously, not over a period of time; thus, true causes and effects might not be strongly demonstrated.
Conclusions
This is the first serological investigation of T. canis infection among schoolchildren in Thailand. The high rate of Toxocara seropositivity reflected high levels of exposure to T. canis among schoolchildren in rural areas of southern Thailand. A lack of handwashing before meals appeared to be a significant risk factor for T. canis infection. Appropriate public health education on personal hygiene, especially strengthening hand hygiene practices among schoolchildren, should be implemented to prevent the transmission of T. canis.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- AOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- CO:
-
Cutoff
- COR:
-
Crude odds ratio
- IQR:
-
Interquartile range
References
Ma G, Holland CV, Wang T, Hofmann A, Fan CK, Maizels RM, et al. Human toxocariasis. Lancet Infect Dis. 2018;18(1):e14–24.
Chen J, Liu Q, Liu GH, Zheng WB, Hong SJ, Sugiyama H, et al. Toxocariasis: a silent threat with a progressive public health impact. Infect Dis Poverty. 2018;7(1):59.
Rostami A, Riahi SM, Hofmannc A, Ma G, Wang T. Behniafar H, et al. Adv Parasitol: Global prevalence of Toxocara infection in dogs; 2020. (In Press).
Rostami A, Ma G, Wang T, Koehler AV, Hofmann A, Chang BCH, et al. Human toxocariasis - A look at a neglected disease through an epidemiological 'prism'. Infect Genet Evol. 2019;74:104002.
Strube C, Heuer L, Janecek E. Toxocara spp. infections in paratenic hosts. Vet Parasitol. 2013;193(4):375–89.
Boldis V, Ondriska F, Spitalska E, Reiterova K. Immunodiagnostic approaches for the detection of human toxocarosis. Exp Parasitol. 2015;159:252–8.
Global Health DoPDaM, Centers for Disease Control and Prevention. Toxocariasis 2019 [Available from: https://www.cdc.gov/dpdx/toxocariasis/index.html.
Mohamad S, Azmi NC, Noordin R. Development and evaluation of a sensitive and specific assay for diagnosis of human toxocariasis by use of three recombinant antigens (TES-26, TES-30USM, and TES-120). J Clin Microbiol. 2009;47(6):1712–7.
Gyang PV, Akinwale OP, Lee YL, Chuang TW, Orok AB, Ajibaye O, et al. Seroprevalence, disease awareness, and risk factors for Toxocara canis infection among primary schoolchildren in Makoko, an urban slum community in Nigeria. Acta Trop. 2015;146:135–40.
Fu CJ, Chuang TW, Lin HS, Wu CH, Liu YC, Langinlur MK, et al. Seroepidemiology of Toxocara canis infection among primary schoolchildren in the capital area of the Republic of the Marshall Islands. BMC Infect Dis. 2014;14:261.
Silva MB, Amor ALM, Santos LN, Galvao AA, Oviedo Vera AV, Silva ES, et al. Risk factors for Toxocara spp. seroprevalence and its association with atopy and asthma phenotypes in school-age children in a small town and semi-rural areas of Northeast Brazil. Acta Trop. 2017;174:158–64.
Fan CK, Lan HS, Hung CC, Chung WC, Liao CW, Du WY, et al. Seroepidemiology of Toxocara canis infection among mountain aboriginal adults in Taiwan. American Journal of Tropical Medicine and Hygiene. 2004;71(2):216–21.
Kaplan M, Kalkan A, Kuk S, Demirdag K, Ozden M, Kilic SS. Toxocara seroprevalence in schizophrenic patients in Turkey. Yonsei Med J. 2008;49(2):224–9.
Fajutag AJ, Paller VG. Toxocara egg soil contamination and its seroprevalence among public school children in Los Banos, Laguna, Philippines. Southeast Asian J Trop Med Public Health. 2013;44(4):551–60.
Aghamolaie S, Seyyedtabaei SJ, Behniafar H, Foroutan M, Saber V, Hanifehpur H, et al. Seroepidemiology, modifiable risk factors and clinical symptoms of Toxocara spp. infection in northern Iran. Trans R Soc Trop Med Hyg. 2019;113(3):116–22.
Hosseini-Safa A, Mousavi SM, Bahadoran Bagh Badorani M, Ghatreh Samani M, Mostafaei S, Yousofi Darani H. Seroepidemiology of Toxocariasis in Children (5-15 yr Old) Referred to the Pediatric Clinic of Imam Hossein Hospital, Isfahan, Iran. Iran J Parasitol. 2015;10(4):632–7.
Liu EW, Chastain HM, Shin SH, Wiegand RE, Kruszon-Moran D, Handali S, et al. Seroprevalence of antibodies to Toxocara Species in the United States and associated risk factors, 2011-2014. Clin Infect Dis. 2018;66(2):206–12.
Nicoletti A, Cicero CE, Mantella A, Giuliano L, Rascuna C, Paradisi V, et al. Seroprevalence of Toxocara Canis in the city of Catania. Italy. Mediterr J Hematol Infect Dis. 2019;11(1):e2019031.
Papavasilopoulos V, Pitiriga V, Birbas K, Elefsiniotis J, Bonatsos G, Tsakris A. Soil contamination by Toxocara canis and human seroprevalence in the Attica region. Greece. Germs. 2018;8(3):155–61.
Rostami A, Riahi SM, Holland CV, Taghipour A, Khalili-Fomeshi M, Fakhri Y, et al. Seroprevalence estimates for toxocariasis in people worldwide: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2019;13(12):e0007809.
