Abstract
Background
Human toxocariasis is a zoonotic parasitic disease caused by the Toxocara canis and T. cati nematodes larvae. Dog owners are at a higher risk of acquiring T. canis infection, and there is no available evidence regarding the seroprevalence of T. canis infection among dog owners in Thailand. Therefore, this study aimed to investigate the seroprevalence of T. canis infection and associated risk factors among dog owners in rural areas of Thailand.
Methods
A total of 132 dog owners, including 25 men and 107 women, were recruited for this study. Serum anti-T. canis IgG antibodies were detected using a commercial enzyme-linked immunosorbent assay (ELISA) kit, and information on risk factors was collected using a questionnaire. In addition, hematological parameters were analyzed by the auto hematology analyzer. Risk variables associated with T. canis infection were investigated using univariate and multivariate logistic regression models.
Results
The overall seroprevalence of T. canis was 76.5% (101/132). Men were more likely to be infected with T. canis than women. Univariate analysis revealed that dog owners who did not practice handwashing before meals (p = 0.005) or after contact with soil (p = 0.035) or dogs (p = 0.049) had a substantially higher risk of acquiring T. canis infection. After adjusting for confounders, not practicing handwashing before meals remained a significant risk factor for T. canis infection (p = 0.038). The mean number of eosinophils was significantly higher in the seropositive group than in the seronegative group.
Conclusions
This is the first serological report of T. canis infection among dog owners reflecting the high rate of T. canis seropositivity in rural areas of southern Thailand. This study also provides group-specific data concerning modifiable risk behaviors for more effective T. canis infection control and prevention strategies in Thailand.
Similar content being viewed by others
Background
Toxocariasis in humans is a zoonotic disease caused by the parasitic roundworm of the genus Toxocara, particularly prevalent in tropical and subtropical areas and developing countries [1, 2]. The domestic dog is the definitive host for Toxocara canis and the domestic cat for T. cati [3]. Reportedly, the global seroprevalence of T. canis infection is 11.1% in dogs [4]. In humans, toxocariasis is caused by accidental ingestion of Toxocara embryonated eggs containing larvae, which are contaminated in the soil by dog or cat feces, or via the consumption of larvae worms in raw or poorly cooked meat by paratenic hosts infected with Toxocara larvae [5]. In humans, the estimated global seroprevalence of toxocariasis is 19.0% [6].
Although most patients infected with Toxocara are asymptomatic, four clinically symptomatic forms of human toxocariasis have been reported: Common toxocariasis (covert toxocariasis), visceral larva migrans (VLM), ocular larva migrans (OLM), and neurotoxocariasis [2]. However, Toxocara infection also contributes to the development of allergic diseases such as asthma, which is globally prevalent [7]. Toxocara is unable to complete its lifecycle in humans; hence, no Toxocara eggs have been found in the human stool [2, 8]. The gold standard test for diagnosing toxocariasis is the identification of the organism using microscopy, which is seldom performed for practical reasons. Therefore, an enzyme-linked immunosorbent assay (ELISA) that detects IgG-specific antibodies against T. canis excretory-secretory (TES) antigens is widely available and recommended by the Centers for Disease Control and Prevention [9]. However, TES-based immuno-diagnosis is limited because of the cross-reactivity of Toxocara TES antigens with other roundworms, especially human Ascaris lumbricoides, and this test is also incapable of differentiating between active and previous Toxocara infections.
