Abstract
Background
We report the final results of the randomized phase 2 FIGHT trial that evaluated bemarituzumab, a humanized monoclonal antibody selective for fibroblast growth factor receptor 2b (FGFR2b), plus mFOLFOX6 in patients with FGFR2b-positive (2 + /3 + membranous staining by immunohistochemistry), HER-2–negative gastric or gastroesophageal junction cancer (GC).
Methods
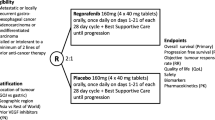
Patients received bemarituzumab (15 mg/kg) or placebo once every 2 weeks with an additional bemarituzumab (7.5 mg/kg) or placebo dose on cycle 1 day 8. All patients received mFOLFOX6. The primary endpoint was investigator-assessed progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate, and safety. Efficacy was evaluated after a minimum follow-up of 24 months.
Results
In the bemarituzumab-mFOLFOX6 (N = 77) and placebo-mFOLFOX6 (N = 78) arms, respectively, 59.7% and 66.7% of patients were FGFR2b-positive in ≥ 10% of tumor cells. The median PFS (95% confidence interval [CI]) was 9.5 months (7.3–13.7) with bemarituzumab-mFOLFOX6 and 7.4 months (5.7–8.4) with placebo-mFOLFOX6 (hazard ratio [HR], 0.72; 95% CI 0.49–1.08); median OS (95% CI) was 19.2 (13.6–24.2) and 13.5 (9.3–15.9) months, respectively (HR 0.77; 95% CI 0.52–1.14). Observed efficacy in FGFR2b-positive GC in ≥ 10% of tumor cells was: PFS: HR 0.43 (95% CI 0.26–0.73); OS: HR 0.52 (95% CI 0.31–0.85). No new safety findings were reported.
Conclusions
In FGFR2b-positive advanced GC, the combination of bemarituzumab-mFOLFOX6 led to numerically longer median PFS and OS compared with mFOLFOX6 alone. Efficacy was more pronounced with FGFR2b overexpression in ≥ 10% of tumor cells. Confirmatory phase 3 trials are ongoing (NCT05052801, NCT05111626).
Clinical trial registration
NCT03694522.
Similar content being viewed by others
Introduction
Gastric cancer (GC), including gastroesophageal junction (GEJ) cancer, is often diagnosed at an advanced stage and is associated with poor prognosis [1]. Systemic chemotherapy has been the standard first-line treatment for advanced GC, although the clinical benefits have been limited [2, 3]. Recent therapeutic approaches including immune checkpoint inhibitors or targeted therapies that are directed towards different mechanistic pathways of GC have demonstrated promising outcomes, especially in biomarker-enriched patient populations [4, 5]. Human epidermal growth factor receptor 2 (HER-2)–directed therapies such as trastuzumab and trastuzumab deruxtecan have improved overall survival (OS) outcomes in HER-2–positive GC [6, 7]. However, most targeted therapies, such as bevacizumab, everolimus, or panitumumab and cetuximab, have not demonstrated OS benefits in unselected patients with GC, which has been attributed, in part, to intratumoral heterogeneity or a lack of selective biomarkers [4, 5]. Treatment decisions are therefore best guided by biomarker expression and histological classifications to preselect patients most likely to benefit from targeted therapies and adapt treatments to help improve outcomes for GC. New and effective biomarker-targeted treatment options remain an unmet clinical need for advanced GC.
The fibroblast growth factor/FGF receptor (FGF/FGFR) pathway plays a crucial role in the growth and development of cancer cells, and overexpression of proteins in this pathway could lead to disease progression [8]. The IIIb splice isoform of FGFR2 (FGFR2b) was observed to be overexpressed in approximately 30% of HER-2–negative GC [9,10,11,12]. FGFR2b overexpression in GC may be associated with poorly differentiated diffuse-type histology and poor outcomes, including lower OS, and warrants further investigation [10, 11, 13].
Bemarituzumab is a first-in-class, humanized monoclonal antibody specific to human FGFR2b that blocks FGF binding to the receptor. Bemarituzumab acts through a two-pronged approach [14]. First, it selectively inhibits FGFR2b signaling with downstream effects on cancer cell proliferation [14, 15]. Second, the afucosylated structure of bemarituzumab leads to activation of FcγRIIIa/CD16a, which increases the affinity of bemarituzumab for natural killer cells, thereby enhancing its antibody-dependent cellular cytotoxicity against FGFR2b-expressing tumor cells [14, 16].
