Abstract
Background
Rapid economic development in China has resulted in an increase in severe air pollution in city groups such as the Beijing–Tianjin–Hebei Metropolitan Region. PM2.5 (fine particles with an aerodynamic equivalent diameter of 2.5 μm or less) is one of the most important pollutants. The deposition process is an important way of removing particles from the air. To evaluate the effect of an urban forest on atmospheric particle removal, a concentration gradient method was used to measure the deposition velocities of water-soluble inorganics in PM2.5 in two national forest parks in Beijing, China. The following eight water-soluble inorganic ions in PM2.5 were investigated: sodium, ammonium, potassium, magnesium, calcium, chloride, nitrate, and sulfate.
Methods
Samples were taken from two sites in Beijing from the 7th to the 15th May, 2013. The concentrations of water-soluble inorganic ions were analyzed with ion chromatography. We used the concentration gradient technique to estimate the deposition flux and velocity. To determine the relationships between leaf traits and particle accumulation, typical leaf samples from each selected species were studied using scanning electron microscopy.
Results
The total deposition flux and total deposition velocity during the daytime were higher than those at night. Sulfate showed the biggest deposition flux and velocity at both study sites, whereas the other ions showed different trends at each site. Result from higher proportion of coniferous to broadleaved trees, the total deposition flux of the eight ions measured in Jiufeng National Forest Park was greater than that in Olympic Forest Park.
Conclusions
The deposition velocity was affected by meteorological conditions such as wind speed, temperature, and humidity. The deposition velocity was also influenced by tree species. The surface of plants is an important factor influencing particle deposition. The results of this study may help in assessing the effects of forestry systems on particle removal and provide evidence for urban air pollution control and afforestation of urban areas.
Similar content being viewed by others
Background
Rapid economic development in China has resulted in an increase in severe air pollution in city groups such as the Beijing–Tianjin–Hebei Metropolitan Region. PM2.5 pollution in the megacity, Beijing, China, is one of the environmental problems and is associated with haze and low visibility. In 2015, the annual average concentration of PM2.5 was 80.6 μg·m− 3, with concentrations greater than 500 μg·m− 3 at times. Therefore, PM2.5 has received wide attention and the government has acted to control serious air pollution. The deposition process is an important way of removing particles from the air. Numerous studies have demonstrated that plants can purify air by absorbing atmospheric particles (Zhou et al. 2002). McDonald et al. (2007) found that planting trees in one-quarter of an urban area in cities in the UK, reduced PM10 concentrations by 2% to 10%. Nowak et al. (2006) concluded that urban plants removed ~ 215,000 tons of PM10 per year across the USA in 2006.
PM2.5 consists of a wide variety of components, including the water-soluble fraction, elemental carbon (EC), organic carbon (OC), and heavy metals, which are distributed across a wide range of particle sizes and therefore have a different deposition velocity in the forest system. The water-soluble fraction is an important component of PM2.5, and can directly influence the acidity, hygroscopicity, and cloud nucleation capability of aerosols (Novakov and Penner 1993). Considerable researches have been carried out on the water-soluble fraction of PM2.5 in Europe, the USA and in Asia, particularly in Beijing. However, these researches had focused on the concentrations, size distribution characteristics, source analysis, seasonal and diurnal variations in PM2.5 (Yao et al. 2002; Zhang et al. 2011; Li et al. 2013). The deposition of water-soluble inorganic ions has been rarely studied in Beijing (Cai et al. 2011), especially under the high PM2.5 concentrations observed in recent years.
Deposition flux and velocity are major indices of the deposition characteristics which can be affected by many factors. Some studies have found that the deposition velocity is affected by time interval, season, and terrain type (Feliciano et al. 2001; Matsuda et al. 2005). Zhang et al. (2004) studied the dry deposition above different underlying surfaces and showed that there was little difference between different types of particles, but there was some distinction between different gaseous pollutants. Park et al. (2002) measured the deposition velocity of nitrogen, and Jitto et al. (2007) measured the velocity of sulfur deposition. These studies each investigated only one or two species. Cai et al. (2011) sampled and analyzed dry deposition of water-soluble inorganic ions in the Beijing urban area in 2001–2005. This research showed that the monthly variation in the dry deposition of each ion was different. The lowest value of the deposition velocity of each ion occurred mainly in autumn. In 2014, Mkoma et al. (2014) conducted a detailed study on the atmospheric particle dry deposition of major ions in the South Atlantic. They found that particle dry deposition was a very important mechanism representing air-to-sea fluxes of major species.