The seroprevalence of T. canis has been reported in several countries. Regions with high seroprevalence of toxocariasis include the rural areas of Rio, Brazil (71.8%) [10], Makoko, Nigeria (86.1%) [11], and the Republic of the Marshall Islands (86.7%) [12]. In Western countries, the seroprevalence of toxocariasis is low, including the United States (5.1%) [13], Mexico (4.7% in adults and 13.8% in children) [14], Italy (8%) [15], and Greece (16%) [16]. In Southeast Asia, there are many countries that have a slightly higher seroprevalence, such as Nakhon Si Thammarat Province, Thailand (58.2%) [17], Ho Chi Minh City, Vietnam (45.2%) [18], and Los Baños, Laguna, Philippines (49%) [19]. Dogs are popular pets in many countries around the world and are beneficial for owners in several ways, such as security, improved mental health, socialization, and physical wellbeing [20]. Infection with Toxocara spp. in dogs has been reported worldwide, including in the Eastern Mediterranean region, Africa, and Southeast Asia (including Thailand), North America, South America, Europe, and the Western Pacific [21,22,23,24]. Dog owners are prone to be infected with T. canis from their dogs by ingesting animal fecal material. Moreover, close contact, lack of handwashing, and increased risk of pet-associated disease have been reported [6, 17]. However, few studies have explored the seroprevalence of T. canis in dog owners.
Currently, there are no reports on the seroprevalence of T. canis infection in dog owners living in the rural areas of southern Thailand. Therefore, this study aimed to investigate the seroprevalence of T. canis infection and its associated risk factors among dog owners in the rural areas of Nakhon Si Thammarat Province, Thailand.
Methods
Study site and setting
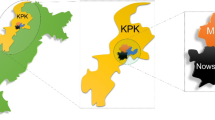
The study was carried out from September to November 2020 in three districts of Nakhon Si Thammarat Province: Tha Sala, Phrom Khiri, and Nopphitam (Fig. 1). The Nakhon Si Thammarat Province is located in southern Thailand, approximately 780 km from Bangkok (8° 25′ 7″ N, 99° 57′ 49″ E). The average temperatures are 27.7 °C, 27.1 °C, and 26.6 °C in September, October, and November, respectively. The average rainfall from September to November 2020 was 429.4 mm, and the cumulative rainfall was 1288.2 mm (Climatological Center, Thai Meteorological Department). The Thailand Department of Provincial Administration estimated in 2019 that the total population in Tha Sala was 118,113, Phrom Khiri was 37,469, and Nopphitam was 33,533. All districts were similar in terms of culture, economic status, and climate.
Population of the study and sample size
The study population consisted of dog owners > 18 years from different houses in the three districts of Nakhon Si Thammarat Province. The sample size was determined using a single-proportion population as follows:
where p is the prevalence of intestinal parasites from a previous study, d is the margin of error, and Z is the standard score, which corresponds to 1.96. This formula was calculated based on a prevalence rate of 9.3% from a previous study [23], with a margin of error of 0.05 and a confidence level of 95%. The calculated sample size was 130. The exclusion criteria were immune system disorders (such as autoimmune disorders and acquired immune deficiency syndrome), steroid treatment for at least 3 months, and acute illnesses on the day of blood collection. In total, 132 dog owners were randomly selected from each district using a systematic random sampling technique in which every third person was selected for the study.
Questionnaire and survey
The questionnaire consisted of 19 close-ended questions and one open-ended question (Additional file 1) to collect information on the general demographic data of the dog owner (gender, age, district, religion, occupation, and education), information on the dog (number of dogs, species, health check/vaccination, deworming, shower, and clearing ticks and fleas), and information on possible risk factors (playing with the dog; kissing or touching the dog; location of feces discharge; sleeping spot; feces management; and habits of handwashing before a meal, after soil contact, and after contact with the dog). The occupations were divided into 2 groups, including unskilled and skilled workers. Unskilled workers refer to workers with no particular skills or no formal education, including agriculturists, farmers, merchants, and housemaids. Skilled workers mean workers with specialized training or learned skill sets, including officials and teachers. Two professional interviewers conducted direct interviews with the participating dog owners to administer the questionnaire.
Blood collection and preparation
Medical technologists collected blood samples (5 mL) from the antecubital vein. The collected blood samples (3 mL) were immediately separated into clot blood tubes, which were used to measure IgG class antibodies against T. canis. The blood samples were kept at 4 °C in a cooling box and were immediately transferred to the laboratory for serum preparation. Serum samples were kept at − 80 °C until further examination. Another 2 mL of collected blood was added to an EDTA tube, stored at 4 °C, and transported immediately to the laboratory to measure hematological parameters.
