Abstract
Variety and durability of color are presumed as key constrains of natural dyes. So, this study attempts to investigate the effect of metallic mordants on the color fastness properties of ecologically dyed cotton fabric using banana floral stem sap. Color difference was measured in terms of hue (ΔH*), chroma (ΔL*) and value (ΔC*) difference. Metal ions in residual mordanting bath, dyeing wastewater and level of trace metals in the finished fabric surface were accessed to justify the environmental safety and speculate the health risk respectively. Pre-mordanted specimens were dyed at 100 °C for 60 min. Optical properties of extracted sap were observed by UV visible spectroscopy. Dye fixation with fiber was determined by FTIR-ATR spectra. Atomic absorption spectroscopy was employed to determine the trace metals in finished fabric. Effect of metallic mordants were calculated in terms of color fastness to wash, water, perspiration, rubbing and light for estimating the color durability. Except light fastness property almost all color fastness values were 4/5, i.e. very good. Light fastness properties were improved for mordanting action with metallic salts. The level of trace metals in finished fabric were within the safe zone.

Metallic mordant assisted natural dyeing of cotton.
Similar content being viewed by others
Introduction
Textile processing industries are one of the major sources for environmental pollution (Bhuiyan et al. 2014). Huge amount of toxic and hazardous wastewater usually has discharged into the rivers, canals and water streams from textile industries. Thus, flora and agricultural land are severely affected. Moreover, the uses of synthetic dyes cause health hazards and negatively affect the ecosystem. Hence, researchers around the globe are trying to set up novel methods for textile coloration (Kumar and Bhowmik 2012).
In this concern, the demand of natural dyes is increasing to fulfill the environmental awareness. Natural dyes are eco-friendly, safe, non-carcinogenic, non-allergic and easy biodegradable. These dyes need no special care, produce uncommon and soothing shades. Simple dye house is involved in their extraction and application processes (Blackburn 2004; Samanta and Agarwal 2009; Saleh et al. 2013). But researchers are facing some problems to introduce the natural dyeing in bulk production. These are low exhaustion, poor color fastness properties, standardized method for dye extraction, complexity of dyeing process, reproducible results and blending problems (Lee 2007; Umbreen et al. 2008; Sachan and Kapoor 2007).
Different types of metallic salts were used to minimize those problems mostly. Aluminum potassium sulfate, stannous chloride, ferrous sulfate and copper sulfate are commonly used mordants. The metal ions can act as electron donor to form coordination bonds with the dye molecules. Mordants assist to form the chemical bridge between dye and fiber molecules. Thus, improves the dye molecule permanency onto fabric surface, i.e. color fastness properties of natural dye (Vankar 2000).
Oppositely, substantial portion of these metals remains unreacted which create environmental impediment during disposal (Bechtold et al. 2003; Rovira et al. 2015). Toxic elements present in textile are not only responsible for environmental pollution, but also a potential health risk. Depending on the concentration level, trace metals in finished textiles may cause several skin and others diseases including dermatitis, irritation, allergy, skin micro-flora reduction, liver damage, pulmonary congestion, cancer and, etc. (Rovira et al. 2015; Von Goetz et al. 2013; Leme et al. 2014; Sengupta and Behera 2014). Therefore, it is necessary to use mordant for natural dyeing of textiles where the metals in wastewater and trace elements level in finished fabric will lies within safe zone.
Among numerous source of natural dyes, banana floral stem sap is a promising sustainable option. Almost 89% shares of banana plant are accounted as agricultural bio-waste (Repon et al. 2016a). BSF comprises of various elements including dye compounds namely flavonoids, tannin, proanthocyanin, etc. (Pinheiro and Justino 2012; Mabry et al. 1970; Lin and Harnly 2012; Antoine et al. 2004). In our previous study, the dyeing time and temperature had optimized for cotton coloration using BFS where lower color fastness properties and color difference were yielded without any mordants (Repon et al. 2016a, b).
This present study attempts to gratify the color fastness properties and produce variety of color using BFS as natural dye together with various metallic mordants. The metals concentration in mordant bath and wastewater were evaluated to find the ecological aspect during disposal. The levels of trace metals into the finished fabric were also measured to speculate the human health risk.
Methods
Materials
100% cotton knitted single jersey fabric was collected from “HI-FASHION COMPOSITE TEXTILES LIMITD”, Joydebpur, Gazipur, Bangladesh. Areal density of this fabric was 170 g/m2. Table 1 depicts the color co-ordinates of the scoured bleached fabric used for this research work.
Dyes and chemicals
Banana (Musa sapientum) floral Stem was collected from Tangail-1902, Bangladesh. Potassium alum AlK(SO4)2·12H2O, copper(II) sulfate pentahydrate (CuSO4·5H2O), tin(II) chloride pentahydrate (SnCl2·5H2O) and iron(II) sulfate heptahydrate (FeSO4·7H2O) were purchased from Merck, Germany. The ISO standard soaping agent without optical brighter (James heal, England) were used for removing unfixed dye from the fabric surface. All chemicals were laboratory grade and used without any further purification.
