Abstract
Background
Metastatic colorectal cancer (mCRC) patients with progressive disease after all available standard therapies need new medication for further treatment. Famitinib is a small-molecule multikinase inhibitor, with promising anticancer activities. This multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial was designed to evaluate the safety and efficacy of famitinib in mCRC.
Methods
Famitinib or placebo was administered orally once daily. The primary endpoint was progression-free survival (PFS). Secondary endpoints included objective response rate (ORR), disease control rate (DCR), overall survival (OS), quality-of-life (QoL), and safety.
Results
Between July 18, 2012 and Jan 22, 2014, a total of 167 patients were screened, and 154 patients were randomized in a 2:1 ratio to receive either famitinib (n = 99) or placebo (n = 55). The median PFS was 2.8 and 1.5 months in the famitinib and placebo groups (hazard ratio = 0.60, 95% confidence interval = 0.41–0.86, P = 0.004). The DCR was 59.8% and 31.4% (P = 0.002) and the ORR was 2.2% and 0.0% (P = 0.540) in the famitinib and placebo groups, respectively. The most frequent grade 3–4 adverse events were hypertension (11.1%), hand-foot syndrome (10.1%), thrombocytopenia (10.1%), and neutropenia (9.1%). Serious adverse events occurred in 11 (11.1%) patients in the famitinib group and 5 (9.1%) in the placebo group (P = 0.788). The median OS of the famitinib and placebo groups was 7.4 and 7.2 months (P = 0.657).
Conclusion
Famitinib prolonged PFS in refractory mCRC patients with acceptable tolerability.
Trial registration This study was registered on ClinicalTrials.gov (NCT01762293) and was orally presented in the 2015 ASCO-Gastrointestinal Symposium
Similar content being viewed by others
Background
Colorectal cancer (CRC) is a common malignancy and the second leading cause of cancer-related deaths worldwide [1]. At least half of patients will eventually develop metastases [2, 3]. Moreover, the incidence and mortality of CRC have been rising quickly in recent years in China [4]. Combination chemotherapy, consisting of 5-fluorouracil (5-FU) or oral 5-FU analogues, irinotecan and oxaliplatin, with or without anti-epidermal growth factor receptor (anti-EGFR) and anti-angiogenesis monoclonal antibody, are adopted as the standard first- or second-line therapy for CRC [5,6,7,8,9]. However, there are no other drugs except for regorafenib being used to treat metastatic CRC (mCRC) after standard chemotherapy failure. Regorafenib has been approved to treat mCRC based on the results of two phase III studies CORRECT [10] and CONCUR [11]. Another clinical study involving fruquintinib also demonstrated promising anticancer effect on mCRC [12].
Famitinib (famitinib l-malate) is a novel and potent receptor tyrosine kinase inhibitor (rTKI) [13]. The targets of famitinib include tyrosine kinase receptor c-kit, vascular endothelial growth factor receptor-2 and -3 (VEGFR-2 and -3), platelet-derived growth factor receptor (PDGFR), FMS-like tyrosine kinase-3 receptor (FLT3), and tyrosine-protein kinase receptor Ret [13, 14]. A phase I study showed that famitinib was generally well-tolerated and has a wide spectrum of antitumor activities [14]. Based on the results of famitinib from pre-clinical and phase I studies and due to the high unmet needs of Chinese mCRC patients, we initiated a multicenter, randomized, double-blinded, placebo-controlled, phase II study to evaluate the efficacy and safety of famitinib in Chinese patients with mCRC who failed standard therapies.
Patients and methods
Patients and study design
This clinical trial involves 19 hospitals/institutions in China. The clinical trial protocol was approved by the institutional review board of each center.
The inclusion criteria are as follows: (1) patients have pathologically confirmed advanced colorectal adenocarcinoma (excluding all other histological types) and have previously received at least two lines of standard chemotherapy (must include 5-FU, irinotecan, and oxaliplatin) and failed treatment (treatment failure is defined as intolerable adverse events [AEs] or disease progression during treatment or within 3 months after the last treatment); (2) according to the response evaluation criteria in solid tumors (RECIST) version 1.1 criteria [15], patients must have at least one target lesion with measurable diameter (long diameter of tumor lesion ≥ 10 mm and short diameter of lymph node lesion ≥ 15 mm on computed tomography [CT], with scan slice thickness no more than 5 mm; without local treatment); (3) age of 18–70 years; (4) Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; and (5) life expectancy ≥ 3 months.