The first-in-human (FIH) study of bemarituzumab monotherapy showed that it was well tolerated and demonstrated activity as later-line therapy in patients with advanced GC, with a confirmed objective response rate (ORR) of 17.9% (95% confidence interval [CI], 6.1–36.9) in 28 patients with FGFR2b-positive tumors and a median duration of response of 12.6 weeks (range, 9.1–19.1) [17]. The phase 1/2 FIGHT trial (NCT03694522) evaluated the efficacy and safety of bemarituzumab in combination with modified 5-fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) chemotherapy as first-line treatment for HER-2 non-positive advanced GC with FGFR2b overexpression and/or FGFR2 gene amplification [18]. The phase 1 safety run-in was an open-label, dose-escalation study of bemarituzumab-mFOLFOX6 in patients with gastrointestinal tumors. The randomized phase 2 study further assessed the efficacy and safety of the combination versus placebo-mFOLFOX6 in HER-2 non-positive FGFR2-selected advanced GC [9]. The primary analysis (median follow-up, 10.9 months) showed that the median progression-free survival (PFS) was 9.5 months (95% CI 7.3–12.9) with bemarituzumab-mFOLFOX6 versus 7.4 months (95% CI 5.8–8.4) with placebo-mFOLFOX6 (hazard ratio [HR], 0.68; 95% CI 0.44–1.04; P = 0.07) [9]. Here, we report the final analysis and updated safety results after a minimum follow-up of 24 months.
Methods
Study design
The study design and protocol have been described previously [9]. Briefly, the phase 2 portion was a randomized, double-blind, placebo-controlled study conducted at 164 sites across 18 countries and was designed to evaluate the efficacy and safety of bemarituzumab-mFOLFOX6 in patients with advanced HER-2 non-positive GC prescreened for FGFR2b overexpression (via immunohistochemistry [IHC]) and/or FGFR2 gene amplification (via circulating tumor DNA [ctDNA] assay). Positive FGFR2b overexpression status by IHC was defined as exhibiting any moderate (2 +) to strong (3 +) membranous staining in more than 0% of tumor cells. Patients were randomized (1:1) to receive either bemarituzumab-mFOLFOX6 or placebo-mFOLFOX6. Treatment continued until disease progression, as determined by Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1), or unacceptable toxicity. During the long-term follow-up period, patients were contacted every 3 (± 1) months for 24 months after the last patient was enrolled, or until death, loss to follow-up, consent withdrawal, or study termination, whichever occurred first. Data cutoff for this final analysis was May 13, 2022.
All patients provided written and informed consent; study protocols (online only) received institutional approval. Complete procedural details are available in the protocol (online only).
Patients
Eligible patients were ≥ 18 years and had histologically confirmed GC or GEJ adenocarcinoma; unresectable, locally advanced/metastatic disease not amenable to curative therapy; FGFR2b overexpression as determined by a centrally performed IHC tissue test and/or FGFR2 gene amplification via a centrally performed ctDNA blood-based assay; measurable or non-measurable, but evaluable disease using RECIST v1.1; an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; adequate organ function; and no prior chemotherapy for metastatic or unresectable disease. Patients were permitted, at the discretion of the investigator, to receive a single dose of mFOLFOX6 while awaiting results of centralized FGFR2 testing. Key exclusion criteria included untreated or symptomatic central nervous system metastases; ≥ grade 2 Common Terminology Criteria for Adverse Events (CTCAE) peripheral sensory neuropathy; corneal abnormalities that may increase the risk of developing a corneal ulcer; and known tumor positivity for HER-2 (as identified by a positive IHC test of 3 + or IHC of 2 + with positive fluorescent in situ hybridization). Complete eligibility criteria are available in the online protocol.
Treatment and randomization
Bemarituzumab or an equivalent placebo was administered at a dose of 15 mg/kg body weight intravenously every 2 weeks (Q2W); an additional dose of 7.5 mg/kg bemarituzumab was administered on cycle 1 day 8. The standard mFOLFOX6 regimen, comprising oxaliplatin (85 mg/m2), leucovorin (400 mg/m2), and 5-fluorouracil (400 mg/m2 bolus followed by 2400 mg/m2 over approximately 48 h), was administered Q2W in both arms. Eligible patients were stratified by geographic region, prior treatment status (de novo versus adjuvant/neoadjuvant), and administration of a single dose of mFOLFOX6 before enrollment (yes or no). A permuted block scheme with a block size of four was used for randomization to ensure an equal sample size and a similar distribution of stratification factors.
Endpoints and assessments
The primary endpoint was PFS, defined as the time from randomization until disease progression based on investigator assessment (using RECIST v1.1) or death, whichever occurred first. Secondary endpoints included OS, defined as the time from randomization until death from any cause; ORR, defined as the proportion of patients with partial response (PR) or complete response (CR) according to investigator assessment of tumor lesions per RECIST v1.1; and incidence of adverse events (AEs). An exploratory endpoint was duration of response (DOR) limited to patients who were responders to treatment, as determined by the investigator per RECIST v1.1, and defined as the time of first response to progression or death from any cause, whichever occurred first. AEs were coded using Medical Dictionary for Regulatory Activities version 25.0 and were graded using CTCAE version 5.0.