As previously mentioned, some researches were conducted on the deposition velocities of gaseous species and particles, but there were limited data relating to PM2.5 and its components. In Beijing, the PM2.5 concentration is much higher than that in most other regions in the world, of which water-soluble inorganic ions occupied 20%–80% of total amount of PM2.5. The “Million Hectares Afforestation Strategy” has been carried out in Beijing, and more of these projects are proposed. However, it is unclear whether the underlying surface of urban forests and urban green spaces influence PM2.5 deposition. An investigation into the actual deposition velocity of PM2.5 and its main components is necessary to evaluate the impact of plants on PM2.5 removal, and obtain accurate model calculation results. In this study, we measured the deposition flux and velocity of water-soluble inorganic ions above a forestry system in Beijing, China, to determine the deposition characteristics of water-soluble inorganic ions.
Methods
Ethics statement
This study did not involve any endangered or protected species.
Sample collection and site description
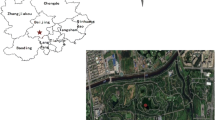
The dry deposition of water-soluble inorganic ions in PM2.5 from the 7th to the 15th May, 2013 was investigated. Samples were taken from two sites shown in Fig. 1. The first study site, Olympic Forest Park (OFP), is managed by the Beijing Olympic Forest Park Development and Management Co., Ltd. It is located in a deciduous forest in the Olympic Forest Park in Haidian District of Beijing (116°24′15″E, 40°2′2″N). The second study site, Jiufeng National Forest Park (JNFP), is managed by the Forestry Committee of Beijing Forestry University and is used for teaching and research by the university. It is located in Haidian District of Beijing (116°6′34″E, 40°4′22″N). The information of two forests is showed in Table 1.
At the OFP site, we established three sampling points on a monitoring tower surrounded by Populus tomentosa, a winter-deciduous tree species. The vertical heights of the sampling points were 9 m (approximately the middle of the tree canopy), 12 m (the upper edge of the tree canopy), and 15 m (about 3 m above the tree canopy). The JNFP monitoring site was similarly positioned with sampling points at heights of 9, 12, and 15 m. The monitoring tower was surrounded by Platycladus orientalis, an evergreen species, and Pinus massoniana (Masson pine). All sampling points were equipped with automatic meteorological station (included Temperature, Humidity, wind speed). The sample filters were replaced at 06:00, 10:00, 14:00, 18:00, and 22:00 every day, at the same time in both sites. The time period from 06:00–18:00 was considered as daytime, others as nighttime.
Materials and instruments
We obtained PM2.5 samples using aerosol samplers coupled with a size-selective inlet. When the air was drawn through the collection medium (TH-150A, Tianhong, Wuhan, China), PM2.5 were collected. The flow rate was set to 100 L·min− 1. Based on a prior comparison of several sample filters, we selected micro-quartz fiber filters (MK360, Munktell & Filtrak Gmbh, Sweden) with a diameter of 90 mm due to their low background inorganic ions, high temperature resistance, and large specific surface area. These filters also maintain stable flow at 100 L·min− 1 during the sampling period. The filters were weighed before and after particle collection by electronic balance (BT125D, Sartorius, German). The meteorological instruments (U30-NBC, ONSET, Cape Cod, Massachusetts, USA) were set on the same height with each sampler, and meteorological data were recorded automatically.
Sample storage and measurement
Before and after sampling, the filters were stored at constant temperature and humidity conditions for at least 24 h. After sampling, the specimens were kept in a clean sealed polypropylene box at − 4°C until required. We extracted the ions twice with 25 mL deionized water, for 15 min each time, and stored the extraction solution in a sealed Erlenmeyer flask. The extraction solution was then stored in a stock bottle below − 4°C. Erlenmeyer flask and stock bottle are glassware.
The concentrations of water-soluble inorganic ions were analyzed with ion chromatography (IC, ICS-3000, Dionex, Sunnyvale, California, USA). Cation column and anion column are CS12A and AS22 respectively. The following eight ions were determined: sodium (Na+), ammonium (NH4+), potassium (K+), magnesium (Mg2+), calcium (Ca2+), chloride (Cl−), nitrate (NO3−), and sulfate (SO42−).
