Abstract
Background
Ecological immunology proposes that the optimal immune defence, and the costs coming with it, vary across environments. In environments with higher pathogen load, the immune system should experience greater challenges and, therefore, investment in maintaining it should be higher. The biomarker neopterin allows monitoring of innate immune responses, and is therefore an ideal tool to investigate the effects of ecological variables on the immune system. Here, we compared urinary neopterin levels of apparently healthy chimpanzees without acute symptoms of sickness across two environments: in captivity (22 zoos) and in the wild (two populations).
Results
Our results revealed that urinary neopterin levels were nearly twice as high in wild compared to captive chimpanzees, independent of chimpanzee subspecies.
Conclusion
We conclude that wild chimpanzees experience more frequent immune challenges in comparison to captive individuals. Therefore, wild individuals have to allocate more energy to immune function and away from reproduction and growth. Our data indicate that the generally delayed development of wild animals in comparison to captive individuals might not only be related to lower energy intake but might result from greater energy allocations to immune function. Finally, our data highlight the importance of understanding immune costs for accurate characterization of energy budgets in animals.
Similar content being viewed by others
Background
Life-history theory (LHT) concepts show the demands and challenges that influence immune function in an ecological context. Increased energy allocation to immune function is an investment in maintenance and finally in survival, but leaves less energy for growth or reproduction [1,2,3]. Various assessments of energy allocation to the immune system suggest that maintenance and stimulation of immune functions are energetically expensive [1, 4, 5], thereby imposing a significant burden to organisms [2, 6, 7]. Therefore, life-history patterns among vertebrates have been shaped, among other factors, by general patterns of immunity and immune responses associated with environment-specific pathogens [5].
The mammalian immune system is divided into two major functional categories: innate (or constitutive) and adaptive (or acquired) immunity. Innate immunity consists of primary mechanisms providing the first line of disease defence. Although not fully developed in neonates, innate immunity develops faster than adaptive immunity. Such defences include both physical barriers and biochemical factors—e.g., anatomical and physiological barriers, inflammatory mediators, and cellular components [8]. Cellular components like macrophages are stimulated by interferon–γ and co-stimulated by tumour-necrosis factor and endotoxins. Interferon–γ is released by T-cells after antigen recognition; they release neopterin in a high and constant rate into body fluids. Therefore, neopterin is an ideal marker to monitor the degree of the innate immune response activation [9, 10]. So far, investigations of immune system responses or changes in immune functioning have been performed mainly invasively—e.g., in blood samples in humans, domestic animals, and wild living birds [11,12,13,14]. However, increases in neopterin concentrations in serum during the clinical course of infections are paralleled by the same patterns in urine [15], and the measurement of urinary neopterin levels in non-human primates with a commercial neopterin assay has been recently validated [16, 17].
In humans, many Indigenous people (for definition, please see Gracey & King [18]), experience low standards of health, which are associated with poverty, malnutrition, overcrowding, poor hygiene, and environmental contamination. They are exposed to a high rate of disease-causing infections. Moreover, infections are more frequently fatal in Indigenous people [18]. However, a comparison of human urinary neopterin levels, as an indicator of nonspecific immune system activation, indicates across studies that individuals living in environments with lower levels of pathogen prevalence indeed have lower levels of immune challenge, as indicated by lower urinary neopterin levels [15, 19]. Because the neopterin measurements were run with different methods (for example LC-MS in Fuchs et al. [15] and enzyme-immuno assay in Filteau et al. [19]), and in different laboratories [20], these comparisons must be interpreted with caution. Additionally, vaccination studies in humans indicate differences in natural immune background across human populations. One factor influencing the variation in immune responses to viral vaccines is genetic polymorphisms of the human leukocyte antigen system. However, in rural human populations, successful vaccination requires de-worming of patients prior to the vaccine’s administration [21, 22], suggesting these populations have a constantly activated innate immune system preventing a specific immune response to the vaccination. Indirect evidence of potential health differences across human populations were shown in gut microbiome, which is vital for immune function, where differences in microbiome diversity were found between non-industrialized, rural communities from Africa and South America; and western industrialized populations [23].