Analysis of hematological parameters
Hematological parameters, including red blood cells (RBC), hemoglobin (Hb), hematocrit (HCT), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), white blood cells (WBC), neutrophils (Neu), lymphocytes (Lymp), eosinophils (Eo), monocytes (Mono), basophils (Baso), and platelets (PLT), were analyzed by the auto hematology analyzer at the Medical Technology Clinic, Walialak University.
Detection of anti-Toxocara canis IgG antibodies
Commercial ELISA kits (NovaLisa® (NOVTOCG0450), NovaTec Immunodiagnostica GmbH, Dietzenbach, Germany) with > 95% sensitivity and specificity were used to measure serum anti-T. canis IgG antibodies, according to the manufacturer’s protocol. In brief, all samples were diluted 1 + 100 with IgG sample diluent, and all controls (T. canis IgG-positive, T. canis IgG-negative, T. canis IgG cutoff, and substrate blank) were prepared. The following requirements must be satisfied for an assay to be considered valid: cutoff was 0.150–1.300, negative controls were < 0.200 and < cutoff, positive controls were > cutoff, and the substrate blank was < 0.100. Next, 100 μL of the control or diluted sample was added to pre-coated 96-well plates and incubated for 1 h at 37 °C. After incubation, the plates were washed three times with washing buffer, and all wells, except for the substrate blank well, were incubated with 100 μL of protein A horseradish peroxidase (HRP) conjugate for 30 min at room temperature. The plates were washed three times and incubated with 100 μL 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate solution for 15 min at room temperature in the dark. Next, the stop solution (100 μL) was added to each well and incubated for 15 min at room temperature to stop the reaction. The assay included negative and positive serum samples (provided by the ELISA kits) and a substrate blank in addition to a blank (no serum sample). The absorbance was measured at 450/620 nm using an automatic microplate reader. For interpretation, the results were calculated to NovaTec units (NTU), samples with > 11 NTU were considered positive. However, if the NTU value was between 9 and 11, the sample was considered equivocal and a fresh sample was repeated. If the results of the repeated test were also equivocal, the sample was considered negative.
Statistical analysis
All data were entered, cleaned, and analyzed using the SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA). The mean and standard deviation (SD) were used to describe quantitative data, whereas frequencies (percentages) were used to describe qualitative data. A Chi-squared test was used to compare the proportions of T. canis infections among subgroups stratified by sex, age, district, religion, occupation, and education. Risk variables associated with T. canis infection were investigated using a univariate logistic regression model. The variables in the univariate logistic regression model with p < 0.1 were included in a multiple logistic regression model that was adjusted for confounding factors. Differences were considered statistically significant when p < 0.05. The hematological parameters of seropositive and seronegative dog owners were compared using the Mann–Whitney U test and independent t test for nonparametric and parametric data analysis, respectively.
Results
Characterization of sociodemographic factors
This study included 132 dog owners, including 25 men (18.94%) and 107 women (81.6%). The median age of the participants was 50 years (interquartile range 20 years). The participants lived in three districts of Nakhon Si Thammarat Province: Tha Sala (n = 53), Nopphitam (n = 37), and Phrom Kiri (n = 42). All participants were Buddhists. Their occupations and educational levels predominantly were unskilled workers and high school diplomas or lower, respectively. The mean number of dogs per household was 1.9. All the participants allowed their dogs to excrete outside the house (Table 1).
Seroprevalence of Toxocara canis infection
The seroprevalence of T. canis infection among dog owners is shown in Table 2. The results showed that the overall seroprevalence of T. canis infection was 76.5%. Male participants (80%) had a higher seropositivity rate than female participants (75.7%). The rates of seropositivity were 69.4%, 89.2%, and 73.8% in the Tha Sala, Nopphitam, and Phrom Kiri districts, respectively, but were not significantly different among the three districts (p = 0.910). Seropositivity for T. canis was observed in all age groups. The highest rate of seropositivity was found among 18–35 years (85%), then 72–89 years (76.9%), whereas the lowest rate of seropositivity was observed among 36–53 years (74.6%). Chi-squared tests showed no significant associations between T. canis seropositivity and sex, age, and education (Table 2).