The exclusion criteria are as follows: (1) with a history or presence of other malignancies, excluding cured skin basal cell carcinoma and carcinoma in situ of the cervix; (2) with previous treatment with VEGFR TKIs (e.g., sorafenib, sunitinib, and regorafenib); (3) with multiple factors influencing oral administration (e.g., inability to swallow, chronic diarrhea, and intestinal obstruction); (4) with definite gastrointestinal bleeding tendency evidenced by local active ulcer lesions and stool occult blood (++), history of melena and hematemesis in the past 2 months, and potential of major gastrointestinal bleeding considered by the investigator; (5) with evidence of central nervous system (CNS) metastasis at baseline or a history of CNS metastasis (for patients with clinically suspected CNS metastasis, CT or magnetic resonance imaging [MRI] scan must be performed within 14 days prior to randomization to exclude CNS metastasis); (6) excessive tumor burden of vital organs (e.g., liver tumor burden > 50%) demonstrated with imaging; or (7) abnormal function of vital organs. Each subject provided written informed consent before enrollment.
Randomization and blinding
Patients were randomly assigned to the famitinib or placebo group in a 2:1 ratio. A centralized randomization system, supplied by the Department of Epidemiology and Health Statistics at Nanjing Medical University, was used. Randomization of subjects was on the basis of pre-allocated block sizes (block size six) and was stratified by previous treatment (no more than or more than three-line therapy) and baseline lactate dehydrogenase (LDH) level (≤ 1.5 or > 1.5 times of the upper limit of normal).
The unblinding of treatment for individual patients was allowed for emergency situations only.
Treatment and follow-up
Patients were treated with 25 mg oral famitinib or matching placebo tablet once daily until progressive disease (PD), death, unacceptable AEs, withdrawal of consent by the patient or a decision by the physician that discontinuation would be in the patient’s best interest. Patients were followed-up every 2 weeks for the first 6 weeks and every 3 weeks thereafter while receiving treatment, and every 6 weeks after cessation of treatment until death or the last follow up of August 21, 2014. Predefined dose modifications were permitted to manage clinically significant treatment-related AEs. This study was conducted in compliance with ethical principles originated or derived from the Declaration of Helsinki (October 1996).
Assessments
Tumor response was assessed radiologically every 6 weeks, using the RECIST version 1.1 criteria [15].
The primary endpoint was progression-free survival (PFS), defined as the duration from treatment initiation to first radiological observation of PD or death from any cause. Secondary endpoints included overall survival (OS), defined as the duration from treatment initiation to death from any cause; objective tumor response rate (ORR), defined as the proportion of patients with complete or partial response; disease control rate (DCR), defined as the proportion of patients with a best response of complete or partial response or stable disease (defined as disease stabled for more than 6 weeks after randomization); and quality of life (QoL) evaluated by questionnaire and safety assessments.
Patients’ health-related QoL and health utility values were measured before enrollment and at the end of each 6-week treatment, according to the European Organization for Research and Treatment of Cancer (EORTC) general health status and quality of life questionnaire QLQ-C30 [16]. AEs were graded with the National Cancer Institute Common Terminology Criteria for Adverse Events (Version 4.0) [17].
Statistical analysis
Based on the investigators’ opinions, the assumed median PFS of patients in the famitinib and placebo groups would be 3.2 and 2.0 months, respectively. Considering that this was a phase II trial, setting one-sided significant level to 0.05, with a famitinib-to-placebo allocation ratio of 2:1, 144 subjects in total could achieve 80% power.