Wild primates routinely sustain wounds and encounter infectious disease [24]. They face a diverse array of parasites and pathogens, which potentially reduce host survival and reproductive success [24,25,26,27,28]. However, wild animals have a long evolutionary history with a vast number of naturally occurring microorganisms, shaping their host-pathogen interaction [29]. In contrast, captive individuals are mainly exposed to pathogens of human origin to which they might not be well adapted. Those human pathogens are especially a threat to great ape species due to recent common ancestry [30]. Furthermore, captive animals may also face increased risk from unfamiliar infectious diseases of animal origin and may be more vulnerable to infection due to inadequate nutrition and environmental conditions [31, 32]. Studies comparing immune system components in the same species in different environments are rare and found contrasting results. First evidence for differences in immunity in wild and captive environments was found in a bird species. Some blood markers of immunity such as eosinophil concentrations were higher in wild birds, and some immune markers such as total leukocyte count were comparable in the two environments [33]. In contrast, in a recent study in serum of dolphins, free living individuals had elevated markers related the immunity such as lymphocytes, eosinophils and platelets compared to captive ones [34]. However, all makers measured in the study, whether related to endocrine system or immune system, were in general higher in wild compared to captive living individuals [34], and therefore results have to be interpreted with caution. Additionally, so far all studies used serum samples, which are impossible to collect in most wild living species. Therefore, one of our aims was to establish a marker that allows repeated non-invasive sampling to investigate the effect of immune energetic burden on life-history patterns.
To evaluate the impact of immune system challenges in different environments, we investigated patterns of urinary neopterin levels as a measure for the unspecific immune response in chimpanzees living in 22 zoos and in two natural environments. We examined potential differences in immune response, which might affect energy allocation and thereby influence life-history trade-offs. We predict that urinary neopterin levels are lower in zoo housed chimpanzees because wild living chimpanzees are exposed to a large number of microorganisms, while zoo chimpanzees are treated, vaccinated, and screened against a variety of pathogens [35]. Alternatively, captive chimpanzees may have higher urinary neopterin levels because they are exposed to a higher variety of human pathogens to which they are not adapted.
Urine samples were collected from chimpanzees housed in zoos and wild living chimpanzees coming from two field sites, one West African chimpanzee (Pan troglodytes verus) field site, and one East African chimpanzee (Pan troglodytes schweinfurthii) field site. This comparative approach controls for potentially unusual urinary neopterin levels due to higher pathogen loads at one field site than elsewhere. In addition, this approach controls for potential genetic differences between chimpanzee subspecies, since captive individuals belong to different chimpanzee subspecies or are sub-specific hybrids.
Results
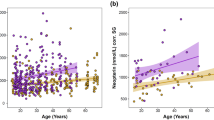
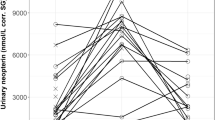
Both mean and median urinary neopterin levels in both wild populations were nearly twice as high as the levels of captive chimpanzees (Table 1). The full/null model comparison (χ2 = 28.608, df = 8, P < 0.001) revealed that the environment was a significant predictor of urinary neopterin levels (Table 2), with significant lower urinary neopterin levels in captive chimpanzees in comparison to wild living individuals (captive vs. Sonso: Estimate = 0.558; SE = 0.122; P < 0.001; captive vs. Taï: Estimate = 0.555; SE = 0.112; P < 0.001; Fig. 1). However, urinary neopterin levels from Sonso were not different from those from Taï (Estimate 0.003; SE 0.118; P = 0.978) (Fig. 1), indicating that urinary neopterin levels of wild chimpanzees do not differ by subspecies or specific pathogens in one field site.
Measures of urinary neopterin levels corrected for creatinine (μmol/mol) from chimpanzees living in captivity (N = 58 samples), Taï (N = 75 samples) and Sonso (N = 45 samples). Individuals: Ncaptivity = 58 (26 males, 32 females), NTaï = 29 (15 males, 14 females), NSonso = 29 (12 males, 17 females). The y-axis is displayed on a log scale. The boxes illustrate the 25th and 75th percentiles, bars indicate medians, and circles indicate outliers
Discussion
The 43% higher urinary neopterin levels in wild chimpanzees most probably do not reflecting acute infections, but suggest a permanently challenged immune system faced with a high diversity of microorganisms and / or a higher disease burden. This assumption is supported by an even further increase in urinary neopterin levels in wild chimpanzees at Taï during a respiratory disease outbreak [36]. However, while there are non-infectious health conditions that lead to elevated neopterin levels such as sepsis, malignancies, autoimmune diseases, heart failure, and coronary artery diseases [37], we do not have any evidence that such conditions created the pattern observed in this study. Independently of the causes of higher neopterin levels in wild chimpanzees, constantly elevated urinary neopterin levels indicate a permanent energetic burden for wild chimpanzees, allocating energy into maintenance, and thereby away from other traits such as growth and reproduction [38, 39].
