Abstract
Background
Falls are a common but serious problem in older adults, and may lead to fractures and bleeding. As many factors, such as medication, aging, and comorbid diseases may simultaneously affect fall-related adverse events (AEs) in older adults, we evaluated the association between fall-related AEs and the use of medication, aging, and comorbid diseases using the Japanese Adverse Drug Event Report (JADER) database.
Methods
We analyzed reports of fall-related AEs associated with α-blockers, diuretics, calcium channel blockers, central nervous system (CNS)-active drugs (opioids, benzodiazepines, hypnotics and sedatives, non-selective monoamine reuptake inhibitors, and selective serotonin reuptake inhibitors (SSRI)) in the JADER database using the reporting odds ratio (ROR). For the definition of falls, we used the Preferred Terms of The Medical Dictionary for Regulatory Activities (MedDRA). We used the association rule mining technique to discover undetected associations, such as potential risk factors.
Results
The JADER database comprised 430,587 reports between April 2004 and November 2016. The RORs (95% CI) of α-blockers, diuretics, calcium channel blockers, opioids, benzodiazepines, hypnotics and sedatives, non-selective monoamine reuptake inhibitors, and SSRIs were 1.63 (1.27–2.09), 0.74 (0.63–0.86), 1.26 (1.15–1.38), 0.93 (0.80–1.07), 1.83 (1.68–2.01), 1.55 (1.12–2.14), 2.31 (1.82–2.95), and 2.86 (2.49–3.29), respectively. From the lift value in the association rule mining, the number of administered CNS-active drugs and patient age were associated with fall-related AEs. Furthermore, the scores of lift for patients with herpes zoster administered calcium channel blockers or benzodiazepines and patients with dementia administered benzodiazepines were high.
Conclusion
Our results suggest that the number of administered CNS-active drugs and patient age are both associated with fall-related AEs. We recommend that patients with herpes zoster treated with calcium channel blockers and benzodiazepines be closely monitored for fall-related AEs.
Similar content being viewed by others
Background
Falls are common health events and a serious problem among older adults [1,2,3]. Falls may cause severe fractures, functional decline, decreased activity, and reduction in quality of life [4,5,6,7]. Risk factors for falls among older adults include intrinsic, extrinsic, and environmental factors [8,9,10]. Intrinsic factors are age-related changes in the sensory motor system leading to gait/balance disorder, dysfunctions of the nervous and muscular systems, dizziness/vertigo, postural hypotension, and visual disorders. Extrinsic factors include medications [9, 11,12,13,14].
Several medication classes, including antidepressants, antipsychotics, benzodiazepines, sedative hypnotics, opioids, some cardiac drugs, and diuretics, have been associated with an increased risk of falls [8,9,10,11,12,13,14,15]. The Japan Geriatric Society published guidelines for safe pharmacotherapy in the elderly. Potentially inappropriate medication uses were summarized in the guideline as follows: benzodiazepines, non-benzodiazepines, and anxiolytics associated with falls and related fractures; antidepressants (tricyclic antidepressants) associated with orthostatic hypotension; loop diuretics and α-blockers associated with orthostatic hypotension and falls [16]. The use of benzodiazepines and sedative hypnotics can increase the risk of falls [9, 17,18,19] due to dizziness, sedation, impaired motor coordination, and postural disturbances [20]. Medications affecting the central nervous system (CNS) can cause dizziness and orthostatic hypotension that increases the risk of falls [8, 9, 21, 22].
According to the American Geriatrics Society (AGS) 2015 Updated Beers Criteria, taking 3 or more CNS-active drugs concomitantly increases the risk of falls [15]. On the other hand, polypharmacy can lead to drug interactions and may be an important risk factor for falls [23]. Previous studies have reported an association between polypharmacy and falls [8, 24,25,26,27,28,29], although some studies found no association [25, 30,31,32]. To our knowledge, the detailed association between the number of concomitant CNS-active drugs and falls remains unclear.
