Abstract
Background
Cocaine- and amphetamine-regulated transcript (CART), discovered initially by via differential display RT-PCR analysis of brains of rats administered cocaine, is expressed mainly in central nervous system or neuronal origin cells, and is involved in a wide range of behaviors, such as regulation of food intake, energy homeostasis, and reproduction. The hens egg-laying rate mainly depends on the developmental status of follicles, expression of CART have not been identified from hen follicles, the regulatory mechanisms of CART biological activities are still unknown. The objective of this study was to characterize the mRNA expression of CART in hen follicular granulosa cells and determine CART peptide localization and regulatory role during follicular development.
Methods
Small white follicles (1–2 mm in diameter) were treated for RNA isolation; Small white follicles (1–2 mm in diameter) and large white follicles (4–6 mm in diameter) were treated for immunohistochemical localization and large white follicles (4–6 mm in diameter), small yellow follicles (6–8 mm in diameter), large yellow follicles (9–12 mm in diameter), mature follicles (F5, F4, F3, F2, F1, >12 mm in diameter) were treated for RNA isolation and Real time PCR.
Results
The results showed that full length of the CDS of hen CART was 336 bp encoding a 111 amino acid polypeptide. In the hen ovary, CART peptide was primarily localized to the theca layer, but not all, the oocyte and granulosa layer, with diffused, weaker staining than relative to the theca cell layer. Further, amount of CART mRNA was more (P < 0.05) in granulosa cells of 6–8 mm follicles compared with that in granulosa cells of other follicles. However, CART mRNA amount was greater in theca cells of 4–6 mm follicles relative to follicles of other sizes (P < 0.05).
Conclusions
Results suggest that CART could play a potential role in developmental regulation of chicken follicles.
Similar content being viewed by others
Background
The reproductive performance of hens, especially the egg-laying rate depends mainly on the developmental status of follicles, Follicular development process has the priority features. According to the diameter, follicles can be divided into mature follicles and immature follicles. Mature follicles, the follicles before ovulation (F1, F2, F3, F4, F5\(\ldots\)), sometimes can be up to 40 mm in diameter. Immature follicles can be divided into small white follicle (SWF, <2 mm), large white follicles (LWF, 3–5 mm), small yellow follicles (SYF, 6–8 mm) and large yellow follicles (LYF, 9–12 mm) [1, 2]. In immature follicles, there is a cuboidal cells layer and basement membrane. As follicle volume increases, granulosa cells begin to proliferate, and theca gradually forms in connective tissue outside the basement membrane, and central egg yolk accumulates. In these small follicles, only a single follicle per day is selected from the cohort of follicles of 6–8 mm in diameter into the pre-ovulatory hierarchy to begin rapid growth and final differentiation. Ovaries were studied and only two types of atresia were identified—non-bursting and bursting. Smaller, non-yolky follicles (<1 mm diameter) showed non-bursting atresia. Atresia in follicles >1 mm diameter was invariably of the bursting type [3]; these two types are related to the developmental stage or size of the follicle that becomes atretic.
CART is an endogenous neuropeptide which is widespread in animals. Kobayashi et al. [4] first discovered CART mRNA expressed in cow ovaries, which was localized in the antral follicles oocytes, granulosa and cumulus cells by immunohistochemistry and in situ hybridization. Further research found that when granulosa cells were treated with a certain dose of CART, the generation of E2 was inhibited in granulosa cells, the effect depends on the stage of cell differentiation, suggesting that CART could play a crucial role in regulating follicle atresia [5]. Lv et al. [6] showed that CART mRNA amounts in subordinate follicles were significantly greater than that in dominant follicles. E2 secretion levels decreased by CART injection in early dominant follicle, and CYP19A1 (Cytochrome P450, family 19, subfamily A, polypeptide 1) mRNA expression levels reduced in granulosa cells, demonstrated that CART could cause bovine follicular atresia. For mammals, E2 was synthesized and secreted by granulosa cells, but for poultry, E2 was mainly synthesized and secreted by theca cells [7]. Jochnson et al. [8] found estradiol was feedback by paracrine pathway to control the secretion of progesterone in granulosa cells, directly affected on the follicle, involved in regulating ovulation. With estrogen increasing, the sensitivity of follicles to hormone increased, play a decisive role to the formation of the dominant follicle, low estrogen synthesis follicles become blocked, and eventually apoptosis [1, 9, 10]. Recently, our laboratory has confirmed that CART plays a crucial role in inhibiting the proliferation of granulosa cells and the secretion of E2 in cattle, pig and sheep by cell culture in vitro [6, 11, 12]. CART acts as a potent inhibitor of promoting granulosa cells apoptosis by down-regulating FSH-induced cAMP amount, E2 accumulation and aromatase mRNA levels [13, 14]. It is unknown if CART is expressed in the follicles of laying hens. The relationship between the CART expression and follicular development in different stages of laying hens remains to be determined. Thus, in this study, we hypothesized that CART is expressed in laying hens’ follicle. Immunohistochemical localization and qRT-PCR were performed to detect the CART mRNA expression in granulosa cells and theca cells in different sizes of hens’ follicles.