All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA). Median OS and PFS with 95% confidence interval (CI) for each group were estimated using the Kaplan–Meier method. Patients without disease progression or death by the last follow-up would be censored in PFS or OS curves. OS and PFS were compared between the famitinib and placebo groups using the log-rank test. Stratified log-rank tests by previous treatment and LDH level were also performed. If the proportionality assumption holds true, Cox proportional hazard model was used to estimate the hazard ratio (HR) and 95% CI with treatment as a fixed factor. Cox proportional hazard model adjusted for stratification factors including age, gender, LDH level, number of metastatic organs and treatment line was also performed. ORR and DCR were compared between treatment groups using the Cochran–Mantel–Haenszel test, adjusted for stratification factors. Baseline characteristics and AEs were compared with the use of analysis of variance or Chi square test as appropriate.
Results
Patient characteristics
Between July 18, 2012 and Jan 22, 2014, 154 patients were enrolled and randomized to receive famitinib (n = 99) or placebo (n = 55), and the flow diagram is shown in Fig. 1. The baseline characteristics were balanced between the two groups (Table 1). Overall, 71 (46.1%) of the 154 patients had previously received monoclonal antibody treatment, and 94 (61.0%) had received more than three lines of treatment for mCRC.
Treatment situation
The mean duration of treatment was 86.8 ± 78.0 days (median 63.0 days; interquartile range [IQR] 40.0–105.0 days) for the famitinib group and 58.2 ± 47.1 days (median 42.0 days; IQR 40.0–82.0 days) for the placebo group. The mean duration of follow-up from the completion of study treatment to last follow-up was 6.2 ± 4.4 months (median 5.1 months; IQR 2.9–9.2 months) for the famitinib group and 7.3 ± 5.5 months (median 5.4 months; IQR 2.9–10.0 months) for the placebo group.
The mean daily dose of famitinib was 23.1 ± 3.2 mg (median 25.0 mg; IQR 20.9–25.0 mg). The mean daily dose of placebo was 24.8 ± 1.4 mg (median 25.0 mg; IQR 25.0–25.0 mg).
As shown in Table 2, dose interruption occurred in 49 (49.5%) patients and dose reduction occurred in 24 (24.2%) patients in the famitinib group; however, in the placebo group, dose interruption occurred in 13 (23.6%) patients and dose reduction occurred in 2 (3.6%) patients. AEs were the most common reasons for dose modification.
Clinical outcome
At last, 92 patients in the famitinib group and 51 in the placebo group had clinical response evaluation. Complete response was not achieved in any group. Two patients in the famitinib group had partial response (ORR = 2.2%), compared to none in the placebo group (P = 0.540). DCR was 59.8% (55/92) in the famitinib group and 31.4% (16/51) in the placebo group (P = 0.002).
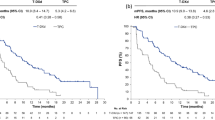
Up to the last follow-up, disease progression and death occurred in 83 (83.8%) and 82 (82.8%) patients in the famitinib group and in 47 (85.5%) and 42 (76.4%) patients in the placebo group. The median PFS was 2.8 and 1.5 months in the famitinib and placebo groups (HR = 0.60, 95% CI = 0.41–0.86, P = 0.004, Fig. 2a), and the median OS was 7.4 and 7.2 months in the famitinib and placebo groups (P = 0.657, Fig. 2b). Stratified analysis including age, gender, LDH level, number of metastatic organs, and treatment line demonstrated that the famitinib group had longer PFS than the placebo group (Fig. 3).
Kaplan–Meier estimates of progression-free survival (PFS) and overall survival (OS) probability of mCRC patients treated with famitinib and placebo. a the median PFS in the famitinib group was significantly longer than that in the placebo group (P = 0.004); b there is no significant difference in the median OS between the two groups
Following famitinib or placebo treatment, 31 (31.3%) and 23 (41.8%) patients in the famitinib and placebo groups received further anti-cancer therapies. As shown in Table 3, 21 (21.2%) patients in the famitinib group and 6 (10.9%) patients in the placebo group received chemotherapy, including S-1, raltitrexed, or other regimens.
Using the EORTC questionnaire QLQ-C30, the mean QoL scores at baseline were 84.9 ± 4.7 in the famitinib group and 85.3 ± 5.5 in the placebo group; the mean scores at the end of study treatment were 83.5 ± 8.1 in the famitinib group and 81.7 ± 5.0 in the placebo group (Fig. 4), indicating a mild decrease in QoL in both groups. Patients’ QoL and health status deteriorated to a similar extent in both groups (P > 0.100 for all visit-specific comparisons; P = 0.534 for overall comparison).