Risk factors analysis
Table 3 shows the summary of findings from the regression analysis to investigate the associations between T. canis infection and potential factors. The univariate analysis revealed that dog owners who did not wash their hands before meals (crude odds ratio (COR) = 8.383; 95% CI 1.89–37.16; p = 0.005), after contact with soil (COR = 5.027; 95% CI 1.12–22.54; p = 0.035), and after contact with the dog (COR = 4.519; 95% CI 1.00–22.34; p = 0.049) had a significantly increased risk of acquiring T. canis infection. However, after adjusting for confounders, only dog owners who did not wash their hands before meals remained at significant risk of acquiring T. canis infection (adjusted odds ratio (AOR) = 6.067; 95% CI 1.10–33.34; p = 0.038). Dog owners who did not wash their hands before meals were 6.067 times more likely to have acquired T. canis infection than those who had not after adjusting for other factors. When stratified by age, the rates of seropositivity for T. canis were approximately equal among the age groups (74.6%, 75%, 76.9%, and 85% for dog owners aged 36–53, 54–71, 72–89, and 18–35 years, respectively). Men were more likely to be infected with T. canis than women. However, there were no statistically significant differences among these factors in the univariate analysis (Table 3). Univariate analysis of the other information on the dogs and the dog owner’s behavior, including bleeding in the dog; vaccination of the dog; deworming of the dog; bathing the dog; tick/flea treatment; behavior of dog owner regarding playing, kissing, and touching the dog; the sleeping place of the dog; frequency of playing with the dog; and management of the dog feces, showed no statistically significant differences (Table 3).
Toxocara canis infection and hematological parameters
The hematological parameters for the T. canis IgG seropositive and T. canis IgG seronegative groups are displayed in Table 4. The results revealed that the mean number of eosinophils was significantly higher in the T. canis IgG seropositive group than in the seronegative group (p < 0.04). There were no significant differences in RBC count, MCHC, Hb, HCT, MCV, RDW, WBC, neutrophil number, lymphocyte number, monocyte number, or basophil number between the two groups (Table 4).
Discussion
Toxocariasis in humans is a neglected zoonotic parasite that affects millions of pediatric and adolescent populations globally, especially in tropical and subtropical regions. This is the first serological investigation of T. canis infection among dog owners in rural areas of southern Thailand. The present study showed that the overall seroprevalence of T. canis infection was 76.52%, which was higher than that reported in Iran (20.43%) [25] and Egypt (29.85%) [26]. In addition, the results of our study were also higher than the globally reported seroprevalence (19.0%) and the pooled seroprevalence in Southeast Asia (34.1%) [6], Thailand (58.2%) [17], Vietnam (45.2%) [18], and the Philippines (49%) [19]. However, the seroprevalence rate in this study was lower than that in other regions, including Makoko, Nigeria (86.1%) [11], and the Republic of the Marshall Islands (86.75%) [12]. Several factors contribute to T. canis infection, including geographic, socioeconomic, climatic, environmental, and cultural factors. The present study covered three districts of Nakhon Si Thammarat Province, and all areas were considered rural communities. These countryside areas are filled with palm oil and rubber plantations. Nakhon Si Thammarat Province has a tropical rainforest climate with temperatures ranging from 24 to 34 °C, suitable for the development of the infective larval stage of Toxocara eggs. Dogs are the most popular pets in this province, with a large number of stray dogs in the area. The mutual affection of humans and their companions, accepted as an operative connection known as the human–animal bond, provide several benefits with regard to mental health, socialization, and physical wellbeing [27]. However, privately owned dogs can play a crucial role in helminthic zoonotic toxocariasis transmission [28, 29]. We hypothesized that the soil, number of dogs in the areas, and the human–animal bond influenced the seropositivity rate in this study.