Sample collection from leaves
To determine the amount of water-soluble components in the particles adsorbed on the leaves, five cations (Na+, NH4+, K+, Mg2+, Ca2+) and three anions (Cl−, NO3−, SO42−), were collected and analyzed from 20 plant species showed in Table 2.
The collected blades were placed in a constant volume (25 mL) of deionized water and shaken. Following filtration using a 0.22 μm pore diameter filter, the solution was placed in a clean plastic storage bottle and stored in a refrigerator. Before analysis, the solution was defrosted at ambient temperature and the concentrations of the water-soluble inorganic ions were then assessed using ion chromatography.
Scanning electron microscopy of leaves
To determine the relationships between leaf traits and particle accumulation, typical leaf samples from each selected species were studied using scanning electron microscopy (SEM) (S-3400 N II, Hitachi Japan Co., Ltd., Tokyo, Japan). Leaf samples were collected at the same time from the same plants as the samples used in the water-soluble inorganics in particle from the leaves mentioned above. Samples were stored in paper bags and dried in a dryer at 60 °C for 48 h. Micrographs were taken at two different magnifications (× 500 SE, × 2.50 k SE).
Quality control
We adopted the following quality control approaches to ensure the accuracy of our data during the study:
-
1)
To prevent contamination, the size-selective inlet, tweezers and bottles used for sample collection were ultrasonically cleaned with deionized water before sampling.
-
2)
The balance was used under constant temperature and humidity conditions. Three blank filter films were weighed, and each filter film was weighed ten times, and then the average weight was calculated. Before weighing the sampling filter films each time, the three blank filter films were weighed to confirm the balance was stable. When the change in weight of these three blank filter films was less than 0.5 mg, the balance was considered stable.
-
3)
To ensure that all the ions were completely extracted into water, we first ultrasonically extracted the water-soluble inorganic ions three times with 25 mL deionized water, for 15 min each time. No ions were detected in the third extraction solution. Therefore, the ions were extracted twice with 25 mL deionized water, for 15 min each time.
-
4)
The standard sample for ion chromatography analysis was measured continuously for 6 days, and all calculated standard deviations were lower than 5% with the exception of SO42−, which showed a standard deviation of 5.03%. For the standard curves, the correlation coefficient for Na+ and NH4+ was 0.9993 and 0.9991, respectively, and all others were higher than 0.9995. The detection limits (S/N = 3) for chloride, nitrate, sulfate, sodium, ammonium, potassium, magnesium, and calcium ions were 0.03, 0.06, 0.05, 0.01, 0.01, 0.02, 0.01, and 0.01 mg·L− 1, respectively.
Calculation of water-soluble inorganic ion fluxes and velocity
Previously published data on exact deposition flux and velocity in China are rare. We used the concentration gradient technique to estimate the deposition flux (Businger 1986; Erisman and Draaijers 1995; Wyers and Duyzer 1997). The flux was derived from measurements of air concentrations at several heights and from meteorological variables (Erisman and Draaijers 1995).
The following equation was used to estimate the flux (F, μg·m− 2·s− 1):
where u* (m·s− 1) is friction velocity, which was measured by the three-dimensional ultrasonic wind speed and direction meter (Gill, HS); and c* (μg·m− 3) was calculated using Eq. (2):
where Z1 and Z2 represent the heights of 9 and 12 m; Ψh is the integrated stability correction function in atmospheric deposition in relation to acidification and eutrophication, following Erisman and Draaijers (1995); Δc is the concentration difference in ions at 12 and 9 m, and L (m) is the Monin–Obukhov length, which can also be measured by the instrument, d (m) is displacement height, and k is the Von Karman constant (0.41). Daytime Ψh,d was calculated with Eqs (3) and (4), and Eq. (5) was applied for night conditions (Ψh,n).
From Eqs (1, 2), the flux (F) is expressed in Eq. (6) as follows:
The dry deposition velocity was calculated using Eq. (7):
Where Vd (cm·s− 1) is the dry deposition velocity, and c (μg·m− 3) is the concentration of ions at the height of 15 m.