Statistical analysis
The planned enrollment for phase 1 was up to 21 patients, and up to 155 patients were to be enrolled in phase 2, with a total enrollment of approximately 167 patients in the study. Efficacy was assessed in the intention-to-treat (ITT) population, which included all randomized patients, while safety was assessed in the safety analysis set, which included patients who had received at least one dose of the assigned treatment. The Kaplan–Meier method was used to estimate the median PFS and OS and the associated 95% CIs in each treatment arm. HRs and 95% CIs were calculated using a stratified Cox regression model. Formal hypothesis testing was not performed at this final analysis. In a prespecified exploratory analysis, PFS and OS were assessed for subgroups of patients who were FGFR2b-positive in ≥ 10% of tumor cells with moderate to strong staining intensity (2 + /3 +) as assessed by IHC. Data on AEs are presented descriptively by number of patients and frequency. Statistical analyses are detailed in the protocol (online only).
Results
Patients
Of the 910 patients prescreened for FGFR2b-positivity, 155 were eligible and were randomized to receive bemarituzumab-mFOLFOX6 (N = 77) or placebo-mFOLFOX6 (N = 78) as part of the ITT analysis set (Fig. 1). The safety analysis set included 153 patients (bemarituzumab-mFOLFOX6, N = 76; placebo-mFOLFOX6, N = 77) who received at least one dose of the study treatment (Fig. 1). At the data cutoff for this final analysis (May 13, 2022), all patients had discontinued the study. The most frequent reasons for treatment discontinuation in the bemarituzumab-mFOLFOX6 arm were AEs (31 [40.3%]) and radiographic disease progression (27 [35.1%]; clinical progression, n = 4), while in the control arm it was radiographic disease progression (46 [59.0%]) (Fig. 1). The primary reason for study discontinuation was death (bemarituzumab-mFOLFOX6, 53 [68.8%]; placebo-mFOLFOX6, 54 [69.2%]), with the most common cause for death being disease progression (bemarituzumab-mFOLFOX6, 39 [50.6%]; placebo-mFOLFOX6, 46 [59.0%]).
Patient disposition. aPatient chose to discontinue treatment but continued in follow-up. bRadiographic disease progression was assessed as per RECIST version 1.1. AE, adverse event; mFOLFOX6, modified FOLFOX (infusional 5-fluorouracil, leucovorin, and oxaliplatin); RECIST, Response Evaluation Criteria in Solid Tumors
Baseline demographics and characteristics were generally balanced between the treatment arms and were reported previously [9]. Of the 155 enrolled patients, 111 (71.6%) were male, and the median (range) age was 60 (23–84) years; 137 (88.4%) had gastric adenocarcinoma and 18 (11.6%) had GEJ adenocarcinoma; 53 (34.2%) and 102 (65.8%) had ECOG performance status of 0 and 1, respectively; 145 (93.5%) had stage IV disease at screening (bemarituzumab-mFOLFOX6, 75 [97.4%]; placebo-mFOLFOX6, 70 [89.7%]); and 71 (45.8%) had received a single dose of mFOLFOX6 before randomization (Table 1). FGFR2b overexpression in ≥ 10% of tumor cells as assessed by IHC (moderate [2 +] to strong [3 +] tumor staining intensity; FGFR2b ≥ 10% subgroup) and regardless of ctDNA gene amplification was found in 46 (59.7%) patients in the bemarituzumab-mFOLFOX6 arm and 52 (66.7%) patients in the placebo-mFOLFOX6 arm. The median duration of exposure was 24.0 weeks (range, 2.0–96.9) in the bemarituzumab-mFOLFOX6 arm and 26.0 weeks (range, 2.0–130.7) in the placebo-mFOLFOX6 arm.
Progression-free survival
At the data cutoff, the median PFS follow-up time was 6.8 months (range, 0–35.9). PFS events were observed in 49 (63.6%) patients in the bemarituzumab-mFOLFOX6 arm and 61 (78.2%) patients in the placebo-mFOLFOX6 arm. The median PFS was 9.5 months (95% CI 7.3–13.7) in the bemarituzumab-mFOLFOX6 arm and 7.4 months (95% CI 5.7–8.4) in the placebo-mFOLFOX6 arm (HR 0.72; 95% CI 0.49–1.08) (Fig. 2a), with a 12-month estimated PFS rate of 45.5% (95% CI 32.7–57.5) and 22.2% (95% CI 12.8–33.3), respectively.
PFS and OS in the ITT population. a PFS in the ITT population. b OS in the ITT population. The intention-to-treat population included all patients who underwent randomization. HRs and 95% CIs were calculated using the Cox proportional hazards model, adjusted for randomization stratification factors, including administration of mFOLFOX6 single dose prior to randomization and geographical region. Vertical bars indicate censoring. CI confidence interval; HR hazard ratio; ITT intention-to-treat; mFOLFOX6 modified FOLFOX (infusional 5-fluorouracil, leucovorin, and oxaliplatin); OS overall survival; PFS progression-free survival
Overall survival
At the data cutoff, the median OS follow-up time was 13.5 months (range, 0–40.5). Fifty-three (68.8%) patients in the bemarituzumab-mFOLFOX6 arm and 54 (69.2%) patients in the mFOLFOX6 and placebo-mFOLFOX6 arm died. The median OS was 19.2 months (95% CI 13.6–24.2) with bemarituzumab-mFOLFOX6 and 13.5 months (95% CI 9.3–15.9) with placebo-mFOLFOX6 (HR 0.77; 95% CI 0.52–1.14) (Fig. 2b). The OS landmarks in the bemarituzumab-mFOLFOX6 and placebo-mFOLFOX6 arms, respectively, were 66.3% and 56.5% at 12 months, and 39.7% and 28.6% at 24 months.