Developmental differences between wild and captive primates are well documented. For example, captive female chimpanzees reach menarche and give first birth at a younger age than chimpanzees in the wild [40]. Additionally, in wild great apes, tooth eruption is in some teeth two years later than in captivity [41, 42], but ranges overlap extensively [43]. These differences in the pace of development between wild and captive individuals were assumed to be related to constant energy abundance without seasonal variation and inter-individual variation in captive animals [43, 44]. However, differences might also exist in how much of the available energy needs to be allocated into immune function. For example, in humans living in developing countries, children with higher levels of immune activation have impaired growth [45].
Age effects on neopterin levels were found in a number of studies. In humans, children have higher urinary neopterin levels, declining with increasing age, which then increase again with senescence in adulthood. However, this effect might be mainly driven by age-related changes in creatinine clearance rate, since creatinine was used as a correction factor for urine concentration [15]. A pattern of increasing urinary neopterin levels with increasing senescence was found as well in Barbary macaques [46]. Such an effect might also be caused by an increase in chronic health problems with increasing age [47, 48]. However, age dependent effects are less pronounced than changes in clinical neopterin levels changes [15] and might therefore be difficult to find in populations experiencing constant immune challenge. For example, no age effect was found in two studies on urinary neopterin levels in wild chimpanzees [36, 49], and urinary neopterin levels were independent of sex and age as well in this study.
Conclusion
Our data support the idea that environmental gradients create varying selective pressures on immune systems that may result in different optimal life history strategies [50]. Individuals confronted with higher pathogen load or higher microorganism diversity and thereby with higher immune system activation, allocate energy into maintenance and less into other factors like growth and reproduction, which would allow captive individuals to grow faster and to reproduce earlier than wild living animals. Therefore, the measurement of immune system activation is essential for a more accurate characterization of energy budgets of animals and a better understanding of the role of immunity in the evolution of life-history strategies [51].
Methods
We measured urinary neopterin levels in 178 samples of apparently healthy chimpanzees collected at random from two different environments (captive and wild). ‘Apparently healthy’ was defined as a chimpanzee not showing any current chronic or acute symptoms of sickness. The captive environment (22 zoos) was represented by 58 urine samples from 32 females and 26 males, collected between 2006 and 2014. Samples representing the wild environment were collected at two sites, communities of the Taï chimpanzee project in Taï National Park, Côte d’Ivoire (West Africa), and the Sonso community, Budongo Forest, Uganda (East Africa). In Taï, a number of acute diseases, including anthrax and respiratory infections, have been documented [52,53,54], which are absent in Sonso. From the Taï chimpanzees, samples of 14 females (35 samples, average of 2.5 samples per individual) and 15 males (40 samples, average 2.7 samples per individual) collected in 2009 were analysed. From the Sonso community, urine samples collected between 2009 and 2010 from 17 females (23 samples, average 1.4 samples per individual) and 12 males (22 samples, average 1.8 samples per individual) were used. The age range of the captive chimpanzees was between 4 and 53 years (median: 15 years), in Sonso individuals ranged between 11 and 50 years (median: 17 years), and in Taï between 2 and 45 years (median: 15 years). For 49 of the 58 captive chimpanzees the exact birthdate was available from the studbook. For three captive and three Taï chimpanzees, only the year and month of birth were known, and for these we set the day of birth to the 15th of the respective month. For the remaining individuals, only the year of birth was known, and in these cases, the day of birth was set to June 15th of the respective year (all Sonso chimpanzees, 15 Taï chimpanzees, and six captive chimpanzees). All urine samples were non-invasively collected in captivity and in the wild. For details on urine collection protocols, see Behringer et al. [55] for captive samples, Deschner et al. [56] for Taï samples, and Crockford et al. [57] for Sonso samples. After collection, the samples were frozen in the zoo or the field sites, and transported frozen to the Max Planck Institute for Evolutionary Anthropology (MPI-EVA) in Leipzig, Germany.