Methods
Animals
All animal experiments in this study were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Six healthy hens were selected, and ovaries were collected and follicles in different diameters were treated for RNA isolation, tissue fixing and the separation of granulosa cells and theca cells, respectively.
RNA isolation and cDNA synthesis
Total RNA was isolated from the small white follicles of hens using Trizol (Takara, Dalian, China) according to the manufacturer’s instructions. Isolated RNA was dissolved in RNase-free sterile water treated with 0.1% (vol/vol) diethylpyrocarbonate. Before cDNA synthesis, 2.5 μg total RNA were incubated with 2.0 μL 5 × gDNA eraser buffer and 1.0 μL gDNA eraser (Takara, Dalian, China) at 42 °C for 2 min to remove genomic DNA. Then 4 μL of 5 × PrimeScript® Buffer 2,1 μL of RT Primer Mix,1 μL of PrimeScript® RT Enzyme Mix I (Takara, Dalian, China), and RNA free water up to 20 μL. The cDNA was synthesized at 37 °C for 15 min and 85 °C for 5 s, transferred to a sterile screw-cap micro-centrifuge tube, and stored at −20 °C for further use.
Cloning of CART cDNA
The chicken CART gene sequence is not available in the National Center for Biotechnology Information (NCBI) GenBank database. Thus, DNAMAN software was used to identify the similarity of bovine, human being, rat and porcine CART cDNA sequences, a pair of primers were designed for PCR amplification of the hen CART cDNA sequence (Table 1). The identity of CART amplicons generated via RT-PCR was determined by agarose gel electrophoresis analysis. Total RNA from small white follicles from adult ovary were reverse transcribed, and respective cDNAs were amplified by PCR. The cycler program used consisted of 35 cycles at 94 °C for 30 s, 57 °C for 30 s, and 72 °C for 1 min, with a final extension at 72 °C for 5 min. The amplified cDNA encoding partial CART was ligated into the pMD 19-T Vector (TAKARA, Dalian, China). Plasmids containing inserts of interest were then subjected to fluorescent dye terminator sequencing via Beijing Genomics Institute (BGI, China).
Immunohistochemical localization of the CART peptide
Small white follicles (1–2 mm in diameter) and large white follicles (4–6 mm in diameter) were collected at a local abattoir from ovaries of three different hens. Samples were placed in a plastic tissue cassette, fixed in Bouin’s buffer for 20–24 h, washed in 70% (vol/vol) alcohol until the yellow color is gone. Then tissues were dehydrated and embedded in paraffin. Immunohistochemical localization of the CART peptide was performed using previously described procedures [15] using rabbit anti rat CART (55–102) polyclonal antisera (Phoenix Pharmaceuticals, Inc., Belmont, CA) at a 1:2000 dilution. Parallel controls were used, including sections incubated with a similar dilution of normal rabbit serum or rabbit anti-CART serum that had been pre-incubated overnight at 4 °C with 10 g/mL rat CART (55–102) peptide (American Peptide Co., Sunnyvale, CA). Ten serial sections from each sample were examined.