Results and discussion
Deposition flux of water-soluble inorganic ions
Based on the concentrations of ions in PM2.5 and the meteorological data, the deposition flux and velocity of eight water-soluble inorganic ions in PM2.5 were calculated. When there was a downward deposition flux of ions (toward the ground), the value was expressed as “−”. For example, the deposition flux of sulfate was 0.5022 − (− 15.0805) μg·m− 2·s− 1, which indicated that the deposition flux range was 0.5022 (upward flux) to 15.0805 (downward flux).
The deposition flux of water-soluble inorganic ions at both sites was calculated. Eight ions (Na+, NH4+, K+, Mg2+, Ca2+ Cl−, NO3−, SO42−) were measured, and their deposition fluxes were determined to be 0.0078 − (− 0.0429), 0.0450 − (− 0.2540), 0.0936 − (− 0.3642), 0.0006 − (− 0.1020), − 0.0002 − (− 0.1920), 0.0942 − (− 0.2985), 0.1103 − (− 1.5090), and 0.5022 − (− 15.0805) μg·m− 2·s− 1, respectively. Due to the adsorption of PM2.5 by the forest, in most cases the concentrations of ions at 15 m were higher than those at 9 m. Figure 2 also shows this trend, but in a few cases, the ion concentrations decreased with increased height, which may have been due to the effect of instantaneous meteorological conditions. In general, the total deposition flux of the eight ions measured at the JNFP site was greater than that at OFP. This may have been due to differences in tree species and spatial density of trees, as mentioned in the Methods section. At JNFP, the sampling site was surrounded by P. orientalis and P. massoniana. At OFP, the surrounding plants were mainly Populus tomentosa. Trees with a complex leaf structure have a high capacity for adsorbing PM2.5. The leaf structure of P. orientalis and P. massoniana is more complex than that of P. tomentosa. This was demonstrated by the leaf SEM results. The SEM results showed that P. orientalis and P. massoniana in JNFP had more deep folds and villi than P. tomentosa in OFP which retained more particles. The spatial density of trees in JNFP was 600–750 ha− 1, and was 750–900 ha− 1 in OFP. The higher tree density provided more leaf area, and absorbed more particles.
The total deposition flux of all ions in JNFP during the night was lower than that in OFP. The OFP is located in an urban area; thus, the concentrations of ions were higher. The concentrations of particle are known to influence the deposition flux. Table 3 shows the average deposition flux of all water-soluble inorganic ions during the daytime and night at the two sampling sites. It can be seen that the deposition flux was higher during the daytime than at night. In both the JNFP and OFP, sulfate showed the largest deposition flux compared with the other ions. The highest deposition flux of sulfate was found in the JNFP. In addition to sulfate, ammonium, sodium and nitrate ions also showed a higher deposition flux in JNFP than in OFP. In contrast, potassium, magnesium, calcium and chloride ions showed a higher deposition flux in OFP than in JNFP.
As we seen from Table 4, time had significant effect on every ions deposition, and site had significant effect on 3 ions (K+, Mg2+, SO42−). It shows that time was an important factor affecting the deposition flux, while the site had less impact.
Deposition velocity of water-soluble inorganic ions
As shown in Table 5, the deposition velocities of all ions measured in the JNFP were higher than those in the OFP and the deposition velocities were higher during the daytime than at night. Matsuda et al. (2010) showed the same trend in a deciduous forest at the eastern foot of Mt. Asama (36°240′N, 138°350′E, 1380 m above sea level), Nagano Prefecture, central Japan, where the dominant canopy tree species was birch (Betula ermanii). They found that the deposition velocity of sulfate was 0.9 ± 1.0 cm·s− 1 during the daytime and 0.3 ± 0.3 cm·s− 1 at night. P. orientalis and P. massoniana, which were the main tree species at the JNFP sampling site, are both evergreen species. In the OFP, the main tree species at the sampling site was P. tomentosa, which is a winter-deciduous tree species. Cheng et al. (2004) showed that coniferous forest have a stronger capacity for capturing PM2.5. The results of our study support this finding.
We also measured meteorological parameters during the study period (Fig. 3) including wind speed, humidity, and temperature.