Response rate
Overall, 66 (85.7%) patients in the bemarituzumab-mFOLFOX6 arm and 60 (76.9%) in the placebo-mFOLFOX6 arm had measurable disease at baseline. In the bemarituzumab-mFOLFOX6 arm, the ORR was 48.1% (95% CI 36.5, 59.7), with a CR and PR in 4 (5.2%) and 33 (42.9%) patients, respectively (Table 2). In the placebo-mFOLFOX6 arm, the ORR was 33.3% (95% CI 23.1, 44.9), with a CR and PR in 2 (2.6%) and 24 (30.8%) patients, respectively. The median DOR was 11.9 months (95% CI 6.9, 17.3) with bemarituzumab-mFOLFOX6 (n = 37) and 7.5 months (95% CI 4.3, 13.8) with placebo-mFOLFOX6 (n = 26).
Efficacy for patients in FGFR2b ≥ 10% subgroup
The baseline demographics and disease characteristics were balanced between treatment arms for the ≥ 10% subgroup (data not shown). A prespecified subgroup efficacy analysis was performed for patients in the FGFR2b ≥ 10% subgroup. In these patients, the median PFS was 14.0 months (95% CI 7.2–19.0) with bemarituzumab-mFOLFOX6 and 7.3 months (95% CI 5.4–8.2) with placebo-mFOLFOX6 (HR 0.43; 95% CI 0.26–0.73) (Fig. 3a), with a 12-month estimated PFS rate of 54.4% (95% CI 36.6–69.2) and 17.8% (95% CI 7.9–31.0), respectively. The median OS was 24.7 months (95% CI 14.2–30.1) with bemarituzumab-mFOLFOX6 and 11.1 months (95% CI 8.4–13.8) with placebo-mFOLFOX6 (HR 0.52; 95% CI 0.31–0.85) (Fig. 3b). The OS landmarks for the bemarituzumab-mFOLFOX6 and placebo-mFOLFOX6 arms, respectively, were 71.5% and 49.2% at 12 months, and 51.3% and 21.3% at 24 months. In the bemarituzumab-mFOLFOX6 arm, the ORR was 56.5% (95% CI 41.1–71.1), with a CR and PR in 2 (4.3%) and 24 (52.2%) patients, respectively (Table 2). In the placebo-mFOLFOX6 arm, the ORR was 36.5% (95% CI 23.6–51.0), with a CR and PR in 1 (1.9%) and 18 (34.6%) patients, respectively. The best percentage change in tumor size from baseline is shown in Supplementary Fig. 1.
PFS and OS in the FGFR2b ≥ 10% subgroup. a PFS in the FGFR2b ≥ 10% subgroup. b OS in the FGFR2b ≥ 10% subgroup. The FGFR2b ≥ 10% subgroup included patients with FGFR2b tumor staining score of 2 + or 3 + in at least 10% of tumor cells by immunohistochemistry. HRs and 95% CIs were calculated using the unstratified Cox proportional hazards model. Vertical bars indicate censoring. CI confidence interval; FGFR2b IIIb splice isoform of fibroblast growth factor receptor 2; HR hazard ratio; mFOLFOX6 modified FOLFOX (infusional 5-fluorouracil, leucovorin, and oxaliplatin); OS overall survival; PFS progression-free survival
Subsequent therapies
In all randomized patients, subsequent therapy after progression was well balanced between treatment arms, with 44 (57.1%) patients receiving at least one other line of therapy in the bemarituzimab-mFOLFOX6 arm versus 45 (57.7%) patients in the placebo-mFOLFOX6 arm (Supplementary Table 1). The most common agents received were taxanes (41.3%), the vascular endothelial growth factor/receptor (VEGF/VEGFR) inhibitor ramucirumab (25.2%), the topoisomerase 1 (TOP1) inhibitor irinotecan (20.6%), and programmed death receptor 1 (PD-1) or its ligand (PD-L1) inhibitors (11.6%). In the FGFR2b ≥ 10% subgroup, 28 (60.9%) and 27 (51.9%) patients received at least one new anticancer therapy in the bemarituzumab-mFOLFOX6 and placebo-mFOLFOX6 arms, respectively (Supplementary Table 2). The most common agents received were taxanes (40.8%), the VEGF/VEGFR inhibitor ramucirumab (27.6%), the TOP1 inhibitor irinotecan (17.3%), and PD-1/PD-L1 inhibitors (11.2%).