Among the participating dog owners in the three districts, the highest T. canis seropositivity (89.2%) was observed in the Nopphitam District. However, there were no statistically significant differences between the three districts. This might be explained by the similarities in the areas, such as climate, geography, values, and culture among the districts. Most dog owners who were unskilled workers with low education levels had a relatively higher rate of T. canis infection. This correlation was similarly observed in a previous study conducted in a rural area of southern Thailand [17]. However, this study revealed that educational level and occupation did not significantly influence the seropositivity rate of T. canis. This might be due to the small number of dog owners who were skilled workers and had obtained college degrees. While comparing age groups, our study showed no significant difference in the seropositivity rate of T. canis infection among different age groups. The seropositivity rates of T. canis infection were similar across all age groups: 74.6% in dog owners aged 36–53 years, 75% in those aged 54–71 years, 76.9% in those aged 72–89 years, and 85% in those aged 18–35 years. However, this result was in contrast to several studies that showed that older age was a significant risk factor for T. canis infection [11, 13, 30, 31]. Serum Toxocara IgG can persist with age, which increases the detection of Toxocara via serology over time. A possible explanation for these similar seropositivity rates among different age groups may be that the dog owners came in contact with T. canis eggs at a young age, as IgG persists over a long period of life. Our study revealed that men (80%) were more likely to be infected with T. canis than women (75.7%). This trend has also been reported in previous studies [31, 32]. It has been hypothesized that males engage in more outdoor activities than females and, therefore, are more likely to be in contact with contaminated soil and dogs [33, 34]. However, univariate and multivariate analyses demonstrated that sex was not significantly associated with seropositivity for anti-T.canis IgG antibodies in our study.
Regarding modifiable risk behaviors, handwashing before meals appeared to be the only significant risk factor associated with T. canis infection in both the univariate and multivariate analyses. Dog owners who did not practice handwashing before meals were six times more likely to be infected with T. canis than those who did. The risk ratio in this study was higher than that in a previous study conducted on schoolchildren in Nakhon Si Thammarat Province, Thailand [17]. Interestingly, the univariate analysis in this study revealed that not practicing handwashing after coming in contact with soil and dogs was significantly associated with T. canis infection. However, these associations were not statistically significant in the multivariate analysis. A similar result was observed in a previous study [17]. Humans acquire T. canis by accidentally ingesting infected eggs, which can be found in several public places [35]. A previous study conducted in rural areas of southern Thailand reported that the soil samples from public schools were contaminated with Toxocara eggs, especially that in the playgrounds; this correlated with our results as all dog owners reportedly allowed the dog to excrete outside the house (100%) [34]. This study suggests that people might be infected with T. canis in ways other than by caring for dogs and emphasizes the necessity of hand hygiene to prevent parasite eggs from entering the human body via the fecal–oral route. Further investigation for the presence of Toxocara eggs in dogs’ feces and soil samples is needed to understand the potential etiological factors of the disease transmission to humans.
The results of the hematological profile showed a higher eosinophil count in the seropositive group than in the seronegative group. This finding is similar to those of previous studies on other parasites [36, 37], suggesting that eosinophils play a major role in the immune response against tissue parasites. These results are similar to the previous study in which eosinophil appeared to increase during hookworm infection [37].
The study had several limitations. First, ELISA is a method for detecting anti-T. canis IgG antibodies, which is not the reference laboratory test for T. canis detection, Toxocara spp. larval excretory-secretory antigens Western blot, and could yield false-positive results due to cross-reactivity with other helminths, especially A. lumbricoides [38, 39]. However, our study area was not endemic to A. lumbricoides [40,41,42]. Consequently, the probability of false positivity is likely minimal. Second, the study performed cross-sectional research, and the seroprevalence and the risk factors were evaluated concurrently and not over a period of time; hence, the true causes and effects might not be strongly determined.