Sampling
Different samples are identified as tabulated in Table 2.
Natural dye extraction
Dye was retrieved according to our previous investigation (Repon et al. 2016a, b). Briefly, the banana (M. sapientum) floral stems were separated from banana tree and washed. Fresh floral stems were cut and sliced (Fig. 1). Sap was extracted from floral stem by roller squeezer machine. Then sap solution was filtrated by a nylon strainer and stored for application.
Mordanting
To promote the dye uptake, cotton specimens were treated with various positively charged metallic salts. Figure 2 expresses the chelating between cellulose and Al3+ via co-ordination linkage. Cotton fabric samples were subjected to pre-mordanting with 5 g/L metallic mordants at 100 °C for 60 min (Repon et al. 2016a, b). Figure 3 represents the process curve of pre-mordanting action. After mordanting action samples were impregnated into bath for overnight. Then the mordanted samples were squeezed and air dried in flat dryer machine (MESDAN, Italy). The material to liquor ratio were maintained as 1:20.
Co-ordination bond formation during mordanting of cotton with alum (Chung et al. 2004)
Dyeing
The dyeing action was ended according to our previous study (Repon et al. 2016a, b). Briefly, natural dyeing was performed according to exhaust method by Infra-red Lab Sample Dyeing Machine (XIAMEN RAPID, China) at 100 °C for 60 min (Fig. 4). Then the dye bath was cooled at 40 °C. Samples were washed at room temperature and air dried in flat dryer machine (MESDAN, Italy). Then soaping was performed for removing unfixed dye from the fabric surface by 0.5 g/L ISO standard soap at 80 °C for 10 min. For both dyeing and soaping, the material to liquor ratio were kept as 1:20. No additional water was used in the dyeing bath. The probable bonding among tannic acid, flavonoids (Luteolin) of BFS with cellulose (Sinha et al. 2016; de Assis et al. 2014) have shown in Fig. 5. Figure 6 indicates photographs different dyed specimens for different mordanting agent’s variation.
Optical properties of banana floral stem sap
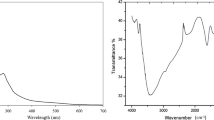
Physical and optical phenomena of the extracted natural dye from banana floral stem were accessed by the UV absorption spectra. UV–visible Spectrometer (T-60 UV–visible Spectrometer, PG Electronics, UK) was employed to take the UV spectra. Absorbance was taken over the wavelength 230–700 nm.
Conformation of dye molecule into fabric
FTIR-ATR spectra of the scoured-bleached and dyed specimens for several mordants were observed to assure the presence of dye molecule into fabric by using the FTIR spectrophotometer (PerkinElmer Spectrum Two, UK). Samples were directly fitted on the respective place of universal ATR of the machine. Transmittance % was taken over the wavenumber 400–4000/cm for identifying the targeted functional groups.
Determination of hue, chroma and value difference of dyed fabric
The hue (ΔH*), chroma (ΔC*) and value difference (ΔL*) of the dyed samples were determined according to the CIE lab system by a dual beam reflectance Data-color Spectroflash SF 650X (USA) (Repon et al. 2016a, b). Each sample was folded twice to give an opaque view with four plies and values were measured automatically. Sample A was considered as the standard sample. The hue, chroma and value difference of the dyed samples were calculated by using the following Eqs. 1, 2 and 3 respectively.
Hue difference,
where, Hs = sample and Hstd = standard.
Chroma difference,
where, Cs = sample and Cstd = standard.
And value difference,
where, Ls = sample and Lstd = standard.
Determination of color fastness properties
Standard methods were employed for evaluating color fastness properties of the selected dyed specimens. Color fastness to washing, rubbing (dry and wet), water, perspiration and light fastness tests were carried out by using grey scale of color change and color staining according to ISO 105-C06: 2010 (AATCC 2013a), ISO-105x12: 1995 (AATCC 2013b), EN ISO 105-E01: 2013 (AATCC 2008), ISO 105-E04: 2013 (AATCC 2006) and EN ISO 105-B02: 2013 (AATCC 1996) method respectively.
Determination of environmental impact of wastewater
During mordanting of cotton with various metals, considerable volume of metal leftover as unreacted in the residual mordanting bath (Bechtold et al. 2003). Besides, these exhausted amounts of metals reside onto fabric surface by both physical and chemical bond. Consequently, during coloration process, the existed metals will not participate fully into the dye fiber binding purposes (Rather et al. 2016). Hence, the effluents after dyeing and subsequent washing were employed to determine the concentration of metal ions in the dyeing wastewater.
Metals loading in various stages were estimated by using the UV–visible Spectrometer (T-60 UV–visible Spectrometer, PG Electronics, UK). Firstly, maximum absorption (λ max) wavelengths of different metals were determined. The λ max values of aluminum, iron, copper and tin were noticed as 244, 510, 643 and 210 nm respectively which are harmonized with the relevant previous studies (McIntyre et al. 1982; Lande et al. 2016; Al 2014; Spectrophotometric Analysis of Copper). Secondly, metals concentrations were determined against particular λ max values for each element.