Safety
All 76 patients in the bemarituzumab-mFOLFOX6 arm and 76 (98.7%) patients in the placebo-mFOLFOX6 arm had at least one treatment-emergent AE (TEAE; Table 3). At least one grade ≥ 3 TEAE occurred in 63 (82.9%) and 58 (75.3%) patients in the bemarituzumab-mFOLFOX6 and placebo-mFOLFOX6 arms, respectively (Table 3). TEAEs related (TRAEs) to any study agent were reported in 72 (94.7%) and 73 (94.8%) patients in the bemarituzumab-mFOLFOX6 and placebo-mFOLFOX6 arms, respectively. TEAEs leading to discontinuation of bemarituzumab or placebo were reported in 31 (40.8%) and 4 (5.2%) patients in the bemarituzumab-mFOLFOX6 and placebo-mFOLFOX6 arms, respectively (Table 3). Corneal AEs accounted for the majority of TEAEs that led to discontinuation of bemarituzumab (24/31 [77.4%] patients), whereas no patients discontinued treatment due to corneal AEs in the placebo-mFOLFOX6 arm. TEAEs led to dose reductions and dose delays of bemarituzumab/placebo in 9 (11.8%) and 51 (67.1%) patients, respectively, in the bemarituzumab-mFOLFOX6 arm, and in 7 (9.1%) and 41 (53.2%) patients in the placebo-mFOLFOX6 arm.
Serious TEAEs occurred in 26 (34.2%) and 28 (36.4%) patients in the bemarituzumab-mFOLFOX6 and placebo-mFOLFOX6 arms, respectively. Fatal TEAEs were reported in 5 (6.6%) patients in the bemarituzumab-mFOLFOX6 arm and in 4 (5.2%) patients in the placebo-mFOLFOX6 arm.
Any-grade corneal AEs were reported in 51 (67.1%) patients in the bemarituzumab-mFOLFOX6 arm, with a median time to onset of 16.9 weeks (interquartile range [IQR], 10.1–24.0), and in 8 (10.4%) patients in the placebo-mFOLFOX6 arm, with a median time to onset of 11.6 weeks (IQR, 7.7–16.6); Grade 3 corneal AEs were reported in 21 (27.6%) patients in the bemarituzumab-mFOLFOX6 arm and none were reported in the placebo-mFOLFOX6 arm. No serious or grade ≥ 4 corneal AEs were observed in either arm. In the bemarituzumab-mFOLFOX6 arm, corneal AEs resolved in 27 patients, with a median time to resolution of 24.4 weeks (IQR, 13.0–41.1), and the median time to resolution or downgrade to grade 1 (from grade ≥ 2) was 20.3 weeks (IQR, 9.1–31.1) in 22 patients. In the placebo-mFOLFOX6 arm, corneal AEs resolved in two patients, with a median time to resolution of 1.4 weeks (IQR, 0.9–2.0), and the median time to resolution or downgrade to grade 1 (from grade ≥ 2) was 2.0 weeks (IQR, 2.0–2.0) in one patient. All corneal AEs resolved in 23 (30.3%) patients in the bemarituzumab-mFOLFOX6 arm and in 3 (3.9%) patients in the placebo-mFOLFOX6 arm. Commonly reported corneal TEAEs and time to resolution are summarized in Table 4.
Discussion
This randomized, double-blind, placebo-controlled phase 2 trial was designed to evaluate the bemarituzumab-mFOLFOX6 combination in patients with HER-2 non-positive, FGFR2b-selected, treatment-naïve advanced GC. Because the study design was changed from a confirmatory phase 3 to a phase 2 study, it was not powered to assess statistically significant improvements in PFS and OS [9]. The primary analysis showed a clinically meaningful improvement in PFS with bemarituzumab-mFOLFOX6 treatment compared with placebo-mFOLFOX6 (HR 0.68; 95% CI 0.44–1.04; P = 0.07) [9]. At this final analysis conducted after a minimum follow-up of 24 months, bemarituzumab-mFOLFOX6 treatment continued to show promising clinical efficacy and manageable toxicities versus placebo-mFOLFOX6 in FGFR2b-positive advanced GC. The benefits in PFS (HR 0.43; 95% CI 0.26–0.73), OS (HR 0.52; 95% CI 0.31–0.85), and ORR (difference between arms in ORR 20.0%; 95% CI 0.6–39.4) with bemarituzumab-mFOLFOX6 versus placebo-mFOLFOX6 were more pronounced in the FGFR2b ≥ 10% subgroup than in the overall population. No new safety signals were reported. Adequately powered phase 3 confirmatory studies are ongoing to determine whether there are statistically significant improvements in efficacy with this combination strategy.