The spontaneous reporting system (SRS), such as the US Food and Drug Administration (FDA) adverse event reporting system (FAERS), has been used in pharmacovigilance assessments [33, 34]. Based on the FAERS database, we previously reported that the concomitant use of antipsychotic drugs may increase the risk of hyperglycemic adverse events (AEs) using established pharmacovigilance indexes of the reporting odds ratio (ROR) [35]. The regulatory authority in Japan, the Pharmaceuticals and Medical Devices Agency (PMDA), controls the SRS of the Japanese Adverse Drug Event Report (JADER) database. By assessing the adjusted RORs using the JADER database, we demonstrated that polypharmacy may be more closely associated with an increased risk of renal disorder than hepatic disorder [36].
Association rule mining has been proposed as a new analytical approach for identifying undetected associations between variables in large databases, such as potential risk factors. Recently, this algorithm has been applied to assess the association rules of AEs in the JADER database [37,38,39,40]. As many factors, such as medications, aging, and comorbid diseases, may be simultaneously affecting fall-related AEs in older adults [41,42,43,44], we included these factors in our analysis.
In the present study, we aimed to explore the association between fall-related AEs and the use of medications such as antidepressants, antipsychotics, benzodiazepines, sedative hypnotics, opioids, calcium channel blockers, and diuretics with ROR using the SRS database. To the best of our knowledge, this is the first study to evaluate potential associations among the number of concomitant CNS-active drugs, aging, and fall-related AEs using the association rule mining technique. Furthermore, we explore the association rules among fall-related AEs, the use of medication, and comorbid diseases.
Methods
Data sources
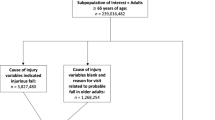
Data from the JADER database between April 2004 and November 2016 were downloaded from the PMDA website (www.pmda.go.jp). The database consists of 4 data tables: patient demographic information (DEMO), drug information (DRUG), AEs (REAC), and primary disease (HIST). We constructed a relational database integrating the 4 tables using FileMaker Pro Advanced 13 (FileMaker, Inc. Santa Clara, CA). The description of age was recorded in the data table of DEMO that includes patient demographic information. The reports were stratified by age as follows: ≤19, 20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, and ≥ 90 years. If the description of age was included as young adults, adults, elderly, first trimester, second trimester, third trimester, or unknown, the patient was excluded because these descriptions could not be categorized into precise 10-year intervals.
Drugs
We used the Anatomical Therapeutic Chemical (ATC) Classification System described by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology for drug definition. All generic names of drugs were verified and subsequently linked with the corresponding ATC classification code. According to the listed drugs in the AGS 2015 Updated Beers Criteria, 98 drugs were selected and categorized into 8 ATC-drug classes: α-blockers (“α-adrenoreceptor antagonists” (ATC code: C02CA, https://www.whocc.no/atc_ddd_index/?showdescription=yes&code=C02CA)); diuretics (“sulfonamides, plain” (ATC code: C03CA, https://www.whocc.no/atc_ddd_index/?code=C03CA), “aryloxyacetic acid derivatives” ATC code: C03CC, https://www.whocc.no/atc_ddd_index/?showdescription=yes&code=C03CC)); calcium channel blockers (“calcium channel blockers” (ATC code: C08, https://www.whocc.no/atc_ddd_index/?code=C08)); opioids (“opioids” (ATC code: N02A, https://www.whocc.no/atc_ddd_index/?code=N02A)); benzodiazepines (“benzodiazepine derivatives” (ATC code: N05CD, https://www.whocc.no/atc_ddd_index/?code=N05CD), “benzodiazepine related drugs” (ATC code: N05CF, https://www.whocc.no/atc_ddd_index/?code=N05CF)); hypnotics and sedatives (“barbiturates, plain” (ATC code: N05CA, https://www.whocc.no/atc_ddd_index/?code=N05CA), “aldehydes and derivatives” (ATC code: N05CC, https://www.whocc.no/atc_ddd_index/?code=N05CC), “melatonin receptor agonists” (ATC code: N05CH, https://www.whocc.no/atc_ddd_index/?code=N05CH), “other hypnotics and sedatives” (ATC code: N05CM, https://www.whocc.no/atc_ddd_index/?code=N05CM)); non-selective monoamine reuptake inhibitors (“non-selective monoamine reuptake inhibitors” (ATC code: N06AA, https://www.whocc.no/atc_ddd_index/?code=N06AA)); SSRI (“selective serotonin reuptake inhibitors” (ATC code: N06AB, https://www.whocc.no/atc_ddd_index/?showdescription=yes&code=N06AB)) (Table 1).