Same with deposition flux, time had significant effect on deposition velocity, but sampling site had little effect. It can be seen from Table 6, time had significant effect on 5 ions Vd (Na+, K+, Cl−, NO3−, SO42−). It means that there was difference between daytime and nighttime deposition velocities. Only deposition velocities of Na+ and NO3− had significant correlation with sampling site. It can be seen that, the difference of impact of two forest systems on water-soluble ions deposition was insignificant.
Table 7 shows the meteorological conditions in the OFP and JNFP. The wind speed and temperature were higher during the daytime than at night in both the OFP and JNFP, whereas humidity showed the opposite trend. We analyzed the relationship between the deposition velocity and meteorological conditions. The results indicated that deposition velocity was positively correlated with wind speed and relative humidity. Higher wind speed can contribute to particle deposition, and higher humidity may enhance deposition. The higher wind speed will increase the air turbulence, and the higher humidity will make the ultra-fine particles larger by absorb the water. These will enhance the deposition of particles. Recent studies have shown that the deposition velocity of aerosol particles is strongly affected by aerodynamic conditions (Slinn 1982; Wesely et al. 1985; Ruijgrok et al. 1997). The friction velocity can be influenced by the crown canopy of different tree species. Therefore, the deposition velocity may be influenced by sub-layer surface roughness (Matsuda et al. 2005). We also found that the particle deposition velocity was affected by friction velocity (Fig. 4).
Similar to deposition flux, the deposition velocity of sulfate ions was highest. The deposition velocities of ammonium, chloride and nitrate ions were lower. Since there have been few studies on the dry deposition of water-soluble inorganic ions in PM2.5 in China, our results were compared with other researches in Asia. Endo et al. (2011) reported that the deposition velocity ranges of sulfate, nitrate and ammonium were 0.1–2.4, 0.2–2.9, and 0.1–2.3 cm·s− 1, respectively. The deposition velocities of sulfate, nitrate and ammonium in our study were within these ranges. Table 8 showed the results of this work and other studies for reference treeless green spaces. Overall, the deposition velocities in our research is almost equal to Xing et al. (2018). Compared with the result of Chu et al. (2008), the velocities of nitrate ions were slightly lower, and velocities of sulfate ions were slightly higher. Whereas velocities of other ions were much lower. The differences may be due to different underlying surface and sampling periods. Xing et al. (2018) investigated in Jiaozhou Bay during a year. The sampling site (36°03′19.46″N, 120°20′25.11″E) was located on the roof of a ~ 15 m high building at the Institute of Oceanology, Chinese Academy of Sciences, where the shortest distance to the nearest shoreline is only 20 m. Additionally, no obvious barriers are located nearby. And Chu et al. (2008) investigated in August, September and October. The sampling position was set on the roof of a building (25 m) in the campus of Hungkuang University which is on the top of Da Du Mountain. The mountain is 500 m high. This sampling position was near highway and Taiwan Straits for about 100 m and 10 km, respectively.
Effect of leaf surface on deposition
The surface of plants is an important factor influencing particle deposition. Due to the large canopy surface area of leaves, stems and branches and the air turbulence created by their structure, trees have a greater ability to capture particles than smaller vegetation (Tallis et al. 2011; Sun et al. 2014; Mo et al. 2015). Different leaf characteristics (such as wrinkles, roughness, pubescence, mucus and the wettability of leaves) can also result in a different capacity to retain particles. In addition, leaf structure, canopy shape, density of branches and leaves can also influence the ability of a plant to capture particles. Research has shown that a rough leaf blade can retain particles more easily. The main tree species in the JNFP, P. orientalis, is associated with greater deposition due to the scale patterns and grooves on its leaves. In contrast, the leaf shape of P. tomentosa is ovoid and the leaf surface is smooth; therefore the capacity of these trees in the OFP to retain particles was weaker than those in the JNFP. This supports the finding of Mitchell et al. (2010) who found that surface morphology appears to be a dominant factor in particle deposition on the leaf surface, and that ridged and hairy leaves exhibit greatest deposition velocities. In terms of canopy shape, P. orientalis also showed greater deposition capacity because of its wide circular canopy shape compared with the conical canopy of P. tomentosa. Trees can capture airborne particles, especially fine particles (diameter ≤ 2.5 μm), due to their large leaf area index (LAI; the ratio of total one-sided leaf area to the projected area of the canopy on the ground), which corresponds to available deposition surfaces (Freiman et al. 2006). During the sampling period, the leaf area index of trees in the JNFP and OFP was 3.8 and 1.8, respectively. Deposition is stronger if a higher proportion of coniferous to broadleaved trees is present (Tallis et al. 2011). P. massoniana is a coniferous species and P. tomentosa is a broadleaved tree species. Yang et al. (2015) ranked common urban tree species on the basis of their ability to control PM2.5 pollution. The results showed that conifer species demonstrated high PM2.5 removal efficiency (Yang et al. 2015), which was in agreement with field observations (Beckett et al. 2000; Sæbø et al. 2012). We also found that the JNFP site with P. orientalis and P. massoniana as the main tree species had a higher deposition flux and velocity than the OFP site with P. tomentosa and Sophora japonica as the main tree species.