Conclusions
This is the first serological investigation of T. canis infection among dog owners in southern Thailand with a high rate of T. canis seropositivity, reflecting high levels of T. canis exposure among dog owners in this region. Not practicing handwashing before meals appeared to be a significant risk factor for T. canis infection. This study also provides group-specific data concerning modifiable risk behaviors for more effective T. canis infection control and prevention strategies in Thailand.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- VLM:
-
Visceral larva migrans
- OLM:
-
Ocular larva migrans
- ELISA:
-
Enzyme-linked immunosorbent assay
- TES:
-
T. canis excretory–secretory
- RBC:
-
Red blood cells
- Hb:
-
Hemoglobin
- HCT:
-
Hematocrit
- MCV:
-
Mean cell volume
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- RDW:
-
Red cell distribution width
- WBC:
-
White blood cells
- NTU:
-
NovaTec units
References
Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010;104(1):3–23.
Ma G, Holland CV, Wang T, Hofmann A, Fan CK, Maizels RM, et al. Human toxocariasis. Lancet Infect Dis. 2018;18(1):e14–24.
Deplazes P, van Knapen F, Schweiger A, Overgaauw PA. Role of pet dogs and cats in the transmission of helminthic zoonoses in Europe, with a focus on echinococcosis and toxocarosis. Vet Parasitol. 2011;182(1):41–53.
Rostami A, Riahi SM, Hofmann A, Ma G, Wang T, Behniafar H, et al. Global prevalence of Toxocara infection in dogs. Adv Parasitol. 2020;109:561–83.
Zibaei M, Sadjjadi SM, Maraghi S. The occurrence of Toxocara species in naturally infected broiler chickens revealed by molecular approaches. J Helminthol. 2017;91(5):633–6.
Rostami A, Riahi SM, Holland CV, Taghipour A, Khalili-Fomeshi M, Fakhri Y, et al. Seroprevalence estimates for toxocariasis in people worldwide: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2019;13(12): e0007809.
Asher MI. Recent perspectives on global epidemiology of asthma in childhood. Allergol Immunopathol. 2010;38(2):83–7.
Wu T, Bowman DD. Chapter Four - Visceral larval migrans of Toxocara canis and Toxocara cati in non-canid and non-felid hosts. Boca Raton: Academic Press; 2020. p. 63–88.
Zhan B, Ajmera R, Geiger SM, Gonçalves MTP, Liu Z, Wei J, et al. Identification of immunodominant antigens for the laboratory diagnosis of toxocariasis. Trop Med Int Health. 2015;20(12):1787–96.
Araujo AC, Villela MM, Sena-Lopes A, Farias N, Faria LMJ, Avila L, et al. Seroprevalence of Toxoplasma gondii and Toxocara canis in a human rural population of Southern Rio Grande do Sul. Rev Inst Med Trop Sao Paulo. 2018;60: e28.
Gyang PV, Akinwale OP, Lee YL, Chuang TW, Orok AB, Ajibaye O, et al. Seroprevalence, disease awareness, and risk factors for Toxocara canis infection among primary schoolchildren in Makoko, an urban slum community in Nigeria. Acta Trop. 2015;146:135–40.
Fu C-J, Chuang T-W, Lin H-S, Wu C-H, Liu Y-C, Langinlur MK, et al. Seroepidemiology of Toxocara canis infection among primary schoolchildren in the capital area of the Republic of the Marshall Islands. BMC Infect Dis. 2014;14:261.
Berrett AN, Erickson LD, Gale SD, Stone A, Brown BL, Hedges DW. Toxocara Seroprevalence and associated risk factors in the United States. Am J Trop Med Hyg. 2017;97(6):1846–50.
Ponce-Macotela M, Martínez-Gordillo MN. Toxocara: Seroprevalence in Mexico. Adv Parasitol. 2020;109:341–55.
Nicoletti A, Cicero CE, Mantella A, Giuliano L, Rascunà C, Paradisi V, et al. Seroprevalence of Toxocara Canis in the city of Catania, Italy. Mediterr J Hematol Infect Dis. 2019;11(1):e2019031.