From Beer Lambert law, the absorbance of any solution is directly proportional to its concentration. That means, the absorbance and concentration values of any particular chemical solution will follow the equation of straight line (Concentration Calculation from UV Vis Absorbance; Photometry). At any fixed λ max, the absorbance values of various solutions of known concentrations (reference sample) were calculated by the machine. Later, the standard calibration (concentration vs absorbance) curves were automatically developed by the machine software from the inputted concentration values of reference samples and calculated absorbance values of solution for different metals. Afterward, with respect to that calibration curve, metal concentrations of different stages were calculated. Metal ion concentrations in the residual mordant bath were evaluated in order to access the metals exhaustion percentages onto cotton surface. The exhausted amounts of metals were accessed by the Eq. 4 (Rather et al. 2016; Bechtold et al. 2003).
where, C0 and C1 are the concentration at the maximum wavelength (λ max) for different metals before and after any certain treatment. For accessing the environmental impact, the final concentrations of metals in effluent were compared with the legal limits for textile effluents released to a typical effluent treatment plant of Bangladesh (Tonetti and Innocenti 2009).
Determination of trace metals level in finished fabric
Atomic absorption spectroscopy was used to determine the level of trace metals in the finished fabric that will be in contact with skin. Samples were prepared by following the previous study (Rather et al. 2016). Briefly, samples were dried to remove the adsorbed moisture. Then, specimens were digested with 32.5% HNO3 (Merck, Germany). Fabric to acid ratio was kept as 1:20. Digestion was carried out in a microwave digester (Berghof, Germany) step wise at various temperatures and times. Extracts were cooled, filtrated and preserved until element analysis. Atomic absorption spectrometer (Varian AA 240 FS, Australia) was employed to access the concentration of Al3+, Fe2+, Cu2+ and Sn2+. Detection limits (mg/kg) were varied for various metals. Finally, outcomes were compared with five previous relevant studies (Rather et al. 2016; Rovira et al. 2015; Rezic and Steffan 2007; Menezes et al. 2010; Tuzen et al. 2008) to speculate the health risk.
Results and discussion
Optical properties of the dye
The UV visible absorption spectrum (Fig. 7) of natural dye solution corresponds to extracted sap from banana floral stem. The extracted floral stem sap was thick liquid and very light brown or dusty in color. The absorption spectrum is clearly indicating the Band I in 300–450 nm range where BFS revealed sharp absorption peaks in UV region with a maximum wavelength at 375 nm. Another Band II in the range of 240–295 nm was appeared by signifying appreciable absorption with a maximum wavelength at 275 nm. The presences of flavonoids in BFS are liable for the existences of these two major characteristic bands (Pinheiro and Justino 2012; Mabry et al. 1970). Absorption due to B-ring cinnamoyl system and A-ring benzoyl system are corresponds to B and I and B and II respectively (Mabry et al. 1970). Various major and minor characteristics peaks in the different ranges are exemplifying that BSF is comprised of various flavonoids (300–450 nm) namely flavones and flavonols (240–285 nm) and flavanones (270–295 nm) (Pinheiro and Justino 2012). The wavelength maximums can be grouped as: flavanols and proanthocyanidins at 278 nm, hydrolyzable tannins at 274 nm, flavanones at 288 nm and 3-hydroxylated flavonoles at 352 nm (Pinheiro and Justino 2012; Mabry et al. 1970; Lin and Harnly 2012; Antoine et al. 2004). Above characteristics picks are appeared due to fragmentation of flavonoid. Flavonoid glycosides possess acylated glycosyl moieties. Various acyl groups are formed due to diverse glycoside acylation which leads such fragmentation patterns of flavonoid. Flavonoids, tannins and proanthocyanidins are water soluble polyphenolic compounds (Cuyckens and Claeys 2004; Cuyckens and Claeys 2005; Barhanpurkar et al. 2015). They have high molecular weight (about 500–3000) phenolic hydroxyl groups which enable them to procedure effective crosslinking with cotton fiber substrate via forming co-ordination bond (Basak et al. 2015).
Conformation of dye molecule into fabric
Figure 8 represents the IR spectra of scoured bleached (1) and dyed specimen using BFS (2).
Broad band at 3335.4/cm is attributed to –OH stretching vibration for H bonded H2O uptake (Bilba et al. 2007). Two peaks at 2915.2 and 2858.6/cm corresponds asymmetric and symmetric stretching of long methylene (–CH2–) chain for residual wax on cotton (Chung et al. 2004). For adsorbed H2O into the fabric specimen, peaks appeared at 1638/cm (Chung et al. 2004). Peak at 1423/cm is attributed for C–H wagging of carbohydrate and lignin (Chung et al. 2004; Ibrahim et al. 2010). The presence of band at 1323/cm is endorsed for lignin (Miller and Wilkins 1952). Both peaks at 1153 and 1109/cm corresponds for asymmetric ethar linkage (–C–O–C–) (Chung et al. 2004). Spectra (1) and (2) (Fig. 8) exhibited strong band at 1028.3/cm due to C–O–C symmetric stretching di-alkyl ether linkages and C–O stretching vibration for cellulose, hemicellulose and minor lignin contribution (He et al. 2007). Peak at 898/cm attributes for β glycosidic linkage and asymmetric out of phase ring stretch of C1–O–C4 (Ali 2008).