The isolation of granulosa cells and theca cells
Ovaries were collected at a local abattoir based on the follicular diameter: the first largest, second largest, third largest, fourth largest, fifth largest follicles (F1, F2, F3, F4, F5) and the 9–12, 6–8, 4–6 mm follicles (n = 5) were dissected, and washed with 0.9% saline. Follicular fluid was aspirated from each follicle, stored in −20 °C refrigerator. Follicle shell was cut so that it was almost bisected, but not completely cut through. The inner wall of the follicle was gently scraped to remove the granulosa cells (scrape slightly only one time so as not to get theca cells). The follicle shell was then removed from the watch glass and placed in a petri dish with media for theca isolation. Medium containing granulosa cells was transferred to a sterile 15 mL tube on ice containing 2 mL medium using pipette. The watch glass with remaining cells was rinsed with medium which was then transferred into a 15 mL tube. The theca cells were then isolated under a stereomicroscope. Using 2 pairs of fine forceps to peel the theca interna (yellow) from the theca externa (white), starting at the edges of the cut flaps. The isolated granulosa cells and theca cells were frozen in liquid nitrogen for 5 s, then stored in −80 °C refrigerator before RNA extraction.
Quantitative real-time PCR
Real-time RT-PCR was used to quantify amounts of CART mRNA in granulosa cells and theca cells. Total RNA from both types of cells were used for analysis (n = 5 each). Synthesis of cDNA was performed as described above. Primers were designed using the Primer premier 5.0 program (http://www.premierbiosoft.com) with chicken CART nucleotide sequence obtained above. The PCR mixture contained 100 ng cDNA, 10 μL SYBR® Premix Ex TaqII (TAKARA, Dalian, China), ROX Reference Dye II 0.4 μL, 8 pM forward and reverse primer (CART-R-F, CART-R-R, Table 1) in a total reaction volume of 20 μL. As an internal control, the amount of β-actin mRNA in each sample was quantified using chicken β-actin gene specific primers (primers are listed in Table 1). Reactions were performed in duplicate for each sample in an ABI PRISM 7000 Sequence Detection System (Applied Bio-systems). The thermal cycler program consisted of 45 cycles of 95 °C for 5 s and 60 °C for 30 s. The amounts of CART and β-actin mRNA in each sample were determined by comparison of cycle threshold for each sample with respective β-actin mRNA’s. The relative mRNA expression level of AGTR2 was calculated using the comparative 2−ΔΔCT method [16].
Statistical analysis
The amount of CART mRNA and β-actin mRNA in follicles was analyzed using the general linear model procedure of SPASS (version 17.0, USA). Amounts of CART mRNA were normalized relative to β-actin mRNA, and data were log-transformed before analyses. Data are shown as mean ± SE.
Results
Cloning and sequence analysis of hens CART CDS
A complete hen CART CDS was obtained by PCR, 336 bp in length. The nucleotide sequence of hen CART displayed 90.8% similarity to Parus major and 75.3–79.2% of shared identity with others species (Fig. 1). In order to examine the relationship of hen CART and its counterparts in various other organisms, a phylogenetic tree of CART peptides from hen and other species was constructed (Fig. 2). The topology of the tree demonstrated that there were six groups in the entire alignment of animals including mammalia, verschiedene fischgerichte, primates, reptiles, birds and rodents. The phylogenetic analysis showed that hen CART peptide was closely related to Parus Major CART.
Multiple alignment of nucleotide sequences of hen ovarian follicular CART with other species. Identical/similar sequences were highlighted in black/pink, white and blue background in corresponding species. Hyphens indicated gaps in order to optimize the alignment. The last line indicated consensus nucleotide of different species
Intra-follicular expression of CART peptide
The intra-ovarian localization of the CART peptide was determined using immunohistochemistry (Fig. 3). Prominent CART immune-reactivity was localized to the theca layer. CART immune-reactivity was also localized to the granulosa layer, but with diffused, weaker staining than the theca cell layer. Significant immune-reactivity in the granulosa cells, cumulus cells, and theca cells were not detected when adjacent sections were incubated with normal rabbit serum or when the CART antiserum was pre-absorbed with excess CART peptide.