CNS-active drugs were defined in the AGS 2015 Updated Beers Criteria [15]. According to the AGS criteria, we defined CNS-active drugs by combining opioids (ATC code: N02AA, N02AB, N02AC, N02AD, N02AE, N02AF, N02AG, and N02AJ), benzodiazepines (ATC code: N05CD and N05CF), hypnotics and sedatives (ATC code: N05CA, N05CC, N05CH, and N05CM), non-selective monoamine reuptake inhibitors (ATC code: N06AA) and SSRI (ATC code: N06AB) for the association analysis of the number of concomitant CNS-active drugs. For the association analysis of the number of calcium channel blockers, we defined calcium channel blockers (ATC code: C08CA, C08DA, C08DB, and C08EA). In the DRUG table, the causality of each drug was assigned a code according to its association with the AEs, such as a “suspected drug,” “concomitant drug,” or “interacting drug.” Reports with the drug code “suspected drug,” “concomitant drug,” and “interacting drug” were included in this analysis.
Definition of adverse event
AEs in the JADER database are coded with terms in the Medical Dictionary for Regulatory Activities (MedDRA), which is the terminology dictionary used in the JADER database (the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Introductory Guide MedDRA Version 19.0). We extracted reports of fall-related AEs using the following preferred terms (PT): fall (PT code: 10016173), dizziness (PT code: 10013573), and orthostatic hypotension (PT code: 10031127).
Reporting odds ratio
To detect fall-related AEs, we calculated the ROR, which is widely used in post-marketing studies, by using the disproportionality analysis. The ROR is the ratio of odds of reporting an AE (fall-related AEs) versus all other events associated with the given drug compared with the reporting odds for all other drugs in the JADER database. To compare 1 of the index groups with the reference group, we calculated the crude ROR as (a/c)/(b/d) (Fig. 1) [33]. ROR was expressed as point estimates with a 95% confidence interval (CI). Safety signals were considered significant when the lower limit of the 95% CI of the estimated ROR was greater than 1. Two or more cases were required to define the signal.
Association rule mining
Association rule mining focuses on finding frequent co-occurring associations among a collection of items. Given a set of transactions T (each transaction is a set of items), an association rule can be expressed as X [the antecedent (left-hand-side, lhs) of rule:] → Y [the consequent (right-hand-side, rhs) of rule:], where X and Y are mutually exclusive sets of items. Support is defined as the percentage of transactions in the data that contain all items in both the antecedent (lhs) and the consequent (rhs) of the rule. The support indicates how frequently the rule occurs in the transaction. The formula for calculating support is as follows:
D is the total number of the transaction.
Confidence corresponds to the conditional probability P (Y|X). Confidence measures the reliability of the interference made by a rule. The formula for calculating confidence is as follows:
The lift of an association rule is frequently used to gauge the interestingness of a rule and represents the ratio of probability. The lift is the confidence divided by the proportion of all cases that are covered by the rhs. In other words, lift is the ratio between the confidence of the rule and the support of the itemset in the consequent of the rule. The formula for calculating lift is as follows:
Lift is a measure of the importance of the association and it is independent of coverage. Since P (Y) appears in the denominator of the lift measure, the lift can be expressed as the confidence divided by P (Y). The lift can be evaluated as follows: lift = 1, if X and Y are independent; lift > 1, if X and Y are positively correlated; lift < 1, if X and Y are negatively correlated. The statistical significance of the association rule can be estimated by using the Chi-squared analysis [45, 46]. The Chi-squared statistic is defined in terms of the confidence, support, and lift of the single rule. We calculated the Chi-squared values to evaluate the association rules:
The association rule mining was performed using the apriori function of the arules package of R version 3.3.3 software. The first step of apriori algorithm searches for item sets that have more than a given minimum support, while in the second step, rules are generated by selecting “confident” item sets from those found in the first step. Support and lift were visualized using the R-extention package arulesViz which implements novel visualization techniques to explore association rules. The arguments of plot in the arulesViz were set as follows: method = “graph,” measure = “support,” shading = “lift.” The measures of support were used in visualization as area of circle. The measures of lift were used for the shading of color of the circle.