For BFS stronger bands were seen at 1028.3, 1109, 1161.6, 1323.2 and 1638.4/cm for spectra of dyed fabric (2) than scoured bleached fabric (1) (Fig. 8) by declining over all transmittance % due to presence of tannin, flavonoid, lignin, etc. (de Assis et al. 2014). For dyed cotton with BFS (2), four major changes were appeared at peaks 882.83, 1000, 1056.6, 1173.7 and 1396/cm respectively, which endorse the investigation of Basak et al. (2015), Paul et al. (2013) and de Assis et al. (2014) respectively. Due the presence of inorganic salts into BFS peaks were exhibited at 873, 1000 and 1176/cm for potassium chloride, sodium phosphate and magnesium chloride respectively (He et al. 2007; Bhattacharya and Shah 2000). Peaks at 1056.6 corresponds –C–H and –C–O deformation band (de Assis et al. 2014) and 1396/cm was responsible for CH deformation of –CH2– (Miller and Wilkins 1952). Figure 9 represents the IR spectra of dyed specimens for mordanting agent variation. Characteristic peaks were appeared for different metals in wavenumbers ranging 400–800/cm.
Hue, chroma and value difference
The hue angles of the specimens were decreased for treatment with metallic mordants (Table 3). The orders of decreasing hue difference \((\Delta H^\circ )\) of samples were found as B>E>C>D. The hue angles were decreased by 7.02, 3.36, 2.05 and 4% for B, C, D and E respectively than the control sample A. For B, the hue value was decreased 5.08% than D.
The orders of chroma difference (ΔC*) of samples were found as E>D>C>B. Mordanting agent has positive impact on color saturation. For improved dye fiber linkage via coordination bond, E was 11.36% more saturated with color than A. Sample C and D were 7.97 and 8.36% more saturated than the specimen A correspondingly. The sample treated with stannous chloride was 10.83% more saturated than potassium alum treated.
The orders of value difference (∆L*) of the samples were found as C>D>E>B. Lightness were decreased due to treat with metallic mordants. Maximum value difference was observed for the sample C. The samples B, C, D and E were 4.32, 10.97, 9.42 and 6.44% deeper than the reference sample A. Lower lightness value was observed for iron mordanted samples as it has coordination number 6, i.e. stronger capability of dye-mordant-fiber interactions than that of copper, tin and alum respectively. The color saturation (C*) and lightness (L*) have followed opposing fashion. Alum mordanted specimens were exhibited higher L*, as it block dye molecules by bonding with more dye than that of the fiber molecule. The alteration of yellowness and redness had followed same fashion (Uddin 2015; Ali 2008).
Color fastness to wash
The overall results of color fastness to wash of dyed samples were very good shown in Table 4. The control specimen A showed color change rating of 4 which is explained as good washing fastness. 4–5 color change rating were observed for all mordanted samples. Sample C indicated 4 (good) and B, D and E exhibited 4/5 (very good) rating in color change. The color staining rating was detected 3/4 for A. For B and C, the ratings were noticed as 4 which indicate the slightly staining of dye on to the adjacent wool fiber of multifibre fabric. No staining was occurred on other fiber of multifibre fabrics for all samples.
Color fastness to rubbing and light
The rating of color fastness to rubbing (dry and wet) and light of the control and mordanted specimens were evaluated and presented in the Table 5. The overall results of color fastness to rubbing of all samples were very good. Unmordanted dyed samples (A) demonstrated the rating of 4 (good) for both dry and wet rubbing properties. Very good wet and dry rubbing properties were exhibited by all mordanted dyed specimens such as B, C, D and F respectively.
Regarding light fastness, the rating of 2 was showed by the sample A, which indicates poor light fastness property. Specimens B, C, D and E displayed rating of 2/3 that means light fastness were improved a little bit due to dye-mordant-fiber interaction, i.e. mordanting with metallic salts (46). In case of BFS, lower intra-molecular H bond exist between dye molecule and fiber which increases the electron density at the chromospheres. Consequently, sensitivity of dye towards photochemical oxidation is increased (Ghaly et al. 2014).
Color fastness to water
The rating of color fastness to water of unmordanted and mordanted samples illustrates in Table 6. In case of color change, the samples A, C and E showed 4 (good) rating and B and D showed very good rating (4/5). The color staining rating were observed as four for the specimens A and C, which indicates slightly staining of dye molecules on to the adjacent wool fiber of multifibre fabric. All other mordanted samples directed no staining of color on other fibers of multifibre fabric.
Color fastness to perspiration
Table 7 illustrates the color fastness to perspiration.
Unmordanted dyed specimens showed rating of 4 in color change for both acidic and alkaline perspiration. Very good color staining on cotton was observed. The samples A, D and E showed rating of 4 in acid perspiration. Expediently, all samples presented very good grade, i.e. 4/5 in both color change and staining for both acid and alkali condition.