Sowemimo OA, Lee YL, Asaolu SO, Chuang TW, Akinwale OP, Badejoko BO, et al. Seroepidemiological study and associated risk factors of Toxocara canis infection among preschool children in Osun State. Nigeria. Acta Trop. 2017;173:85–9.
Romero Nunez C, Mendoza Martinez GD, Yanez Arteaga S, Ponce Macotela M, Bustamante Montes P, Ramirez DN. Prevalence and risk factors associated with Toxocara canis infection in children. ScientificWorldJournal. 2013;2013:572089.
Martinez M, Garcia H, Figuera L, Gonzalez V, Lamas F, Lopez K, et al. Seroprevalence and risk factors of toxocariasis in preschool children in Aragua state, Venezuela. Trans R Soc Trop Med Hyg. 2015;109(9):579–88.
Pumidonming W, Salman D, Gronsang D, Abdelbaset AE, Sangkaeo K, Kawazu SI, et al. Prevalence of gastrointestinal helminth parasites of zoonotic significance in dogs and cats in lower Northern Thailand. J Vet Med Sci. 2017;78(12):1779–84.
Rojekittikhun W, Chaisiri K, Mahittikorn A, Pubampen S, Sa-Nguankiat S, Kusolsuk T, et al. Gastrointestinal parasites of dogs and cats in a refuge in Nakhon Nayok, Thailand. Southeast Asian J Trop Med Public Health. 2014;45(1):31–9.
Punsawad C, Phasuk N, Thongtup K, Nagavirochana S, Viriyavejakul P. Prevalence of parasitic contamination of raw vegetables in Nakhon Si Thammarat province, southern Thailand. BMC Public Health. 2019;19(1):34.
Fan CK, Liao CW, Kao TC, Li MH, Du WY, Su KE. Sero-epidemiology of Toxocara canis infection among aboriginal schoolchildren in the mountainous areas of north-eastern Taiwan. Ann Trop Med Parasitol. 2005;99(6):593–600.
Lee JY, Yang MH, Hwang JH, Kang M, Paeng JW, Yune S, et al. The prevalence of toxocariasis and diagnostic value of serologic tests in asymptomatic Korean adults. Allergy Asthma Immunol Res. 2015;7(5):467–75.
Azam D, Ukpai OM, Said A, Abd-Allah GA, Morgan ER. Temperature and the development and survival of infective Toxocara canis larvae. Parasitol Res. 2012;110(2):649–56.
Mendonca LR, Figueiredo CA, Esquivel R, Fiaccone RL, Pontes-de-Carvalho L, Cooper P, et al. Seroprevalence and risk factors for Toxocara infection in children from an urban large setting in Northeast Brazil. Acta Trop. 2013;128(1):90–5.
Berrett AN, Erickson LD, Gale SD, Stone A, Brown BL, Hedges DW. Toxocara Seroprevalence and Associated Risk Factors in the United States. Am J Trop Med Hyg. 2017;97(6):1846–50.
Cassenote AJ, Lima AR, Pinto Neto JM, Rubinsky-Elefant G. Seroprevalence and modifiable risk factors for Toxocara spp. in Brazilian schoolchildren. PLoS Negl Trop Dis. 2014;8(5):e2830.
Fakhri Y, Gasser RB, Rostami A, Fan CK, Ghasemi SM, Javanian M, et al. Toxocara eggs in public places worldwide—a systematic review and meta-analysis. Environ Pollut. 2018;242(Pt B):1467–75.
Watthanakulpanich D, Smith HV, Hobbs G, Whalley AJ, Billington D. Application of Toxocara canis excretory-secretory antigens and IgG subclass antibodies (IgG1-4) in serodiagnostic assays of human toxocariasis. Acta Trop. 2008;106(2):90–5.
Punsawad C, Phasuk N, Bunratsami S, Thongtup K, Siripakonuaong N, Nongnaul S. Prevalence of intestinal parasitic infection and associated risk factors among village health volunteers in rural communities of southern Thailand. BMC Public Health. 2017;17(1):564.
Punsawad C, Phasuk N, Bunratsami S, Thongtup K, Viriyavejakul P, Palipoch S, et al. Prevalence of intestinal parasitic infections and associated risk factors for hookworm infections among primary schoolchildren in rural areas of Nakhon Si Thammarat, southern Thailand. BMC Public Health. 2018;18(1):1118.
Acknowledgements
The authors wish to thank the schoolchildren from the five districts of Nakhon Si Thammarat Province, including Phrom Khiri, Tha Sala, Sichon, Khanom, and Nopphitam, who participated in the study. The authors would like to acknowledge the directors and the teachers of the 15 schools for their support throughout the course of this study. This research was partially supported by the New Strategic Research (P2P) project, Walailak University, Thailand.
Funding
This study was funded by Walailak University (WU-IRG-62-009). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
CP and NP conceived of and designed the study and performed the data collection. CP performed the experiments. CP and NP analyzed the data. CP and NP wrote the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Ethics Committee on Human Rights Related to Research Involving Human Subjects, Walailak University, prior to the recruitment of participants (WUEC-18-131-01). All participants and relevant parties were informed of the purpose of the study. Written informed consent was obtained from the parents or legal guardians of the children after clearly explaining the research objectives.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Phasuk, N., Punsawad, C. Seroprevalence of Toxocara canis infection and associated risk factors among primary schoolchildren in rural Southern Thailand. Trop Med Health 48, 23 (2020). https://doi.org/10.1186/s41182-020-00211-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-020-00211-0