Results
The JADER database contained 430,587 reports between April 2004 and November 2016. The number of reports for the ≥60 year-old-group was 247,170. The number of reports including fall-related AEs was 3715 overall and 2340 in the ≥60 year-old-group. The RORs (95% CI) of α-blockers, diuretics, calcium channel blockers, opioids, benzodiazepines, hypnotics and sedatives, non-selective monoamine reuptake inhibitors, and SSRI were 1.63 (1.27–2.09), 0.74 (0.63–0.86), 1.26 (1.15–1.38), 0.93 (0.80–1.07), 1.83 (1.68–2.01), 1.55 (1.12–2.14), 2.31 (1.82–2.95), and 2.86 (2.49–3.29), respectively (Table 2).
The association rule mining technique was applied to fall-related AEs (as consequent) using demographic data, such as the age category and the number of CNS-active drugs or calcium channel blockers administered (Table 3). To extract association rules efficiently, the thresholds for the optimized support and confidence thresholds were set at 0.000001 and 0.001, respectively, and maxlen (a parameter in the arules package) was restricted to 3. The number of extracted rules was 58 (Table 3). We visualized the result in the heat map of the lift and support obtained from the number of administered drugs (CNS-active drugs) and the stratified age group (Table 3, Fig. 2).
Association rules for falls-related adverse events based on JADER database between April 2004 and November 2016. Support and lift were visualized using the R-extention package arulesViz which implements novel visualization techniques to explore association rules. The arguments of plot in the arulesViz were set as follows: method = “graph,” measure = “support,” shading = “lift.” The measures of support were used in visualization as area of circle. The measures of lift were used for the shading of color of the circle
For fall-related AEs caused by CNS-active drugs, a greater number of administered CNS-active drugs showed a trend of higher lift because the lift of 1 medication, 2 medications, 3 medications, 4 medications, and 5 medications consisting of CNS-active drugs were 1.66, 1.68, 1.69, 2.05, and 2.63, respectively (Table 3, Fig. 2). The lift values increased according to the interaction between aging and the number of administered CNS-active drugs.
For fall-related AEs by calcium channel blockers, the lift of monotherapy and 2 calcium channel blockers were 1.22 and 1.25, respectively, and were almost equal (Table 3). There was no association between increasing the number of calcium channel blockers and fall-related AEs. However, when 1 calcium channel blocker was administered, a higher lift was found among the ≥70 year-old-group than among the < 70 year-old-group.
To evaluate risk factors of fall-related AEs in the ≥60 year-old-group (247,170 cases) using demographic data, such as patient history and administered drugs, we applied the apriori algorithm (minimum support, confidence threshold, 0.000001, 0.01, respectively) and the maxlen was restricted to 4. The number of extracted rules was 45 (Table 4). The association rules of the combination of {benzodiazepines, dementia} → {fall-related AEs}, {benzodiazepines, herpes zoster} → {fall-related AEs}, {calcium channel blockers, benign prostate hypertrophy} → {fall-related AEs}, {opioids, back pain} → {fall-related AEs}, {calcium channel blockers, herpes zoster} → {fall-related AEs}, {opioids, SSRI} → {fall-related AEs} demonstrated high lift scores (Table 4, id [1–6]).
Discussion
Falls can cause serious injuries and are associated with considerable morbidity and mortality, particularly among older adults. The present analysis showed that α-blockers, calcium channel blockers, and CNS-active drugs had high and significant RORs of fall-related AEs. Our study further indicated that the number of administered CNS-active drugs and aging are both associated with the lift value of fall-related AEs. For calcium channel blockers, we also found an age-related increase of lift value. The risk of falls on initiation of antihypertensive drugs in the elderly was reported [47]. Age-related physical and physiological changes increase the incidence of falls. Aging decreases hepatic metabolism and renal drug elimination. These changes lead to higher drug exposure and an increased risk of falls among older adults. These risks should be considered carefully in clinical practice.