Control samples exhibited slightly infirrior color fastness properties than the mordanted samples due to dye–fiber interaction only. For the dye-mordant-fiber intereation, all mordanted samples were exhibited upgraded outcomes interestingly. BFS comprises of many organic and inorganic compounds (de Assis et al. 2014). Hydroxyl and carbonyl groups in the polyphenolic flavonoids and cellulose structure are capable of forming chelate by co-ordination bond with positively charged metals. Some alteration were appeared in color coordinates value for mordanted dyed specimens as compared (B, C, D, and E) to the control sample (A). Hence, one molecule of metal can form a bond with two or more molecules of dye and fiber simultaneously according to their co-ordination numbers. Both iron and copper metals have excellent ability to form complexes by readily chelating with the dye molecule. As the coordination numbers of copper and iron are 4 and 6 respectively, some co-ordination sites remain vacant when they interact with the cotton. Thus, metals can form a complex simultaneously with the cotton in one site by keeping the dye (tannin, flavonoids) on the other site. Such firm binding promote dye uptake accordingly. Iron exhibited stronger fabric-mordants-dye interaction than copper (Uddin 2015; Ali 2008). Thus, all mordanted samples were exhibited better color fastness properties. Exceptionally, iron has stronger coordination linkage formation capability for higher coordination numbers 6 than other metals. In case of tin, slightly change in color was also appeared for its lower coordination bond strength.
Environmental impact of various metals in wastewater
Metal loading in different stages such as, the exhausted metals % onto fabric surface, concentration of residual metals in mordant bath and concentration of metals in dyeing wastewater were tabulated in Table 8. The concentrations of metals in effluent were compared with legal limits for textile effluents released to a typical effluent treatment plant in Bangladesh (Tonetti and Innocenti 2009). The metals exhaustion % onto fabric surface were noticed as 61.79, 74.47, 69.19 and 65.85% for Al3+, Fe2+, Cu2+ and Sn2+ respectively.
Fluctuations in exhaustion percentages for different metals correspond to the co-ordination bond formation capability with fiber molecule. The co-ordination bond formation phenomena of metals depend on the co-ordination number. In case of Fe treated specimens, more metals were exhausted as iron has higher co-ordination number amongst the employed metals. Almost 60–75% of metals were exhausted onto fabric surface. These unreacted metals in mordant bath are great headache of environmental impediment. Moreover, during coloration process, the exhausted amount of metals will not partake fully into the dye–fiber binding purposes. So, the effluent after dyeing and washing were used to determine the concentration of metal ions into dyeing wastewater before discharge. The concentration of metals ions in residual mordant bath were found as 1.91, 1.28, 1.54 and 1.71 g/L for Al3+, Fe2+, Cu2+ and Sn2+ separately. Due to the lower exhaustion %, more amount residual metals were found for Al3+. The amounts of metal ions that remain unexhausted in the mordant baths were more than that of the legal limits for textile effluents discharged to a typical effluent treatment plant of home and abroad (Tonetti and Innocenti 2009). Though the amount of Fe2+ in mordant bath was within the acceptable range of Bangladesh limit but fatefully it was up to almost four times higher than the international limits (Rather et al. 2016; Tonetti and Innocenti 2009).
Regarding the concentrations of metals in dyeing wastewater, the amount of Al3+, Fe2+, Cu2+ and Sn2 were found as 0.11, 0.29, 0.19 and 0.021 g/L correspondingly. The recorded concentrations metals in dyeing wastewater lie within the acceptable range. But regrettably employed metallic salts also release substantial amount of SO4 2− which are beyond the acceptable limit (Rather et al. 2016). Therefore, these metals will lead disposal and environmental related obstacles (Rather et al. 2016; Bechtold et al. 2003; Tonetti and Innocenti 2009).
Level of trace metals in the finished fabric
Level of trace metals in finished fabric is tabulated in the following Table 9. Findings were compared with five previous studies on detection of trace elements in the fabric (Rather et al. 2016; Rovira et al. 2015; Rezic and Steffan 2007; Menezes et al. 2010; Tuzen et al. 2008). For determining the elements, optical emission spectrometry was employed by Rather et al. (2016), Rovira et al. (2015) and Rezic and Steffan (2007) where atomic absorption spectrometry was used by Menezes et al. (2010) and Tuzen et al. (2008). In our present study, atomic absorption spectrometry was followed. The ranges of trace element such as Al3+, Fe2+, Cu2+ and Sn2+ were found as 7.27–73.8, 5.54–29.82, 1.27–78.5 and 0.12–0.53 mg/kg respectively. Outcomes of this study were harmonious with the values of previous relevant experiment (Rather et al. 2016; Rovira et al. 2015; Rezic and Steffan 2007; Menezes et al. 2010; Tuzen et al. 2008). The level of Cu2+ was higher than that of other metals. These metals are prone to migrate onto the human skin under sweating or wet condition. According to previous investigation, depending on the migration rate of trace elements, several skin and other diseases can be occurred which were studied by dermal exposure through the skin-contact clothes (Rovira et al. 2016; Von Goetz et al. 2013; Avagyan et al. 2015; Leme et al. 2014; Sengupta and Behera 2014; Clausen et al. 2016). Averagely, the detected trace metals are typically migrated ranging 0.6–60% (Tuzen et al. 2008). Among these four metals, only Cu2+ is in the suggested list of heavy metals by the Oeko-Tex Standard 100 level (Oeko-Tex Standard 100 (Edition-1) 2011) to protect consumers and to limit the use of some chemical substances that are dangerous to human safety (Tonetti and Innocenti 2009). Moreover, the migration rate of Cu2+ was lower than that of Al3+, Fe2+ and Sn2+ respectively. Therefore, Cu2+ was in the safe zone of hazard quotient (Rezic and Steffan 2007).