Papavasilopoulos V, Pitiriga V, Birbas K, Elefsiniotis J, Bonatsos G, Tsakris A. Soil contamination by Toxocara canis and human seroprevalence in the Attica region. Greece Germs. 2018;8(3):155–61.
Phasuk N, Punsawad C. Seroprevalence of Toxocara canis infection and associated risk factors among primary schoolchildren in rural Southern Thailand. Trop Med Int. 2020;48:23.
Nguyen T, Cheong FW, Liew JWK, Lau YL. Seroprevalence of fascioliasis, toxocariasis, strongyloidiasis and cysticercosis in blood samples diagnosed in Medic Medical Center Laboratory, Ho Chi Minh City, Vietnam in 2012. Parasit Vectors. 2016;9(1):486.
Fajutag AJ, Paller VG. Toxocara egg soil contamination and its seroprevalence among public school children in Los Baños, Laguna, Philippines. Southeast Asian J Trop Med Public Health. 2013;44(4):551–60.
Stull JW, Peregrine AS, Sargeant JM, Weese JS. Household knowledge, attitudes and practices related to pet contact and associated zoonoses in Ontario, Canada. BMC Public Health. 2012;12(1):553.
Rojekittikhun W, Chaisiri K, Mahittikorn A, Pubampen S, Sa-Nguankiat S, Kusolsuk T, et al. Gastrointestinal parasites of dogs and cats in a refuge in Nakhon Nayok, Thailand. Southeast Asian J Trop Med Public Health. 2014;45(1):31–9.
Pumidonming W, Salman D, Gronsang D, Abdelbaset AE, Sangkaeo K, Kawazu S-I, et al. Prevalence of gastrointestinal helminth parasites of zoonotic significance in dogs and cats in lower Northern Thailand. J Vet Med Sci. 2017;78(12):1779–84.
Eslahi AV, Badri M, Khorshidi A, Majidiani H, Hooshmand E, Hosseini H, et al. Prevalence of Toxocara and Toxascaris infection among human and animals in Iran with meta-analysis approach. BMC Infect Dis. 2020;20(1):20.
Gillespie S, Bradbury RS. A survey of intestinal parasites of domestic dogs in Central Queensland. Trop Med Infect Dis. 2017;2(4):1.
Berenji F, Pouryousef A, Fata A, Mahmoudi M, Salehi M, Khoshnegah J. Seroepidemiological study of Toxocariasis in the owners of domestic cats and dogs in Mashhad. Northeastern Iran Iran J Parasitol. 2016;11(2):265–8.
Awadallah MAI, Salem LMA. Zoonotic enteric parasites transmitted from dogs in Egypt with special concern to Toxocara canis infection. Vet World. 2015;8(8):946–57.
Paul M, King L, Carlin EP. Zoonoses of people and their pets: a US perspective on significant pet-associated parasitic diseases. Trends Parasitol. 2010;26(4):153–4.
Fan CK, Liao CW, Kao TC, Li MH, Du WY, Su KE. Sero-epidemiology of Toxocara canis infection among aboriginal schoolchildren in the mountainous areas of north-eastern Taiwan. Ann Trop Med Parasitol. 2005;99(6):593–600.
Dar Z, Tanveer S, Yattoo G, Sofi BA, Wani S, Fomda B. Seroprevalence of toxocariasis in children in Kashmir, J&K State, India. Iran J Parasitol. 2008;3:18.
Aghamolaie S, Seyyedtabaei SJ, Behniafar H, Foroutan M, Saber V, Hanifehpur H, et al. Seroepidemiology, modifiable risk factors and clinical symptoms of Toxocara spp. infection in northern Iran. Trans R Soc Trop Med Hyg. 2019;113(3):116–22.