The effect of the concomitant use of CNS-active drugs on postural balance may be additive and the concomitant use of CNS-active drugs increases the risk of falls [48] and fractures [49, 50], which are associated with high morbidity and mortality rates [51]. The risk of falls may be attributed to potential drug interactions between antidepressants and benzodiazepines. Our findings are consistent with recent research examining concomitant CNS-active drug use and falls among older adults [52,53,54,55,56], which suggests that pharmacodynamic drug interactions (e.g. involving CNS medication, muscle relaxants, opioids, and SSRI) with benzodiazepines contribute to the increased risk of falls. To the best of our knowledge, there have been no previously published reports on association rule mining analyses for CNS-active drugs using the SRS database. Our results suggest that the risk of fall-related AEs with CNS-active drug monotherapy should not be underestimated. The information derived from this study using association rule mining could complement earlier reports.
Studies regarding the association between polypharmacy and falls have been conducted, however, conclusive results have not been obtained due to small sample sizes [26,27,28, 30, 54], selective study populations [25, 26], or study design (cross-sectional analyses). For example, the use of benzodiazepines was associated only with an increased risk of injurious falls when used with concomitant medication. However, the use of benzodiazepines was also associated with a greater number of falls irrespective of polypharmacy [57]. In a prospective study involving a community-dwelling group aged > 60 years, polypharmacy was not associated with an increased risk of falls after adjusting for co-morbidity [57]. Polypharmacy is generally defined according to the total number of concurrent medications. We investigated the association rules with the number of CNS-active drugs or the number of calcium channel blockers. After considering the causality restraints of the current analysis, further robust epidemiological studies are recommended.
Polypharmacy is associated with an increased risk of administration of potentially inappropriate medication. According to the Beers criteria [58] and the Screening Tool of Older Person’s Prescriptions (STOPP)/Screening Tool to Alert doctors to Right Treatment (START) criteria [59, 60], healthcare professionals should optimize the medication of their patients and minimize polypharmacy to reduce AEs.
Among older adults, treatment is complicated by the high frequency of comorbidity [58]. We detected the association rules of combination of {benzodiazepines, herpes zoster} → {fall-related AEs} and {calcium channel blockers, herpes zoster} → {fall-related AEs}. Several studies have reported that valacyclovir and pregabalin induce dizziness which leads to falls [27]. Healthcare professionals should pay attention to the risk of falls among patients with herpes zoster administered calcium channel blockers or benzodiazepines. Healthcare professionals should conduct a thorough medication review, including past patient history, age-related physical changes, drug–drug interactions, and AEs considered as risk factors for falls [61,62,63,64]. Optimized interventions to reduce the incidence of falls among older adults should be introduced, such as stopping, switching, or reducing the number of administrated drugs and adding vitamin D [65].
A number of limitations of the analysis using SRS, such as the JADER database, should be noted. The SRS is subject to over-reporting, under-reporting, missing data, exclusion of healthy individuals, lack of a denominator, and the presence of confounding factors [66]. The target drugs in our study were selective, and not comprehensive, and was not intended to diminish the clinical importance of known drug–drug interactions not listed. Despite these limitations, we obtained reasonable results that complement or corroborate those reported in the literature. Our results provide valuable insights into prescribing drugs to older adults in a real-world clinical setting.
Conclusion
This study is the first to evaluate the correlation between fall-related AEs and the number of concomitant CNS-active drugs, aging, and comorbid diseases using ROR and association rule mining technique based on the JADER database. Despite the inherent limitations of SRS, the number of administered CNS-active drugs and patient age were both associated with the lift value of fall-related AEs. The present analysis demonstrates that the incidence of fall-related AEs associated with benzodiazepines and calcium channel blockers use should be closely monitored in patients with herpes zoster. We believe that the data presented in this study will help healthcare professionals to improve the care of older patients administered different medications concomitantly.
Abbreviations
- AE:
-
Adverse Event
- AGS:
-
American Geriatrics Society
- ATC:
-
The Anatomical Therapeutic Chemical
- CI:
-
Confidence Intervals
- CNS:
-
Central Nerves System
- FAERS:
-
FDA Adverse Event Reporting System
- FDA:
-
The US Food and Drug Administration
- ICH:
-
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- JADER:
-
The Japanese Adverse Drug Event Report
- lhs:
-
left-hand-side
- MedDRA:
-
The Medical Dictionary for Regulatory Activities
- PMDA:
-
The Pharmaceuticals and Medical Devices Agency
- PT:
-
Preferred Term
- rhs:
-
Right-hand-side
- ROR:
-
Reporting Odds Ratio
- SRS:
-
Spontaneous Reporting System
- START:
-
Screening Tool to Alert doctors to Right Treatment
- STOPP:
-
The Screening Tool of Older Person’s Prescriptions
- WHO:
-
The World Heath Organization
References
O’Loughlin JL, Robitaille Y, Boivin JF, Suissa S. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137:342–54.