We applied the method reported by Dzierżanowski et al. (2011) to collect particles on the leaf surface, and then measured the concentration of water-soluble ions in the particles. The particles obtained using this method contained not only fine particles, but also included particles with a diameter > 2.5 μm. Figure 5 shows the percentages of the eight different ions. It is clear that nitrate, calcium, and sulfate ions had the highest concentrations. The high nitrate concentration in the particles on the leaf surface may be from coarse particles. During the process of adsorption and desorption, a considerable amount of nitrate is transferred from fine particles to coarse particles. In their researches on the size distribution of water-soluble inorganic ions conducted in Fukang, China, Miao et al. (2014) reported that all ions except potassium showed higher concentrations in coarse particles than in fine particles, especially nitrate, with a concentration in coarse particles twice that in fine particles. This phenomenon appeared only in the spring ploughing period, which was the same as our observation period.
We investigated the surface of 20 species of plant leaves using SEM. The corresponding size microstructure of the PM2.5 on leaf surfaces was clearly observed. Four species of plants which are representative and typical were evaluated in this study. The upper surface of the four plants was magnified 500 times (Fig. 6). Figure 6 also shows that the particles were adsorbed on the leaves of all the studied plants. The surface microstructure, roughness and degree of wrinkle of the different plant leaves were dissimilar. Of these, the upper surface of Populus canadensis leaves was smooth with only slightly raised lines. Fraxinus mandshurica leaves had deep folds, with a high density of deep trenches. Wrinkling of the upper leaves in the other plants was moderate. Of these, the leaves of Crataegus pinnatifida had large folds on the upper surface accompanied by obvious fine folds. From the SEM images of the leaf surfaces of the studied plants, a distinct gully near the vein was observed.
On the basis of Fig. 6, the upper surface of the plant blade was enlarged further, and we observed the leaf surface morphology when magnified 2500 times. Figure 7 clearly shows the PM2.5 adsorbed on the leaf surface, and the microscopic structure of the leaves. The microstructure of leaves and the adsorption of particles were as follows: The amount of PM2.5 adsorbed on the leaves was greater than that of PM10 and larger particles. PM2.5 was observed on the smooth area, the shallow and deep folds and the villi. PM1 was observed on the villi, and less PM1 was observed in other areas. More large particles were buried in the leaf folds, and many small particles were observed in the vicinity of large particles.
Conclusion
In this study, we investigated the deposition flux and velocity of atmospheric particles in the OFP and JNFP in Beijing, China, using a concentration gradient method. By calculating the deposition flux and velocity of eight water-soluble inorganic ions (sodium, ammonium, potassium, magnesium, calcium, chloride, nitrate, and sulfate) in PM2.5 during different time periods, the deposition characteristics of these ions above the forestry system were determined. In general, the total deposition flux of the eight ions measured in the JNFP was greater than that in the OFP. This can be explained by the difference in tree species at the sampling sites, and the different meteorological conditions. The total deposition flux during the daytime was higher than that at night. Sulfate showed the largest deposition flux in both the JNFP and OFP, whereas other ions showed different characteristics at these two sampling sites. Similar to deposition flux, the deposition velocities of all eight ions were higher in the JNFP than in the OFP and were higher in the daytime than at night. We also demonstrated that deposition velocity was affected by friction velocity, tree species and meteorological conditions (wind speed, temperature, and humidity).