Romero Núñez C, Mendoza Martínez GD, Yañez Arteaga S, Ponce Macotela M, Bustamante Montes P, Ramírez DN. Prevalence and risk factors associated with Toxocara canis infection in children. Sci World J. 2013;2013: 572089.
Silva MB, Amor ALM, Santos LN, Galvão AA, Oviedo Vera AV, Silva ES, et al. Risk factors for Toxocara spp. seroprevalence and its association with atopy and asthma phenotypes in school-age children in a small town and semi-rural areas of Northeast Brazil. Acta Trop. 2017;174:158–64.
Hernández SA, Gabrie JA, Rodríguez CA, Matamoros G, Rueda MM, Canales M, et al. An integrated study of Toxocara infection in honduran children: human seroepidemiology and environmental contamination in a coastal community. Trop Med Infect Dis. 2020;5(3):1–8.
Phasuk N, Kache R, Thongtup K, Boonmuang S, Punsawad C. Soil contamination with Toxocara eggs in public schools in rural sreas of southern Thailand. J Trop Med. 2020;2020:9659640.
Fakhri Y, Gasser RB, Rostami A, Fan CK, Ghasemi SM, Javanian M, et al. Toxocara eggs in public places worldwide – a systematic review and meta-analysis. Environ Pollut. 2018;242:1467–75.
O’Connell EM, Nutman TB. Eosinophilia in infectious diseases. Immunol Allergy Clin North Am. 2015;35(3):493–522.
Phasuk N, Apiwattanakul N, Punsawad C. Profiles of CD4(+), CD8(+), and regulatory T cells and circulating cytokines in hookworm-infected children in southern Thailand. Med Microbiol Immunol. 2021;211(1):19–28.
Mohamad S, Azmi NC, Noordin R. Development and evaluation of a sensitive and specific assay for diagnosis of human toxocariasis by use of three recombinant antigens (TES-26, TES-30USM, and TES-120). J Clin Microbiol. 2009;47(6):1712–7.
Watthanakulpanich D, Smith HV, Hobbs G, Whalley AJ, Billington D. Application of Toxocara canis excretory-secretory antigens and IgG subclass antibodies (IgG1-4) in serodiagnostic assays of human toxocariasis. Acta Trop. 2008;106(2):90–5.
Punsawad C, Phasuk N, Bunratsami S, Thongtup K, Siripakonuaong N, Nongnaul S. Prevalence of intestinal parasitic infection and associated risk factors among village health volunteers in rural communities of southern Thailand. BMC Public Health. 2017;17(1):564.
Punsawad C, Phasuk N, Bunratsami S, Thongtup K, Viriyavejakul P, Palipoch S, et al. Prevalence of intestinal parasitic infections and associated risk factors for hookworm infections among primary schoolchildren in rural areas of Nakhon Si Thammarat, southern Thailand. BMC Public Health. 2018;18(1):1118.
Kache R, Phasuk N, Viriyavejakul P, Punsawad C. Prevalence of soil-transmitted helminth infections and associated risk factors among elderly individuals living in rural areas of southern Thailand. BMC Public Health. 2020;20(1):1882.
Acknowledgements
The authors thank all study participants for their support throughout the course of this study.
Funding
This research was funded by the Walailak University (Grant Number WU-IRG-63-054). The funder had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
PN, NP and CP conceived and designed the study. PN, UN, NP and CP performed the laboratory work. PN, UN, NP and CP analyzed the data. PN were involved in the drafting of the manuscript. UN, NP and CP were involved in the critical review of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Human Research Ethics Committee of the Walailak University, Thailand (Approval Number: WUEC-20-120-1). Informed consent was obtained from all the subjects involved in the study. Written informed consent was obtained from all participants to publish this paper.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Questionnaire Form.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Na-Ek, P., Narkkul, U., Phasuk, N. et al. Seroprevalence of anti-Toxocara canis antibodies and associated risk factors among dog owners in the rural community of Nakhon Si Thammarat province, southern Thailand. Trop Med Health 50, 32 (2022). https://doi.org/10.1186/s41182-022-00425-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-022-00425-4