Conclusions
This investigation were carried out to satisfy the color fastness properties and limited color variety which are presumed as key constrains in application of natural dyes on textile coloration. Here, cotton fabric was pre-mordanted with metallic salts to improve dye (BFS) fiber binding through fiber-metal-dye interaction via coordination bond. The color differences were represented by ΔH*, ΔC* and ΔL* respectively. Except light fastness all other color fastness properties were gratified greatly. But color variety was not attained in higher extent. For environmental hazard analysis, the metals loading were calculated from residual mordanting bath and dyeing wastewater respectively. The amounts of residual metal ions in mordant bath were beyond the acceptable limit of environmental standards. Though in dyeing wastewater iron and copper existed within the acceptable range but unfortunately the substantial amount of SO4 2− incurred from these metal salts would beyond the acceptable limit. However, the levels of trace metals present in the finished fabric were supposed as not harmful for human health. Therefore, further exploration could be continued to develop novel pathways of color multiplicity by ensuring augmented color fastness as well as to depose the forthcoming uses of metal salts.
Abbreviations
- BFS:
-
banana floral stem sap
- FTIR:
-
Fourier transform infrared
- ATR:
-
attenuated total reflectance
- L*:
-
lightness
- a*:
-
redness
- b*:
-
blueness
- c*:
-
chroma
- H:
-
hue
- ΔH*:
-
hue difference
- ΔL*:
-
value difference
- ΔC*:
-
chroma difference
- UV:
-
ultraviolet
- WI:
-
whiteness index
- BI:
-
brightness index
- AlK(SO4)2·12H2O:
-
potassium alum
- CuSO4·5H2O:
-
copper(II) sulfate pentahydrate
- SnCl2·5H2O:
-
tin(II) chloride pentahydrate
- FeSO4·7H2O:
-
iron(II) sulfate heptahydrate
- CIE:
-
Commission Internationale de l’éclairage
- ISO:
-
International Organization for Standardization
- Öko Tex Standard:
-
Internationale Gemeinschaft für Forschung und Prüfung auf dem Gebiet der Textilökologie (Association for the Assessment of Environmentally Friendly Textiles)
References
AATCC. (1996). AATCC Test Method 125-2013 Colorfastness to Light.
AATCC. (2006). AATCC Test Method 15-2013 Colorfastness to Perspiration.
AATCC. (2008). AATCC Test Method 107-2013 Colorfastness to Water.
AATCC. (2013a). AATCC Test Method 61-2013 Colorfastness to Laundering: Accelerated.
AATCC. (2013b). AATCC Test Method 8-2013 Colorfastness to Rubbing.
Al, G. A. A. W. A. (2014). Preparation and characterization of (Pva-Sncl2) composites. Academic Research International, 5(2), 66–71.
Ali, S. (2008). Evaluation of cotton dyeing with aqueous extracts of natural dyes from indigenous plants. (Doctoral dissertation, University of Agriculture, Faisalabad).
Antoine, M. L., Simon, C., & Pizzi, A. (2004). UV spectrophotometric method for polyphenolic tannin analysis. Journal of Applied Polymer Science, 91(4), 2729–2732.
Avagyan, R., Luongo, G., Thorsén, G., & Östman, C. (2015). Benzothiazole, benzotriazole, and their derivates in clothing textiles—a potential source of environmental pollutants and human exposure. Environmental Science and Pollution Research, 22(8), 5842–5849.
Barhanpurkar, S., Kumar, A., & Purwar, R. (2015). Charcterisation of banana pseudostem sap used as a mordant for dying. SSRG International Journal of Polymer and Textile Engineering, 2, 1–7.
Basak, S., Samanta, K., Saxena, S., Chattopadhyay, S. K., Narkar, R., Mahangade, R., et al. (2015). Flame resistant cellulosic substrate using banana pseudostem sap. Polish Journal of Chemical Technology, 17(1), 123–133.
Bechtold, T., Turcanu, A., Ganglberger, E., & Geissler, S. (2003). Natural dyes in modern textile dyehouses—how to combine experiences of two centuries to meet the demands of the future? Journal of Cleaner Production, 11(5), 499–509.
Bhattacharya, S. D., & Shah, A. K. (2000). Metal ion effect on dyeing of wool fabric with catechu. Coloration Technology, 116(1), 10–12.