Bergland A. Risk factors for serious fall related injury in elderly women living at home. Inj Prev. 2004;10:308–13.
Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl 2):ii37–41.
Guideline for the prevention of falls in older persons. American Geriatrics Society, British geriatrics society, and American Academy of Orthopaedic surgeons panel on falls prevention. J Am Geriatr Soc. 2001;49:664–72.
Kannus P, Sievänen H, Palvanen M, Järvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366:1885–93.
Ferreri S, Roth MT, Casteel C, Demby KB, Blalock SJ. Methodology of an ongoing, randomized controlled trial to prevent falls through enhanced pharmaceutical care. Am J Geriatr Pharmacother. 2008;6:61–81.
Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people. Epidemiology. 2010;21:658–68.
Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci. 2007;62:1172–81.
Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169:1952–60.
Ham AC, Swart KMA, Enneman AW, van Dijk SC, Oliai Araghi S, van Wijngaarden JP, et al. Medication-related fall incidents in an older, ambulant population: the B-PROOF study. Drugs Aging. 2014;31:917–27.
Bloch F, Thibaud M, Dugué B, Brèque C, Rigaud AS, Kemoun G. Psychotropic drugs and falls in the elderly people: updated literature review and meta-analysis. J Aging Health. 2011;23:329–46.
de Vries M, Seppala LJ, Daams JG, van de Glind EMM, Masud T, van der Velde N; EUGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Fall-risk-increasing drugs: a systematic review and meta-analysis: I. cardiovascular drugs. J Am Med Dir Assoc 2018;19:371.e1–371.e9.
Seppala LJ, Wermelink AMAT, de Vries M, Ploegmakers KJ, van de Glind EMM, Daams JG, et al.; EUGMS task and Finish group on fall-risk-increasing drugs. Fall-risk-increasing drugs: a systematic review and meta-analysis: II. Psychotropics. J Am Med Dir Assoc 2018;19:371.e11–371.e17.
Seppala LJ, van de Glind EMM, Daams JG, Ploegmakers KJ, de Vries M, Wermelink AMAT, et al.; EUGMS Task and Finish Group on Fall-Risk-Increasing Drugs. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19:372.e1–372.e8.
The American geriatrics society 2015 Beers criteria update expert panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–46.
Japan Geriatric Society. Guidelines for Medical Treatment and its Safety in the elderly 2015 (In Japanese). Tokyo: Medical View Co., Ltd; 2005.
Berdot S, Bertrand M, Dartigues JF, Fourrier A, Tavernier B, Ritchie K, et al. Inappropriate medication use and risk of falls--a prospective study in a large community-dwelling elderly cohort. BMC Geriatr. 2009;9:30.
Ray WA, Thapa PB, Gideon P. Benzodiazepines and the risk of falls in nursing home residents. J Am Geriatr Soc. 2000;48:682–5.
Stockl KM, Le L, Zhang S, Harada AS. Clinical and economic outcomes associated with potentially inappropriate prescribing in the elderly. Am J Manag Care. 2010;16:e1–10.
Ray WA. Psychotropic drugs and injuries among the elderly: a review. J Clin Psychopharmacol. 1992;12:386–96.
Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. psychotropic drugs. J Am Geriatr Soc. 1999;47:30–9.
Park H, Satoh H, Miki A, Urushihara H, Sawada Y. Medications associated with falls in older people: systematic review of publications from a recent 5-year period. Eur J Clin Pharmacol. 2015;71:1429–40.
Neutel CI, Perry S, Maxwell C. Medication use and risk of falls. Pharmacoepidemiol Drug Saf. 2002;11:97–104.
Ziere G, Dieleman JP, Hofman A, Pols HA, van der Cammen TJ, Stricker BH. Polypharmacy and falls in the middle age and elderly population. Br J Clin Pharmacol. 2006;61:218–23.