Abbreviations
- JNFP:
-
Jiufeng National Forest Park
- OFP:
-
Olympic Forest Park
- SEM:
-
Scanning electron microscope
References
Beckett KP, Freersmith PH, Taylor G (2000) Effective tree species for local air quality management. J Arboricult 26(1):12–19
Businger J (1986) Evaluation of the accuracy with which dry deposition can be measured with current micrometeorological techniques. J Clim Appl Meteorol 25(8):1100–1124
Cai YY, Yang FM, He KB, Ma YL, Tomoaki O, Shigeru T (2011) Characteristics of water-soluble ions in dry deposition in urban Beijing. China Environm Sci 31(7):1071–1076
Cheng Z, Wu J, Liu Y, Li H, Xiong Y, Li H, Li L (2004) Effects of main afforestation tree species on dust blocking in Yueyang City. J Chin Urban Forest 2:009
Chu CC, Fang GC, Chen JC, Yang IL (2008) Dry deposition study by using dry deposition plate and water surface sampler in Shalu, Central Taiwan. Environ Monit Assess 146(1–3):441–451
Dzierżanowski K, Popek R, Gawrońska H, Sæbø A, Gawroński SW (2011) Deposition of coniferous matter of different size fractions on leaf surfaces and in waxes of urban forest species. Int J Phytoremediat 13(10):1037–1046
Endo T, Yagoh H, Sato K, Matsuda K, Hayashi K, Noguchi I, Sawada K (2011) Regional characteristics of dry deposition of sulfur and nitrogen compounds at EANET sites in Japan from 2003 to 2008. Atmos Environ 45(6):1259–1267
Erisman JW, Draaijers GP (1995) Atmospheric deposition: in relation to acidification and eutrophication. Elsevier, Amsterdam
Feliciano MS, Pio CA, Vermeulen AT (2001) Evaluation of SO2 dry deposition over short vegetation in Portugal. Atmos Environ 35(21):3633–3643
Freiman MT, Hirshel N, Broday DM (2006) Urban-scale variability of ambient particulate matter attributes. Atmos Environ 40(29):5670–5684
Jitto P, Vinitnantarat S, Khummongkol P (2007) Dry deposition velocity of sulfur dioxide over rice paddy in the tropical region. Atmos Res 85(1):140–147
Li X, Wang L, Ji D, Wen T, Pan Y, Sun Y, Wang Y (2013) Characterization of the size-segregated water-soluble inorganic ions in the Jing-Jin-Ji urban agglomeration: spatial/temporal variability, size distribution and sources. Atmos Environ 77:250–259
Matsuda K, Fujimura Y, Hayashi K, Takahashi A, Nakaya K (2010) Deposition velocity of PM2.5 sulfate in the summer above a deciduous forest in Central Japan. Atmos Environ 44(36):4582–4587
Matsuda K, Watanabe I, Wingpud V, Theramongkol P, Khummongkol P, Wangwongwatana S, Totsuka T (2005) Ozone dry deposition above a tropical forest in the dry season in northern Thailand. Atmos Environ 39(14):2571–2577
Mcdonald AG, Bealey WJ, Fowler D, Dragosits U, Skiba U, Smith RI, Donovan RG, Brett HE, Hewitt CN, Nemitza E (2007) Quantifying the effect of urban tree planting on concentrations and depositions of PM in two UK conurbations. Atmos Environ 41(38):8455–8467
Miao HY, Wen TX, Wang YS, Liu ZR, Wang L, Lan ZD (2014) Size distributions of water-soluble inorganic ions in atmospheric aerosols in Fukang. Environm Sci 35(6):2045–2051
Mitchell R, Maher BA, Kinnersley R (2010) Rates of particulate pollution deposition onto leaf surfaces: temporal and inter-species magnetic analyses. Environ Pollut 158(5):1472–1478
Mkoma SL, Da Rocha GO, Domingos JSS, Santos JVS, Cardoso MP, Da Silva RL, De Andrade JB (2014) Atmospheric particle dry deposition of major ions to the South Atlantic coastal area observed at Baía de Todos os Santos, Brazil. An Acad Bras Ciênc 86(1):37–55
Mo L, Ma Z, Xu Y, Sun F, Lun X, Liu X, Chen J, Yu X (2015) Assessing the capacity of plant species to accumulate particulate matter in Beijing, China. PLoS One 10(10):e0140664
Novakov T, Penner JE (1993) Large contribution of organic aerosols to cloud-condensation-nuclei concentrations. Nature 365(6449):823