Urinary neopterin analyses
For the measurement of urinary neopterin levels in chimpanzees, we used a commercial competitive neopterin ELISA (Neopterin ELISA, Ref. RE59321, IBL International GmbH, Hamburg, Germany), validated for the measurement of neopterin in urine of chimpanzees [16]. All urine samples were thawed, vortexed, and centrifuged. The captive chimpanzee urine was diluted 1:100 with the assay buffer of the supplier. Sonso samples were diluted 1:200 or 1:400, and the urine of the Taï chimpanzees 1:400 or 1:600. The assays were performed following the instructions from the supplier and as described in Behringer et al. [16]. All samples, standards, and controls were measured in duplicate. To compensate for variation in volume and concentration of the collected urine, as well as to make the results comparable to human studies [19, 58,59,60,61,62], creatinine was measured, and results are expressed in μmol/mol creatinine.
Statistical analyses
We used a general linear mixed model (GLMM, [63]) to assess the impact of environment (test predictor with fixed effect) on the log transformed response variable urinary neopterin. The model was fitted in R [64] using the function “lmer” provided in the package “lme4” [65]. To control for the potential influence of day time, storage-time, age of the animal at sampling, and the sex of the animal, these parameters were included as control predictors with fixed effects. As a random effect, we included animal ID, and we included random slopes of age at sampling time, storage time, and day time within animal ID, to keep type I error rates at the nominal level of 5% [66, 67]. Age as well as the time of sample collection (to control for diurnal variation) was z-transformed to a mean of zero and a standard deviation of one to achieve comparable estimates [68].
The required normal distribution and homogeneity of residuals for the model were assessed by visual inspections of a histogram, a q-q plot of the residuals, and by plotting residuals against fitted values. All model assumptions were met. We assessed collinearity by determining variance inflation factors (VIF, [69]) using the function “vif” of the R-package “car” [70] based on a standard linear model excluding the random effects, which revealed no problems (maximum VIF: 2).
We investigated the significance of the fixed effect environment, by comparing the full model with a null model, excluding the predictor variable, but retaining time of sample collection, age and sex, and the random effect animal ID, as well as the random slopes component, using a likelihood ratio test [71]; (R function “anova”). To further explore the effect of each specific environment on urinary neopterin levels, we built two additional models, one compared the captive neopterin levels with the levels from Taï and Sonso, and the second model compared the levels of Sonso and captive chimpanzees with Taï, with the same fixed effects, random effect, and random slopes as in the full model. Significance for all tests was set at the P = 0.05 level.
Abbreviations
- EAZA:
-
European Association of Zoos and Aquaria
- EEP:
-
European Endangered Species Programmes
- ELISA:
-
Enzyme-linked Immunosorbent Assay
- ESB:
-
European Studbooks
- GLMM:
-
General linear mixed model
- LC-MS:
-
Liquid chromatography/mass spectrometry
- LHT:
-
Life-history theory
- MPI-EVA:
-
Max Planck Institute for Evolutionary Anthropology
- VIF:
-
Variance inflation factors
References
McDade TW. Life history theory and the immune system: steps toward a human ecological immunology. Am J Phys Anthropol. 2003;122:100–25.
Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–21.
Stearns SC, Koella JC. The evolution of phenotypic plasticity in life-history traits: predictions of reaction norms for age and size at maturity. Int J Org Evol. 1986;40:893–913.
Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Phys. 1997;273(5 Pt 2):R1631–7.
Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98.
Svensson E, RAberg L, Koch C, Hasselquist D. Energetic stress, immunosuppression and the costs of an antibody response. Funct Ecol. 1998;12:912–9.
Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol Evol. 2003;18:27–32.
Actor JK. Introductory immunology: basic concepts for interdisciplinary applications. London: Academic Press Inc; 2014.
Fuchs D, Hausen A, Reibnegger G, Werner ER, Dierich MP, Wachter H. Neopterin as a marker for activated cell-mediated immunity: application in HIV infection. Immunol Today. 1988;9:150–5.
Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–87.
Norris K, Evans MR. Ecological immunology: life history trade-offs and immune defense in birds. Behav Ecol. 2000;11:19–26.
Sadd BM, Schmid-Hempel P. PERSPECTIVE: principles of ecological immunology: principles of ecological immunology. Evol Appl. 2008;2:113–21.