Lawlor DA, Patel R, Ebrahim S. Association between falls in elderly women and chronic diseases and drug use: cross sectional study. BMJ. 2003;327:712–7.
Baranzini F, Diurni M, Ceccon F, Poloni N, Cazzamalli S, Costantini C, et al. Fall-related injuries in a nursing home setting: is polypharmacy a risk factor? BMC Health Serv Res. 2009;9:228.
Kojima T, Akishita M, Nakamura T, Nomura K, Ogawa S, Iijima K, et al. Association of polypharmacy with fall risk among geriatric outpatients. Geriatr Gerontol Int. 2011;11:438–44.
Kojima T, Akishita M, Nakamura T, Nomura K, Ogawa S, Iijima K, et al. Polypharmacy as a risk for fall occurrence in geriatric outpatients. Geriatr Gerontol Int. 2012;12:425–30.
Helgadóttir B, Laflamme L, Monárrez-Espino J, Möller J. Medication and fall injury in the elderly population; do individual demographics, health status and lifestyle matter? BMC Geriatr. 2014;14:92.
Weber V, White A, McIlvried R. An electronic medical record (EMR)-based intervention to reduce polypharmacy and falls in an ambulatory rural elderly population. J Gen Intern Med. 2008;23:399–404.
Tromp AM, Pluijm SM, Smit JH, Deeg DJ, Bouter LM, Lips P. Fall-risk screening test: a prospective study on predictors for falls in community-dwelling elderly. J Clin Epidemiol. 2001;54:837–44.
Zia A, Kamaruzzaman SB, Tan MP. The consumption of two or more fall risk-increasing drugs rather than polypharmacy is associated with falls. Geriatr Gerontol Int. 2017;17:463–70.
Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–36.
Fukuda A, Tahara K, Hane Y, Matsui T, Sasaoka S, Hatahira H, et al. Comparison of the adverse event profiles of conventional and liposomal formulations of doxorubicin using the FDA adverse event reporting system. PLoS One. 2017;12:e0185654.
Kato Y, Umetsu R, Abe J, Ueda N, Nakayama Y, Kinosada Y, et al. Hyperglycemic adverse events following antipsychotic drug administration in spontaneous adverse event reports. J Pharm Heal Care Sci. 2015;1:15.
Abe J, Umetsu R, Uranishi H, Suzuki H, Nishibata Y, Kato Y, et al. Analysis of polypharmacy effects in older patients using Japanese adverse drug event report database. PLoS One. 2017;12:e0190102.
Fujiwara M, Kawasaki Y, Yamada H. A pharmacovigilance approach for post-marketing in Japan using the Japanese adverse drug event report (JADER) database and association analysis. PLoS One. 2016;11:e0154425.
Hatahira H, Abe J, Hane Y, Matsui T, Sasaoka S, Motooka Y, et al. Drug-induced gingival hyperplasia: a retrospective study using spontaneous reporting system. J Pharm Heal Care Sci. 2017;3:19.
Hasegawa S, Matsui T, Hane Y, Abe J, Hatahira H, Motooka Y, et al. Thromboembolic adverse event study of combined estrogen-progestin preparations using Japanese adverse drug event report database. PLoS One. 2017;12:e0182045.
Naganuma M, Motooka Y, Sasaoka S, Hatahira H, Hasegawa S, Fukuda A, et al. Analysis of adverse events of renal impairment related to platinum-based compounds using the Japanese adverse drug event report database. SAGE Open Med. 2018;6:1–11.
Lamoth CJ, van Deudekom FJ, van Campen JP, Appels BA, de Vries OJ, Pijnappels M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil. 2011;8:2.
Ijmker T, Lamoth CJ. Gait and cognition: the relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture. 2012;35:126–30.
Montero-Odasso M, Muir SW, Hall M, Doherty TJ, Kloseck M, Beauchet O, et al. Gait variability is associated with frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2011;66:568–76.
de Groot MH, Van der Jagt-Willems HC, van Campen JP, Lems WF, Beijnen JH, Lamoth CJ. A flexed posture in elderly patients is associated with impairments in postural control during walking. Gait Posture. 2014;39:767–72.