Safety
Table 4 shows treatment-related AEs that occurred in the two groups during the study. The most frequent AEs in the famitinib group were hematologic toxicity, proteinuria, hypertension, hand-foot syndrome, diarrhea, fatigue, hand-foot skin reaction, and liver dysfunction. In the placebo group, the most frequent AEs were liver dysfunction, fatigue, and proteinuria.
Treatment-related grade 3–4 AEs occurred in 51 (51.5%) patients in the famitinib group and 20 (36.4%) in the placebo group (Table 4). The most frequent famitinib-related grade 3–4 AEs were hypertension, hand-foot syndrome, thrombocytopenia, neutropenia, proteinuria, and liver dysfunction. The most frequent AEs leading to dose modification were dermatological, gastrointestinal, and metabolic or laboratory events.
Serious adverse events (SAEs) occurred in 11 (11.1%) patients in the famitinib group and 5 (9.1%) in the placebo group (P = 0.788). The most notable SAE in the famitinib group was intestinal obstruction (n = 5). Other SAEs included infection, hemoptysis, hypertension, fatigue, renal failure, upper gastrointestinal hemorrhage, and hepatic encephalopathy in the famitinib group, whereas cerebral infarction, cerebral hemorrhage, fatigue, and thrombocytopenia were observed in the placebo group. The majority of SAEs were resolved, whereas four patients died of SAEs (three in the famitinib group and one in the placebo group).
Discussion
VEGF and its receptors play a critical role in angiogenesis in CRCs [18,19,20,21,22,23,24]. Bevacizumab is a humanized monoclonal antibody designed to block VEGF and has shown efficacy on mCRC [25,26,27]. Sorafenib and sunitinib, two small-molecule TKIs, have been studied in the treatment of mCRC patients [28,29,30,31,32]. Regorafenib was approved to treat mCRC, with median OS prolongation by 1.4 months in the CORRECT study [10] and 2.5 months in the CONCUR study [11]. Recently, fruquintinib as third-line treatment was reported to prolong the survival of mCRC patients [12]. Based on the recent unpublished FRESCO study, the median OS was 9.3 months (95% CI, 8.2–10.5 months) in the fruquintinib group versus 6.7 months (95% CI 5.9–8.1 months) in the placebo group, in a total of 416 mCRC patients; the median PFS was 3.7 months (95% CI 3.6–4.6 months) in the fruquintinib group versus 1.8 months (95% CI 1.8–1.8 months) in the placebo group.
In the present study, the addition of famitinib to supportive care significantly prolonged PFS in patients with mCRC who had failed all standard chemotherapy agents with or without monoclonal antibody. The median PFS prolongation was 1.3 months by famitinib in the present study, 1.9 months by fruquintinib based on the unpublished FRESCO study, and 1.4 months by regorafenib in the CORRECT study [10], showing that VEGFR-blocking TKIs are effective in treating mCRC after chemotherapy failure. However, in the present study, famitinib failed to prolong the median OS, which may be related to that many patients in the placebo group received further anti-tumor therapy, especially targeted therapy. At the same time, we did observe a trend of survival prolongation in patients treated with famitinib who had been heavily pre-treated with systematic chemotherapy. The patients in the famitinib group with ≥ 6 cycles of first-line chemotherapy and ≥ 3 cycles of second-line chemotherapy had 2.0 months increase in median OS compared with patients in the placebo group (data not shown).
The median OS prolongation by famitinib in the present study was slightly shorter than those by regorafenib [10] and fruquintinib (data not published). The higher percentages of patients with ECOG score of 1 and elderly patients in the present study than those in the other two studies may influence OS. In addition, only 30.2% of patients had received anti-angiogenesis therapy before fruquintinib treatment in the unpublished FRESCO study, but 39.4% of patients had received anti-angiogenesis therapy before famitinib treatment in the present study, which might also influence OS.