Berdowska A, Zwirska-Korczala K. Neopterin measurement in clinical diagnosis. J Clin Pharm Ther. 2001;26:319–29.
Hamerlinck FFV. Neopterin: a review. Exp Dermatol. 1999;8:167–76.
Fuchs D, Weiss G, Reibnegger G, Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit Rev Clin Lab Sci. 1992;29:307–44.
Behringer V, Stevens JMG, Leendertz FH, Hohmann G, Deschner T. Validation of a method for the assessment of urinary neopterin levels to monitor health status in non-human-primate species. Front Physiol. 2017;8:1–11.
Higham JP, Kraus C, Stahl-Hennig C, Engelhardt A, Fuchs D, Heistermann M. Evaluating noninvasive markers of nonhuman primate immune activation and inflammation. Am J Phys Anthropol. 2015;158:673–84.
Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet. 2009;374:65–75.
Filteau SM, Raynes JG, Simmank K, Wagstaff LA. Vitamin a status does not influence neopterin production during illness or health in south African children. Br J Nutr. 1998;80:75.
Fanson KV, Németh Z, Ramenofsky M, Wingfield JC, Buchanan KL. Inter-laboratory variation in corticosterone measurement: implications for comparative ecological and evolutionary studies. Methods Ecol Evol. 2017;8:1745–54.
Borkow G, Bentwich Z. Chronic parasite infections cause immune changes that could affect successful vaccination. Trends Parasitol. 2008;24:243–5.
Urban JF, Steenhard NR, Solano-Aguilar GI, Dawson HD, Iweala OI, Nagler CR, et al. Infection with parasitic nematodes confounds vaccination efficacy. Vet Parasitol. 2007;148:14–20.
Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, et al. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5. https://doi.org/10.1038/ncomms4654.
Nunn CL. Primate disease ecology in comparative and theoretical perspective. Am J Primatol. 2012;74:497–509.
Altizer S, Nunn CL, Thrall PH, Gittleman JL, Antonovics J, Cunningham AA, et al. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu Rev Ecol Evol Syst. 2003;34:517–47.
Chapman CA, Gillespie TR, Goldberg TL. Primates and the ecology of their infectious diseases: how will anthropogenic change affect host-parasite interactions? Evol Anthropol Issues News Rev. 2005;14:134–44.
Gillespie TR, Chapman CA, Greiner EC. Effects of logging on gastrointestinal parasite infections and infection risk in African primates. J Appl Ecol. 2005;42:699–707.
Nunn CL, Altizer SM. Infectious diseases in primates: behavior, ecology and evolution. Oxford. New York: Oxford University Press; 2006.
Sironi M, Cagliani R, Forni D, Clerici M. Evolutionary insights into host–pathogen interactions from mammalian sequence data. Nat Rev Genet. 2015;16:224–36.
Gilardi KV, Gillespie TR, Leendertz FH, Macfie EJ, Travis DA, Whittier CA, et al., editors. Best practice guidelines for health monitoring and disease control in great ape populations: IUCN International Union for Conservation of Nature; 2015. https://doi.org/10.2305/IUCN.CH.2015.SSC-OP.56.en.
Bais B, Tak L, Mahla S. Study of preventive health measures for wildlife in captivity: a review of management approaches. Int J Avian Wildl Biol. 2017;2. https://doi.org/10.15406/ijawb.2017.02.00020.
Mason GJ. Species differences in responses to captivity: stress, welfare and the comparative method. Trends Ecol Evol. 2010;25:713–21.
Buehler DM, Piersma T, Irene Tieleman B. Captive and free-living red knots Calidris canutus exhibit differences in non-induced immunity that suggest different immune strategies in different environments. J Avian Biol. 2008;39:560–6.
Fair PA, Schaefer AM, Houser DS, Bossart GD, Romano TA, Champagne CD, et al. The environment as a driver of immune and endocrine responses in dolphins (Tursiops truncatus). PLoS One. 2017;12:e0176202.
OIE. Zoonoses transmissible from non-human primates. In: OIE- Terrestiral Manual 2017. 26th ed; 2017. p. 1–5.