Nowak DJ, Crane DE, Stevens JC (2006) Air pollution removal by urban trees and shrubs in the United States. Urban For Urban Green 4(3–4):115–123
Park SU, Lee YH, Lee EH (2002) Estimation of nitrogen dry deposition in South Korea. Atmos Environ 36(31):4951–4964
Ruijgrok W, Tieben H, Eisinga P (1997) The dry deposition of particles to a forest canopy: a comparison of model and experimental results. Atmos Environ 31(3):399–415
Sæbø A, Popek R, Nawrot B, Hanslin HM, Gawronska H, Gawronski SW (2012) Plant species differences in particulate matter accumulation on leaf surfaces. Sci Total Environ 427:347–354
Slinn WGN (1982) Predictions for particle deposition to vegetative canopies. Atmos Environ 16(7):1785–1794
Sun F, Zhe Y, Lun X, Yang Z, Li R, Shi F, Yu X (2014) Deposition velocity of PM2.5 in the winter and spring above deciduous and coniferous forests in Beijing, China. PLoS One 9(5):e97723
Tallis M, Taylor G, Sinnett D, Freer-Smith P (2011) Estimating the removal of atmospheric particulate pollution by the urban tree canopy of London, under current and future environments. Landscape Urban Plan 103(2):129–138
Wesely ML, Cook DR, Hart RL, Speer RE (1985) Measurements and parameterization of particulate sulfur dry deposition over grass. J Geophys Res-Atmos 90(D1):2131–2143
Wyers GP, Duyzer JH (1997) Micrometeorological measurement of the dry deposition flux of sulphate and nitrate aerosols to coniferous forest. Atmos Environ 31(3):333–343
Xing J, Song J, Yuan H, Wang Q, Li X, Li N, Duan L, Qu B (2018) Water-soluble nitrogen and phosphorus in aerosols and dry deposition in Jiaozhou Bay, North China: deposition velocities, origins and biogeochemical implications. Atmos Res 207:90–99
Yang J, Chang Y, Yan P (2015) Ranking the suitability of common urban tree species for controlling PM2.5 pollution. Atmos Pollut Res 6:267–277
Yao X, Chan CK, Fang M, Cadle S, Chan T, Mulawa P, He K, Ye B (2002) The water-soluble ionic composition of PM2.5 in shanghai and Beijing, China. Atmos Environ 36(26):4223–4234
Zhang T, Cao JJ, Tie XX, Shen ZX, Liu SX, Ding H, Han YM, Wang GH, Ho KF, Qiang J, Li WT (2011) Water-soluble ions in atmospheric aerosols measured in Xi'an, China: seasonal variations and sources. Atmos Res 102(1):110–119
Zhang Y, Wang T, Hu Z, Xu CK (2004) Temporal variety and spatial distribution of dry deposition velocities of typical air pollutants over different landuse types. Clim Environm Res 9(4):591–604
Zhou Z, Shao T, Wang P, Gao C, Xu Y, Guo E, Xu L, Ye Z, Peng X, Yu C (2002) The spatial structures and the dust retention effects of green-land types in the workshop district of Wuhan iron and steel company. Acta Ecologica Sinica 22(12):2036-2040
Acknowledgments
We would like to thank the Beijing Municipal Bureau of Landscape and Forestry and Beijing Forestry University for their help and for permitting our access to the park to conduct experiments.
Funding
This work was supported by the grants from the Beijing Collaborative Innovation Center for eco-environmental improvement with forestry and fruit trees (PXM2017_014207_000024), the Special Found for Beijing Common Construction Project for Beijing Laboratory of Urban and Rural Ecological Environment, Beijing Municipal Education Commission.
Availability of data and materials
All data and materials can be obtained by requesting from the author.
Author information
Authors and Affiliations
Contributions
JD, YC and RL contributed to the experiments. JD and XL contributed to the data analysis. All authors contributed to the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ding, J., Lun, X., Ma, W. et al. Deposition of water-soluble inorganic ions in PM2.5 in a typical forestry system in Beijing, China. For. Ecosyst. 5, 36 (2018). https://doi.org/10.1186/s40663-018-0150-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40663-018-0150-2