The most frequent AEs related to famitinib were proteinuria, hand-foot syndrome, fatigue, and hypertension in the present study, which were consistent with those observed in a phase I study [14] and typical AEs of small-molecule VEGF TKIs [10, 11]. These AEs occurred frequently during the early course of treatment and were generally manageable with dose reduction or interruption. The occurrence of hypertension could reflect the anti-angiogenesis effect of VEGF or VEGFR TKIs. The rate of hypertension caused by famitinib in the present study was higher than that caused by regorafenib [10], suggesting that famitinib may have stronger anti-angiogenesis effect than regorafenib. Another multiple-target TKI sunitinib, with similar structure as famitinib, was reported that its efficacy on renal cell carcinoma was related to the occurrence of hypertension [33]. It is worth exploring if there is correlation between the efficacy and hypertension in famitinib treatment in further study.
One limitation of the present study is that the primary tumor site and RAS/v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) mutation were not included in subgroup analysis because of the small sample size.
Conclusions
In summary, famitinib significantly prolonged the median PFS for patients with refractory mCRC who had failed two or more lines of standard chemotherapy, and the toxicities were tolerable. A phase III trial is warranted in further study.
Abbreviations
- 5-FU:
-
5-fluorouracil
- AEs:
-
adverse events
- CR:
-
complete response
- DCR:
-
disease control rate
- ECOG:
-
Eastern Cooperative Oncology Group
- EORTC:
-
European Organization for Research and Treatment of Cancer
- FAS:
-
full analysis set
- FLT3:
-
FMS-like tyrosine kinase-3 receptor
- HR:
-
hazard ratio
- IQR:
-
interquartile range
- IRB:
-
institutional review board
- LDH:
-
lactate dehydrogenase
- mCRC:
-
metastatic colorectal cancer
- MMRM:
-
mixed model for repeated measurements
- mOS:
-
median overall survival
- ORR:
-
objective tumor response rate
- OS:
-
overall survival
- PD:
-
disease progression
- PFS:
-
progression-free survival
- QoL:
-
quality of life
- rTKI:
-
receptor tyrosine kinase inhibitor
- SAE:
-
serious adverse events
- VEGFR-2 and 3:
-
vascular endothelial growth factor receptor-2 and 3
References
Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev. 2009;18(6):1688–94.
Van Cutsem E, Nordlinger B, Cervantes A, ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol. 2010;21(Suppl 5):v93–7.
Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, et al. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42(14):2212–21.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32.
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–65.
Ogino S, Meyerhardt JA, Irahara N, Niedzwiechi D, Hollis D, Saltz LB, et al. KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin Cancer Res. 2009;15(23):7322–9.
Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360(6):563–72.
Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005;23(22):4866–75.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12.
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16(6):619–29.
Xu RH, Li J, Bai Y, Xu J, Liu T, Shen L, et al. Safety and efficacy of fruquintinib in patients with previously treated metastatic colorectal cancer: a phase Ib study and a randomized double-blind phase II study. J Hematol Oncol. 2017;10(1):22–9.
Xie C, Zhou J, Guo Z, Diao X, Gao Z, Zhong D, et al. Metabolism and bioactivation of famitinib, a novel inhibitor of receptor tyrosine kinase, in cancer patients. Br J Pharmacol. 2013;168(7):1687–706.
Zhou A, Zhang W, Chang C, Chen X, Zhong D, Qin Q, et al. Phase I study of the safety, pharmacokinetics and antitumor activity of famitinib. Cancer Chemother Pharmacol. 2013;72(5):1043–53.
An MW, Dong X, Meyers J, Han Y, Grothey A, Bogaerts J, et al. Evaluating continuous tumor measurement-based metrics as phase II endpoints for predicting overall survival. J Natl Cancer Inst. 2015;107(11):djv239.
Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16(1):139–44.
Ghadjar P, Hayoz S, Bernhard J, Zwahlen DR, Holscher T, Gut P, et al. Acute toxicity and quality of life after dose-intensified salvage radiation therapy for biochemically recurrent prostate cancer after prostatectomy: first results of the randomized trial SAKK 09/10. J Clin Oncol. 2015;33(35):4158–66.