Wu DF, Behringer V, Wittig RM, Leendertz FH, Deschner T. Urinary neopterin levels increase and predict survival during a respiratory outbreak in wild chimpanzees (Taï National Park, Côte d’Ivoire). Sci Rep. 2018;8. https://doi.org/10.1038/s41598-018-31563-7.
Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351:17–29.
Bribiescas RG, Ellison PT. How hormones mediate trade-offs in human health and disease. In: Stearns SC, Koella JC, editors. Evolution in health and disease: Oxford University Press; 2007. p. 77–94. https://doi.org/10.1093/acprof:oso/9780199207466.003.0007.
Promislow DEL, Harvey PH. Living fast and dying young: a comparative analysis of life-history variation among mammals. J Zool. 1990;220:417–37.
Atsalis S, Videan E. Reproductive aging in captive and wild common chimpanzees: factors influencing the rate of follicular depletion. Am J Primatol. 2009;71:271–82.
Kelley J, Schwartz GT. Dental development and life history in living African and Asian apes. Proc Natl Acad Sci. 2009;107:1035–40.
Zihlman A, Bolter D, Boesch C. Wild chimpanzee dentition and its implications for assessing life history in immature hominin fossils. Proc Natl Acad Sci U S A. 2004;101:10541–3.
Smith BH, Boesch C. Mortality and the magnitude of the “wild effect” in chimpanzee tooth emergence. J Hum Evol. 2011;60:34–46.
Kimura T, Hamada Y. Growth of wild and laboratory born chimpanzees. Primates. 1996;37:237–51.
Solomons NW, Mazariegos M, Brown KH, Klasing K. The underprivileged, developing country child: environmental contamination and growth failure revisited. Nutr Rev. 1993;51:327–32.
Müller N, Heistermann M, Strube C, Schülke O, Ostner J. Age, but not anthelmintic treatment, is associated with urinary neopterin levels in semi-free ranging barbary macaques. Sci Rep. 2017;7:41973.
Hearps AC, Martin GE, Angelovich TA, Cheng W-J, Maisa A, Landay AL, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function: age-related changes in monocyte phenotype and function. Aging Cell. 2012;11:867–75.
Capuron L, Geisler S, Kurz K, Leblhuber F, Sperner-Unterweger B, Fuchs D. Activated immune system and inflammation in healthy ageing: relevance for tryptophan and neopterin metabolism. Curr Pharm Des. 2014;20:6048–57.
Löhrich T, Behringer V, Wittig RM, Deschner T, Leendertz FH. The use of neopterin as a noninvasive marker in monitoring diseases in wild chimpanzees. EcoHealth. 2018. https://doi.org/10.1007/s10393-018-1357-y.
Billman EJ, Rasmussen JE, Creighton JC, Johnson JB, Belk MC. A multivariate approach to the analysis of within lifetime variation in life history. Methods Ecol Evol. 2014;5:797–805.
Derting TL, Compton S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus). Physiol Biochem Zool. 2003;76:744–52.
Grützmacher KS. Assessing the threat of selected human respiratory viruses to habituated wild gorillas and chimpanzees in sub-saharan africa. Berlin: PhD Thesis. University; 2018.
Köndgen S, Kühl H, N’Goran PK, Walsh PD, Schenk S, Ernst N, et al. Pandemic human viruses cause decline of endangered great apes. Curr Biol. 2008;18:260–4.
Leendertz FH, Ellerbrok H, Boesch C, Couacy-Hymann E, Mätz-Rensing K, Hakenbeck R, et al. Anthrax kills wild chimpanzees in a tropical rainforest. Nature. 2004;430:451–2.
Behringer V, Deschner T, Murtagh R, Stevens JMG, Hohmann G. Age-related changes in thyroid hormone levels of bonobos and chimpanzees indicate heterochrony in development. J Hum Evol. 2014;66:83–8.
Deschner T, Heistermann M, Hodges K, Boesch C. Timing and probability of ovulation in relation to sex skin swelling in wild west African chimpanzees, Pan troglodytes verus. Anim Behav. 2003;66:551–60.
Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbühler K, Deschner T. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc R Soc B Biol Sci. 2013;280:20122765.
Brown AE, Webster HK, Teja-Isavadharm P, Keeratithakul D. Macrophage activation in falciparum malaria as measured by neopterin and interferon-gamma. Clin Exp Immunol. 1990;82:97–101.