Alvarez SA. Chi-squared computation for association rules: preliminary results. Technical report, BCCS-2003–01 July; 2003.
Yildirim P. Association patterns in open data to explore ciprofloxacin adverse events. Appl Clin Inform. 2015;6:728–47.
Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of falls on initiation of antihypertensive drugs in the elderly. Osteoporos Int. 2013;24:2649–57.
Weiner DK, Hanlon JT, Studenski SA. Effects of central nervous system polypharmacy on falls liability in community-dwelling elderly. Gerontology. 1998;44:217–21.
Nurminen J, Puustinen J, Piirtola M, Vahlberg T, Kivelä SL. Psychotropic drugs and the risk of fractures in old age: a prospective population-based study. BMC Public Health. 2010;10:396.
Lai SW, Liao KF, Liao CC, Muo CH, Liu CS, Sung FC. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore). 2010;89:295–9.
Haleem S, Lutchman L, Mayahi R, Grice JE, Parker MJ. Mortality following hip fracture: trends and geographical variations over the last 40 years. Injury. 2008;39:1157–63.
Huang ES, Karter AJ, Danielson KK, Warton EM, Ahmed AT. The association between the number of prescription medications and incident falls in a multi-ethnic population of adult type-2 diabetes patients: the diabetes and aging study. J Gen Intern Med. 2010;25:141–6.
Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf. 2010;19:1248–55.
French DD, Chirikos TN, Spehar A, Campbell R, Means H, Bulat T. Effect of concomitant use of benzodiazepines and other drugs on the risk of injury in a veterans population. Drug Saf. 2005;28:1141–50.
Aparasu RR, Mort JR. Inappropriate prescribing for the elderly: beers criteria-based review. Ann Pharmacother. 2000;34:338–46.
Lau DT, Kasper JD, Potter DE, Lyles A, Bennett RG. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med. 2005;165:68–74.
Richardson K, Bennett K, Kenny RA. Polypharmacy including falls risk-increasing medications and subsequent falls in community-dwelling middle-aged and older adults. Age Ageing. 2015;44:90–6.
American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American geriatrics society updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012;60: 616–631.
Dalleur O, Boland B, Losseau C, Henrard S, Wouters D, Speybroeck N, et al. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: a randomised controlled study. Drugs Aging. 2014;31:291–8.
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–8.
De Geest S, Steeman E, Leventhal ME, Mahrer-Imhof R, Hengartner-Kopp B, Conca A, et al. Complexity in caring for an ageing heart failure population: concomitant chronic conditions and age related impairments. Eur J Cardiovasc Nurs. 2004;3:263–70.
Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly. Drugs Aging. 2012;29:359–76.
Chen Y, Zhu LL, Zhou Q. Effects of drug pharmacokinetic/pharmacodynamic properties, characteristics of medication use, and relevant pharmacological interventions on fall risk in elderly patients. Ther Clin Risk Manag. 2014;10:437–8.
Tan JL, Eastment JG, Poudel A, Hubbard RE. Age-related changes in hepatic function: an update on implications for drug therapy. Drugs Aging. 2015;32:999–1008.
Panel on prevention of falls in older persons, American geriatrics society and British geriatrics society. Summary of the updated American geriatrics society/British geriatrics society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59:148–57.
Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA adverse event reporting system (AERS). INTECH; 2012. p. 265–302.
Acknowledgements
Not applicable.
Funding
This research was partially supported by JSPS KAKENHI Grant number, 17 K08452.
Availability of data and materials
The dataset supporting the conclusions of this article is included within the article.
Author information
Authors and Affiliations
Contributions
MN conceived of the study, and participated in its design and coordination and drafted the manuscript. HH, SH and SS conceived of the study and conducted the statistical analysis and drafted the manuscript. YK, YM, AF, MN, SN, RM, and KS collect and analyzed data. JA performed the statistical analysis. KH helped to interpretation of data. TK participated in its design of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JA is an employee of Medical Database. The rest of the authors have no conflict of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hatahira, H., Hasegawa, S., Sasaoka, S. et al. Analysis of fall-related adverse events among older adults using the Japanese Adverse Drug Event Report (JADER) database. J Pharm Health Care Sci 4, 32 (2018). https://doi.org/10.1186/s40780-018-0129-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40780-018-0129-8