In the ITT population, the HR for OS at the primary analysis was 0.58 (95% CI 0.35–0·95), and was 0.77 (95% CI 0.52–1.14) at this final analysis [9]. This final analysis HR for OS is similar to point estimates recently reported in successful phase 3 trials involving similar control arms, such as the KEYNOTE-859 (HR 0.78; 95% CI 0.70–0.87), CheckMate 649 (HR 0.79; 95% CI 0.71–0.88), SPOTLIGHT (HR 0.75; 95% CI 0.60–0.94), GLOW (HR 0.76; 95% CI 0.35–1.64), and RATIONALE 305 (HR 0.74; 95% CI 0.59–0.94) trials [19,20,21,22,23]. Compared with the primary analysis, the treatment benefit with bemarituzumab-mFOLFOX6 at this final analysis could have been weakened due to a potential impact of subsequent anticancer therapies on OS reflected in the additional follow-up. In the FGFR2b ≥ 10% subgroup, the HR for OS at the primary analysis increased from 0.41 to 0.52 at this final analysis—a finding that still supports promising activity for bemarituzumab in this subset, with a noteworthy 2-year OS rate in bemarituzumab-mFOLFOX6 more than twice that of placebo-mFOLFOX6 (51.3% versus 21.3%). Collectively, together with the FIH dose-escalation/dose-expansion study showing bemarituzumab single-agent activity in an FGFR2b selected gastroesophageal adenocarcinoma population, these data support FGFR2b as a potentially important predictor of response to treatment with bemarituzumab [17].
The safety results were generally similar to those reported at the primary analysis. No new safety findings were observed with longer follow-up. Treatment discontinuation due to TEAEs was higher in the bemarituzumab-mFOLFOX6 arm compared with the placebo-mFOLFOX6 arm. Corneal TEAEs were the primary reason for treatment discontinuation in the bemarituzumab-mFOLFOX6 arm, which may not necessarily be due to the severity of the AE, but could be an unintended outcome of the protocol design wherein no prophylaxis was mandated and treatment was discontinued for any corneal TEAE that was not resolved or improved to grade 1 within 28 days of treatment initiation [9]. In addition, the median time to onset of grade ≥ 2 corneal events was longer than that for any-grade corneal events (23.7 weeks versus 16.9 weeks), suggesting a possible opportunity for early recognition and active management of AEs by prophylactic measures in future studies. Accordingly, the use of ocular lubricants and eyelid hygiene are being assessed in the ongoing phase 3 studies, which also exclude the 28-day requirement for corneal AE resolution in their study designs [24, 25].
This final analysis should be interpreted considering its strengths and limitations. One limitation of this study is the relatively small sample size. In addition, no prophylaxis or mitigation for corneal toxicity was included as a part of the study design. However, this study has several strengths. First, the trial prospectively enrolled a biomarker-selected population, with evaluation of biomarker-enriched subgroups such as those with ≥ 10% of tumor cells expressing FGFR2b. Second, the use of one optional cycle of “induction” FOLFOX is a novel aspect of this trial that facilitates the inclusion of an upfront biomarker-driven cohort of patients and may be used as a template for other upfront biomarker-driven studies in the future. Third, a long minimum follow-up duration of 24 months permitted sufficient follow-up of PFS and OS endpoint outcomes. Lastly, the consistent treatment benefits with respect to all efficacy endpoints in the FGFR2b ≥ 10% subgroup further support the hypothesis that increased scrutinization of FGFR2b as a biomarker may lead to increased efficacy. This analysis is similar to the subset analysis of the ToGA trial which identified that patients with higher HER-2 expression (3 + by IHC) received enhanced benefit from the trastuzumab-chemotherapy combination, reinforcing the concept that increased protein expression of an IHC biomarker can predict greater efficacy [7].
At this final analysis, bemarituzumab-mFOLFOX6 treatment continued to demonstrate promising clinical efficacy and manageable safety in FGFR2-selected, HER-2 non-positive advanced GC, with more pronounced treatment benefit in the patient subset with FGFR2b overexpression (2 + /3 + staining) in ≥ 10% of tumor cells than in those with FGFR2b overexpression in any tumor cell. These data are encouraging and support further evaluation of the bemarituzumab-mFOLFOX6 combination in patients with FGFR2b overexpressed gastric and GEJ cancer in the frontline setting. Phase 3 trials are ongoing to confirm this observed clinical benefit, with a focus on enhanced biomarker selection (NCT05052801, NCT05111626) [24, 25].
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: http://www.amgen.com/datasharing.
References
Wagner AD, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8(8):cd004064. https://doi.org/10.1002/14651858.CD004064.pub4. (in English).
Muro K, et al. Pan-Asian adapted ESMO Clinical practice guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):19–33. https://doi.org/10.1093/annonc/mdy502. (in English).
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. https://doi.org/10.3322/caac.20107. (in English).
Apicella M, Corso S, Giordano S. Targeted therapies for gastric cancer: failures and hopes from clinical trials. Oncotarget. 2017;8(34):57654–69. https://doi.org/10.18632/oncotarget.14825. (in English).
Guan W-L, He Y, Xu R-H. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16(1):57. https://doi.org/10.1186/s13045-023-01451-3. (in English).