Bhuiyan, M. R., Rahman, M. M., Shaid, A., & Khan, M. A. (2014). Application of gamma irradiated textile wastewater for the pretreatment of cotton fabric. Environment and Ecology Research, 2(3), 149–152.
Bilba, K., Arsene, M. A., & Ouensanga, A. (2007). Study of banana and coconut fibers: botanical composition, thermal degradation and textural observations. Bioresource Technology, 98(1), 58–68.
Blackburn, R. S. (2004). Natural polysaccharides and their interactions with dye molecules: applications in effluent treatment. Environmental Science and Technology, 38(18), 4905–4909.
Chung, C., Lee, M., & Choe, E. K. (2004). Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydrate Polymers, 58(4), 417–420.
Clausen, P. A., Spaan, S., Brouwer, D. H., Marquart, H., Le Feber, M., Engel, R., et al. (2016). Experimental estimation of migration and transfer of organic substances from consumer articles to cotton wipes: evaluation of underlying mechanisms. Journal of Exposure Science & Environmental Epidemiology, 26(1), 104–112.
Concentration calculation from UV Vis Absorbance. http://www.instanano.com/characterization/theoretical/concentration-calculation-from-uv-vis-absorbance/. Accessed 20 Feb 2016.
Cuyckens, F., & Claeys, M. (2004). Mass spectrometry in the structural analysis of flavonoids. Journal of Mass Spectrometry, 39(1), 1–15.
Cuyckens, F., & Claeys, M. (2005). Determination of the glycosylation site in flavonoid mono-O-glycosides by collision-induced dissociation of electrospray-generated deprotonated and sodiated molecules. Journal of Mass Spectrometry, 40(3), 364–372.
de Assis, F., Margem, F., Loiola, R., & Monteiro, S. (2014). Characterization of banana fibres functional groups by infrared spectroscopy. Materials Science Forum, 775–776, 250.
Ghaly, A. E., Ananthashankar, R., Alhattab, M. V. V. R., & Ramakrishnan, V. V. (2014). Production, characterization and treatment of textile effluents: A critical review. Journal of Chemical Engineering & Process Technology, 5(1), 1.
He, Z., Honeycutt, C. W., Xing, B., McDowell, R. W., Pellechia, P. J., & Zhang, T. (2007). Solid-state Fourier transform infrared and 31P nuclear magnetic resonance spectral features of phosphate compounds. Soil Science, 172(7), 501–515.
Ibrahim, M. M., Dufresne, A., El-Zawawy, W. K., & Agblevor, F. A. (2010). Banana fibers and microfibrils as lignocellulosic reinforcements in polymer composites. Carbohydrate Polymers, 81(4), 811–819.
International Association for Research and Testing in the Field of Textile Ecology, Oeko-Tex Standard 100, Edition 1.2011, http://www.oeko-tex.com/oekotex100_public/content1.asp?area=hauptmenue&site=grenzwerte&cls=02.
Kumar, K. S., & Bhowmik, D. (2012). Traditional and medicinal uses of banana. Journal of Pharmacognosy and Phytochemistry, 1(3), 51–63.
Lande, S. V., Kvvsbsr, M., Unnikrishnan, S., Sharma, N., & Vaidya, S. D. (2016). Spectroscopic characterization of stability and interaction of Pt–Sn complexes with different capping agents. Journal Nanoscience Current Research, 1(105), 2.
Lee, Y. H. (2007). Dyeing, fastness, and deodorizing properties of cotton, silk, and wool fabrics dyed with coffee sludge (Coffea arabica L.) extract. Journal of Applied Polymer Science, 103(1), 251–257.
Leme, D. M., de Oliveira, G. A. R., Meireles, G., dos Santos, T. C., Zanoni, M. V. B., & de Oliveira, D. P. (2014). Genotoxicological assessment of two reactive dyes extracted from cotton fibres using artificial sweat. Toxicology in Vitro, 28(1), 31–38.
Lin, L. Z., & Harnly, J. M. (2012). Quantitation of flavanols, proanthocyanidins, isoflavones, flavanones, dihydrochalcones, stilbenes, benzoic acid derivatives using ultraviolet absorbance after identification by liquid chromatography–mass spectrometry. Journal of Agricultural and Food Chemistry, 60(23), 5832–5840.
Mabry, T. J., Markham, K. R., & Thomas, M. B. (1970). The structure analysis of flavonoids by ultraviolet spectroscopy. The Systematic Identification of Flavonoids, 35–230.
McIntyre, J. F., Foley, R. T., & Brown, B. F. (1982). Ultraviolet spectra of aluminum salt solutions. Inorganic Chemistry, 21(3), 1167–1172.
Menezes, E. A., Carapelli, R., Bianchi, S. R., Souza, S. N. P., Matos, W. O., Pereira-Filho, E. R., et al. (2010). Evaluation of the mineral profile of textile materials using inductively coupled plasma optical emission spectrometry and chemometrics. Journal of Hazardous Materials, 182(1), 325–330.
Miller, F. A., & Wilkins, C. H. (1952). Infrared spectra and characteristic frequencies of inorganic ions. Analytical Chemistry, 24(8), 1253–1294.