Fuchs D, Hausen A, Kofler M, Kosanowski H, Reibnegger G, Wachter H. Neopterin as an index of immune response in patients with tuberculosis. Lung. 1984;162:337–46.
Mura P, Tallineau C, Reiss D, Piriou A. The rapid determination of neopterine in human urine by isocratic high-performance liquid chromatography. J Liq Chromatogr. 1984;7:2289–96.
Schmutzhard E, Fuchs D, Hengster P, Hausen A, Hofbauer J, Pohl P, et al. Retroviral infections (HIV-1, HIV-2, and HTLV-I) in rural northwestern Tanzania clinical findings. Am J Epidemiol. 1989;130:309–18.
Winkler C, Wirleitner B, Werner ER, Fuchs D. Urinary neopterin concentrations in healthy individuals with household contact. Pteridines. 2003;14:34–8.
Baayen RH. Analyzing Linguistic Data. Cambridge, UK. New York: Cambridge University Press; 2008.
R Development Core Team. R. A language and environment for statistical computing. Vienna: R foundation for statistical computing; 2008. http://www.R-project.org
Bates D, Maechler M, Bolker B, Walker S. lme4: linear mixed-effects models using Eigen and S4; 2013.
Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: keep it maximal. J Mem Lang. 2013;68:255–78.
Schielzeth H, Forstmeier W. Conclusions beyond support: overconfident estimates in mixed models. Behav Ecol. 2009;20:416–20.
Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol. 2010;1:103–13.
Field AP. Discovering statistics using SPSS. 3rd ed. Los Angeles: SAGE Publications; 2009.
Fox J. An R companion to applied regression. 2nd ed. Thousand Oaks: SAGE Publications; 2011.
Dobson AJ, Barnett AG. An introduction to generalized linear models. 3rd ed. Boca Raton: CRC Press; 2008.
Acknowledgments
We thank all field assistants and veterinarians for their help in the field. We thank the Ivorian Ministry of Environment and Forests and Ministry of Higher Education and Scientific Research, the Office Ivoirien des Parcs et Reserves of Côte d’Ivoire and the Centre Suisse de Recherches Scientifiques. We are thankful to the Budongo Conservation Field Station and the Ugandan Authorities (UWA, UNCST). Sincere thanks go to the directors, curators, veterinarians, and keepers of the facilities Aalborg Zoo, Artis Zoo, Badoca Safari Park, Bergzoo Halle, Bioparc Valencia, Boras Djurpark, Burgers Zoo, Dudley Zoological Gardens, Fuengirola Zoo, Furuvik, Gdansk Zoo, Givskud Zoo, Kittenberger Zoo, Kristiansand Dyrepark, Leipzig Zoo, Miejski Ogród Zoologiczny w Warszawie, The Tisch Family Zoological Gardens, Zoo am Meer, Zoo Augsburg, Zoo Copenhagen, Zoo Heidelberg, Zoologicka Zahrada Bratislava. The authors are also grateful to Jacob Negrey for editing the manuscript.
Funding
Funding was provided by Max Planck Society as well as the Deutsche Forschungsgemeinschaft (DFG) and conducted as part of the research group “Sociality and Health in Primates” (FOR2136, DE 1135/2–1 and LE1813/10–1). CC received funding from, the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement No 679787).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
VB carried out the lab work and statistical analyses, VB and TD did data analysis and developed the study concept; TD, RMW, JMGS, CC, FHL and KZ collected data and/or provided essential materials; all authors (VB, JMGS, RMW, CC, KZ, FHL, TD) helped to draft the manuscript and approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol for urine sample collection in captive individuals was approved by the authorities of each zoo and supported by the coordinators of the EAZA Western Chimpanzee EEP and Robust Chimpanzee ESB (Frands Carlsen and Tom de Jongh). Permissions to conduct the research in Taï were granted by the Ministries of Research and Environment of Ivory Coast and Office Ivoirien des Parcs et Reserves. The Uganda Wildlife Authority, the Uganda National Council for Science and Technology, and the President’s Office of Uganda gave permission to collecting samples in Sonso.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Behringer, V., Stevens, J.M.G., Wittig, R.M. et al. Elevated neopterin levels in wild, healthy chimpanzees indicate constant investment in unspecific immune system. BMC Zool 4, 2 (2019). https://doi.org/10.1186/s40850-019-0041-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40850-019-0041-1