Shitara K, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30. https://doi.org/10.1056/NEJMoa2004413. (in English).
Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. https://doi.org/10.1016/s0140-6736(10)61121-x. (in English).
Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov. 2013;3(3):264–79. https://doi.org/10.1158/2159-8290.CD-12-0362. (in English).
Wainberg ZA, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022;23(11):1430–40. https://doi.org/10.1016/S1470-2045(22)00603-9. (in English).
Han N, Kim MA, Lee HS, Kim WH. Evaluation of fibroblast growth factor receptor 2 expression, heterogeneity and clinical significance in gastric cancer. Pathobiology. 2015;82(6):269–79. https://doi.org/10.1159/000441149. (in English).
Ahn S, et al. FGFR2 in gastric cancer: protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod Pathol. 2016;29(9):1095–103. https://doi.org/10.1038/modpathol.2016.96. (in English).
Nagatsuma AK, et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer. 2015;18(2):227–38. https://doi.org/10.1007/s10120-014-0360-4. (in English).
Ishiwata T. Role of fibroblast growth factor receptor-2 splicing in normal and cancer cells. Front Biosci (Landmark Ed). 2018;23(4):626–39. https://doi.org/10.2741/4609. (in English).
Xiang H, et al. Preclinical characterization of bemarituzumab, an anti-FGFR2b antibody for the treatment of cancer. MAbs. 2021;13(1):1981202. https://doi.org/10.1080/19420862.2021.1981202. (in English).
Wesche J, Haglund K, Haugsten EM. Fibroblast growth factors and their receptors in cancer. Biochem J. 2011;437(2):199–213. https://doi.org/10.1042/bj20101603. (in English).
Pereira NA, Chan KF, Lin PC, Song Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs. 2018;10(5):693–711. https://doi.org/10.1080/19420862.2018.1466767. (in English).
Catenacci DVT, et al. Phase I escalation and expansion study of bemarituzumab (FPA144) in patients with advanced solid tumors and FGFR2b-selected gastroesophageal adenocarcinoma. J Clin Oncol. 2020;38(21):2418–26. https://doi.org/10.1200/JCO.19.01834. (in English).
Catenacci DV, et al. Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT phase III study design. Future Oncol. 2019;15(18):2073–82. https://doi.org/10.2217/fon-2019-0141. (in English).
Kater AP, et al. Fixed-duration ibrutinib-venetoclax in patients with chronic lymphocytic leukemia and comorbidities. NEJM Evid. 2022;1(7):EVIDoa2200006. https://doi.org/10.1056/EVIDoa2200006. (in English).
Janjigian YY, et al. Nivolumab (NIVO) plus chemotherapy (chemo) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma (GC/GEJC/EAC): 3-year follow-up from CheckMate 649. J Clin Oncol. 2023;41(4):291. https://doi.org/10.1200/JCO.2023.41.4_suppl.291. (in English).
Moehler MH, et al. Rationale 305: Phase 3 study of tislelizumab plus chemotherapy vs placebo plus chemotherapy as first-line treatment (1L) of advanced gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). J Clin Oncol. 2023;41(4):286. https://doi.org/10.1200/JCO.2023.41.4_suppl.286. (in English).
Rha SY, et al. VP1-2023: Pembrolizumab (pembro) plus chemotherapy (chemo) as first-line therapy for advanced HER2-negative gastric or gastroesophageal junction (G/GEJ) cancer: Phase III KEYNOTE-859 study. Ann Oncol. 2023;34(3):319–20. https://doi.org/10.1016/j.annonc.2023.01.006. (in English).
Shitara K, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401(10389):1655–68. https://doi.org/10.1016/S0140-6736(23)00620-7. (in English).
Wainberg ZA, et al. Trial in progress: phase 1b/3 study of bemarituzumab + mFOLFOX6 + nivolumab versus mFOLFOX6 + nivolumab in previously untreated advanced gastric and gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-102). J Clin Oncol. 2022;40(16):TPS4165. https://doi.org/10.1200/JCO.2022.40.16_suppl.TPS4165. (in English).
Smyth EC, et al. Trial in progress: Phase 3 study of bemarituzumab + mFOLFOX6 versus placebo + mFOLFOX6 in previously untreated advanced gastric or gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-101). J Clin Oncol. 2022;40(16):TPS4164. https://doi.org/10.1200/JCO.2022.40.16_suppl.TPS4164. (in English).
Acknowledgements
We thank the patients, investigators, and study staff who contributed to this study. Medical writing support was provided by Shubha Dastidar, PhD, CMPP, and Utkarsha Singh, PhD, of Cactus Life Sciences (part of Cactus Communications), and was funded by Amgen Inc. We thank Erica Sommermann, MEd, PhD (Amgen Inc.) for operational planning assistance. This study was funded by Five Prime Therapeutics, Inc. Five Prime Therapeutics, Inc. is a wholly owned subsidiary of Amgen Inc.