Bose D, Meric-Bernstam F, Hofstetter W, Reardon DA, Flaherty KT, Ellis LM. Vascular endothelial growth factor targeted therapy in the perioperative setting: implications for patient care. Lancet Oncol. 2010;11(4):373–82.
Martins SF, Garcia EA, Luz MA, Pardal F, Rodrigues M, Filho AL. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genom Proteom. 2013;10(2):55–67.
Sierko E, Wojtukiewicz MZ, Zimnoch L, Thorpe PE, Brekken RA, Kisiel W. Co-localization of prothrombin fragment F1 + 2 and VEGF-R2-bound VEGF in human colon cancer. Anticancer Res. 2011;31(3):843–7.
Eisen T, Joensuu H, Nathan PD, Harper PG, Wojtukiewicz MZ, Nicholson S, et al. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: a single-group phase 2 trial. Lancet Oncol. 2012;13(10):1055–62.
Peterson JE, Zurakowski D, Italiano JE Jr, Michel LV, Connors S, Oenick M, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis. 2012;15(2):265–73.
Yuge R, Kitadai Y, Shinagawa K, Onoyama M, Tanaka S, Yasui W, et al. mTOR and PDGF pathway blockade inhibits liver metastasis of colorectal cancer by modulating the tumor microenvironment. Am J Pathol. 2015;185(2):399–408.
Park M, Kim WK, Song M, Park M, Kim H, Nam HJ, et al. Protein kinase C-delta-mediated recycling of active KIT in colon cancer. Clin Cancer Res. 2013;19(18):4961–71.
Guan ZZ, Xu JM, Luo RC, Feng FY, Wang LW, Shen L, et al. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin J Cancer. 2011;30(10):682–9.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Van Cutsem E, de Haas S, Kang YK, Ohtsu A, Tebbutt NC, Ming XuJ, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a biomarker evaluation from the AVAGAST randomized phase III trial. J Clin Oncol. 2012;30(17):2119–27.
Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–37.
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5(10):835–44.
Al-Marrawi MY, Saroya BS, Brennan MC, Yang Z, Dykes TM, EI-Deiry WS. Off-label use of cetuximab plus sorafenib and panitumumab plus regorafenib to personalize therapy for a patient with V600E BRAF-mutant metastatic colon cancer. Cancer Biol Ther. 2013;14(8):703–10.
Wang ZH, Li Q, Ruan SQ, Xiao Q, Liu Y, Hu YT, et al. Sunitinib mesylate inhibits proliferation of human colonic stromal fibroblasts in vitro and in vivo. J Zhejiang Univ Sci B. 2014;15(8):701–12.
Schmoll HJ, Cunningham D, Sobrero A, Karapetis CS, Rougier P, Koski SL, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III). J Clin Oncol. 2012;30(29):3588–95.
Goldstein D, Rosenberg JE, Figlin RA, Townsend RR, McCann L, Carpenter C, et al. Is change in blood pressure a biomarker of pazopanib and sunitinib efficacy in advanced/metastatic renal cell carcinoma? Eur J Cancer. 2016;53:96–104.
Authors’ contributions
LS and RHX conceived and designed the trial. RHX, LS, KMW, GW, CMS, KFD, LZL, JWW, JPX, CPW, JL, YPL, DW, YB, JPF, YXB, JWB, LWM and JL collected the data. RHX, LS, KMW, GW, CMS, KFD, LZL, JWW, JPX, CPW, JL, YPL, DW, YB, JPF, YXB, JWB, LWM, JL and HY analyzed and interpreted the data. All authors drafted and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank investigators, patients, and their families.
Competing interests
Q. Yang is an employee of Jiangsu Hengrui Medicine Co., Ltd. All remaining authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from Jiangsu Hengrui Medicine Co., Ltd. on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The clinical trial protocol was approved by the institutional review board (IRB) of each center. Each subject provided written informed consent before enrollment.
Funding
The trial was supported by Jiangsu Hengrui Medicine Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xu, RH., Shen, L., Wang, KM. et al. Famitinib versus placebo in the treatment of refractory metastatic colorectal cancer: a multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial. Chin J Cancer 36, 97 (2017). https://doi.org/10.1186/s40880-017-0263-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40880-017-0263-y