Paul, V., Kammy, K., & Redhi, G. G. (2013). Formulation of a novel bio-resin from banana sap. Industrial Crops and Products, 43, 496–505.
Photometry. http://www.chem.uky.edu/courses/che554/2_Photometry/Photometry_Chpt1.pdf. Accessed 20 Feb 2016.
Pinheiro, P. F., & Justino, G. C. (2012). Structural analysis of flavonoids and related compounds-a review of spectroscopic applications. INTECH Open Access Publisher.
Rather, L. J., Shabbir, M., Bukhari, M. N., Shahid, M., Khan, M. A., & Mohammad, F. (2016). Ecological dyeing of Woolen yarn with Adhatoda vasica natural dye in the presence of biomordants as an alternative copartner to metal mordants. Journal of Environmental Chemical Engineering, 4(3), 3041–3049.
Repon, M. R., Al Mamun, M. A., & Islam, M. T. (2016a). Eco-friendly cotton coloration using banana (Musa sapientum) waste: optimization of dyeing temperature. Universal Journal of Engineering Science, 4(1), 14–20.
Repon, M. R., Al Mamun, M. A., & Islam, M. T. (2016b). Optimization of dyeing time of eco-friendly cotton coloration using banana (Musa sapientum) floral stem sap. Chemical and Materials Engineering, 4(2), 26–31.
Rezić, I., & Steffan, I. (2007). ICP-OES determination of metals present in textile materials. Microchemical Journal, 85(1), 46–51.
Rovira, J., Nadal, M., Schuhmacher, M., & Domingo, J. L. (2015). Human exposure to trace elements through the skin by direct contact with clothing: Risk assessment. Environmental Research, 140, 308–316.
Rovira, J., Nadal, M., Schuhmacher, M., & Domingo, J. L. (2016). Trace elements in skin-contact clothes and migration to artificial sweat: risk assessment of human dermal exposure. Textile Research Journal, 87(6), 726–738.
Sachan, K., & Kapoor, V. P. (2007). Optimization of extraction and dyeing conditions for traditional turmeric dye. Indian Journal of Traditional Knowledge, 6, 270–278.
Saleh, S. M., Abd-El-Hady, Y. A., & El-Badry, K. (2013). Eco-friendly dyeing of cotton fabric with natural colorants extracted from banana leaves. International Journal of Textile Science, 2(2), 36–40.
Samanta, A. K., & Agarwal, P. (2009). Application of natural dyes on textiles. Indian Journal of Fibre & Textile Research, 34, 384–399.
Sengupta, A., Behera, J., (2014). Organic baby skin-friendly sustainable textile draws wider attention. Journal of Asia Textile and Apparels, 25.
Sinha, K., Aikat, K., Das, P., & Datta, S. (2016). Dyeing of modified cotton fiber with natural Terminalia arjuna dye: Optimization of dyeing parameters using response surface methodology. Environmental Progress & Sustainable Energy., 35, 719–728.
Spectrophotometric analysis of copper. (2005). http://employees.oneonta.edu/kotzjc/LAB/Spec_Expt.pdf. Accessed 05 Jul 2017.
Tonetti, C., & Innocenti, R. (2009). Determination of heavy metals in textile materials by atomic absorption spectrometry: Verification of the test method. AUTEX Research Journal, 9(2), 66–70.
Tuzen, M., Onal, A., & Soylak, M. (2008). Determination of trace heavy metals in some textile products produced in Turkey. Bulletin of the Chemical Society of Ethiopia, 22(3), 379–384.
Uddin, M. G. (2015). Extraction of eco-friendly natural dyes from mango leaves and their application on silk fabric. Textiles and Clothing Sustainability, 1(1), 1–8.
Umbreen, S., Ali, S., Hussain, T., & Nawaz, R. (2008). Dyeing properties of natural dyes extracted from turmeric and their comparison with reactive dyeing. Research Journal of Textile and Apparel, 12(4), 1–11.
Vankar, P. S. (2000). Chemistry of natural dyes. Resonance, 5(10), 73–80.
Von Goetz, N., Lorenz, C., Windler, L., Nowack, B., Heuberger, M., & Hungerbuhler, K. (2013). Migration of Ag-and TiO2-(Nano) particles from textiles into artificial sweat under physical stress: Experiments and exposure modeling. Environmental Science and Technology, 47(17), 9979–9987.
Authors’ contributions
MRR and MTI planned and carried out the work. Moreover, they have also done the data analysis, interpretation and presentation part. All authors read and approved the final manuscript.
Acknowledgements
Authors would like to highly acknowledge the Bangladesh Atomic Energy Commission and Bangladesh Council of Scientific and Industrial Research for providing some excellent characterization facilities.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Repon, M.R., Islam, M.T. & Mamun, M.A.A. Ecological risk assessment and health safety speculation during color fastness properties enhancement of natural dyed cotton through metallic mordants. Fash Text 4, 24 (2017). https://doi.org/10.1186/s40691-017-0109-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40691-017-0109-x