Funding
This study was funded by Five Prime Therapeutics, Inc. Five Prime Therapeutics, Inc. is a wholly owned subsidiary of Amgen Inc.
Author information
Authors and Affiliations
Contributions
Conception and design: ZAW, AZ-K. Provision of study materials or patients: ZAW, Y-KK, K-WL, SQ, KY, I-HK, AS, SCO, JL, HMT, AT, EH, AAU, GGC, RGS, PCE. Data analysis and interpretation: AZ-K, KT. Manuscript writing, final approval of manuscript, accountable for all aspects of the work: All authors.
Corresponding author
Ethics declarations
Conflict of interest
Zev A. Wainberg: Honoraria (self): Amgen, Arcus, AstraZeneca, Daiichi, Bayer, Bristol Myers Squibb (BMS), Merck, Ipsen, Gilead, Arcus, Astellas, Seagen, Novartis; Advisory/Consultancy: Amgen, Arcus, AstraZeneca, Daiichi, Bayer, BMS, Merck, Ipsen, Novartis, Gilead, Arcus, Astellas, Seagen; Research grant/funding to institution: Amgen, AstraZeneca, Daiichi, Bayer, BMS, Merck, Ipsen, Five Prime, Gilead, Arcus, Astellas, Molecular Templates, Roche/Genentech, Array/Pfizer. Yoon-Koo Kang: Consulting fees (self): Amgen, Novartis, Roche, Daehwa, Zymeworks, Blueprint, Surface Oncology, ALX Oncology, MacroGenics, BMS, Merck, LISCure. Keun-Wook Lee: All support for the present manuscript: Five Prime Therapeutics; Grants or contracts from any entity: All to institution for conducting clinical trials—AstraZeneca, Ono Pharmaceutical, Merck Sharp and Dohme, Merck KGaA, Roche, Pfizer, BeiGene, Leap Therapeutics, ALX Oncology, Zymeworks, Astellas, MacroGenics, Amgen, Seagen, Bolt Therapeutics, Trishula Therapeutics, Oncologie, Pharmacyclics, MedPacto, Green Cross Corp, ABL Bio, Y-Biologics, Daiichi Sankyo, Taiho Pharmaceutical, InventisBio, Elevar Therapeutics, Metafines, Idience, Genome & Company, Exelixis; Honoraria for lectures: Ono Pharmaceutical, Boryung, Daiichi Sankyo, Astellas, Sanofi-Aventis; Participation on a data safety monitoring board or advisory board: ALX Oncology, Metafines. Shukui Qin: None to disclose. Kensei Yamaguchi: Research grants: Taiho Pharmaceutical; Speakers bureau: Daiichi Sankyo Co., Ltd., Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., Eli Lilly Japan K.K., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Merck Biopharm Co., Ltd. In-Ho Kim: None to disclose. Anwaar Saeed: Research grants (to institution): AstraZeneca, BMS, Merck, Clovis, Exelixis, Actuate Therapeutics, Incyte Corporation, Daiichi Sankyo, Five Prime Therapeutics, Amgen, Innovent Biologics, Dragonfly Therapeutics, KAHR Medical, and BioNTech; Advisory board fees: AstraZeneca, BMS, Exelixis, Pfizer, and Daiichi Sankyo. Sang Cheul Oh: None to disclose. Jin Li: Research grants: Roche; Speakers bureau: Eli Lilly, AstraZeneca. Haci Mehmet Turk: None to disclose. Alexandra Teixeira: Consulting fees: Gilead, Daiichi; Non-remunerative positions of influence: Member of SPO (Sociedade Portuguesa de Oncologia). Erika Hitre: None to disclose. Adrian A. Udrea: Honoraria: AstraZeneca, BMS, Lilly, Novartis, Sandoz, Teva; Consulting/Advisory role: Amgen, BMS, Teva; Travel, accommodations, expenses: Astellas Pharma, Teva. Giovanni Gerardo Cardellino: None to disclose. Raquel Guardeño Sanchez: Consulting fees: Ipsen, Novartis. Anita Zahlten-Kümeli: Employee and stockholder of Amgen Inc. Kate Taylor: Employee and stockholder of Amgen Inc. Peter C. Enzinger: Consultant: ALX Oncology, Amgen, Arcus Biosciences, Astellas, AstraZeneca, Boehringer Ingelheim, Blueprint Medicines, BMS, Chimeric Therapeutics, Celgene, Coherus, Daiichi Sankyo, IDEAYA, Istari, Legend, Lilly, Loxo, Merck Sharp & Dohme, Novartis, Ono, Servier, Taiho, Takeda, Turning Point Therapeutics, Xencor, Zymeworks.
Ethics statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wainberg, Z.A., Kang, YK., Lee, KW. et al. Bemarituzumab as first-line treatment for locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma: final analysis of the randomized phase 2 FIGHT trial. Gastric Cancer 27, 558–570 (2024). https://doi.org/10.1007/s10120-024-01466-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-024-01466-w