Expression of CART peptide in chicken ovaries by immunohistochemistry (×400). a, d Micrograph of adjacent sections (to that depicted in b, e) were incubated with normal rabbit serum. b, e representative micrograph of section through stroma of adult hen ovary follicles were incubated with rabbit anti-CART serum. c, f, Micrograph of adjacent section (to that depicted in b, e) were incubated with rabbit anti-CART serum pre-absorbed with an excess of CART peptide. a–c, small white follicles (1–2 mm in diameter); d–f large white follicles (4–6 mm in diameter). GC granulosa cell layer, TC theca cell layer, CC Cumulus cells. a–f: Magnification 400; scale bar 20 µm
Differential expression of CART mRNA
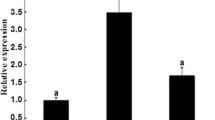
To further determine the association of CART expression with stages of follicular growth and development, the expression of CART mRNA in granulosa cells and theca cells of different size follicles (n = 5 each) was determined. As expected, amount of CART mRNA was more (P < 0.05) in granulosa cells of 6–8 mm follicles compared with that in granulosa cells of other follicles except F1. The CART mRNA amounts were greater in F1 granulosa cells than that in 9–12 mm follicles, F5 and F2 follicles (P < 0.05) (Fig. 4, White column). However, CART mRNA amount was greater in theca cells of 4–6 mm follicles relative to follicles of other sizes (P < 0.05) (Fig. 4, Black column). In every follicle of different size, the expression of CART mRNA was higher in theca cells than in the same size follicle’s granulosa cells (P < 0.05) (Fig. 4).
Relative expression of CART mRNA in granulosa cells (White column) and theca cells (Black column) of the follicles in different sizes. Note: 1: 4–6 mm follicles (large white follicles); 2: 6–8 mm follicles (small yellow follicles); 3: 9–12 mm follicles (large yellow follicles); 4: F5; 5: F4; 6: F3; 7: F2; 8: F1 follicles (mature follicles, F1>F2>F3>F4>F5, and all the five follicles are >12 mm in diameter). (Superscript small letters indicate significantly different, values with the same letters were not significantly different and values with the different letters were significantly different at the level of 0.05)
Discussion
Poultry follicular development is a highly dynamic physiological process, which is coordinated by a variety of hormones and cytokines remote autocrine, paracrine and autocrine and other means to promote granulosa cell proliferation and differentiation, endometrial cells appear and oocyte maturation, direct or indirect control of follicular development occurs and until ovulation [9, 17]. CART mRNA expressed in hypothalamus of multiple mammalian [18], and CART mRNA has been detected in follicles of cattle [11, 14], pig [12] and sheep [19]. We found CART mRNA and protein were expressed in follicles of hen too.
Evidence indicates CART is a novel intraovarian regulator of follicular development in numerous species. The mature CART is a potent negative regulator of FSH-induced [11, 14] and IGF1-induced [20] E2 production in vitro and can inhibit follicular E2 production in vivo [6]. In cattle, follicular fluid CART concentrations in healthy follicles decrease after dominant follicle selection, and CART mRNA is lower in healthy vs atretic follicles collected before and early after initiation of follicle dominance, suggestive of a regulatory role in the selection process [6]. The inhibitory actions of CART on FSH signaling and E2 production depend on the Go/i-subclass of inhibitory G proteins and are linked to multiple components of the FSH signal transduction pathway, resulting in reduced CYP19A1 mRNA and E2 production [11, 14]. CYP19A1 is the steroidogenic enzymes responsible for androgen synthesis and the aromatization of androgens to estrogens [21]. In immature chicken ovaries, exogenous FSH induces steroidogenesis by increasing CYP19A1 mRNA expression and subsequent progesterone synthesis [22]. qRT-PCR results showed that CART mRNA expression level was significantly higher within the largest pre-ovulatory follicle (F1) granulosa cells than that in those follicles with >12 mm in diameter (F5 and F2), this is consistent with Tilly’s results [23], indicating the negative relationship between CART expression levels and estrogen amounts. CART mRNA were greatly expressed in theca cell layer of the follicles (4–6 mm), resulting in an increasing of CART peptide expression of small yellow follicles (6–8 mm) in the next stage. Previous research suggested that theca cell layers of follicles (4–6 mm) were thinner than that in other follicles, and the theca cells layers are the main sources of estrogen and testosterone [23,24,25], inhibiting the synthesis and secretion of E2. It further validates that small yellow follicle is selected and then develops into a preovulatory follicles [26, 27]. Preovulatory follicles rarely become atretic under normal physiological conditions. Follicle recruitment into the preovulatory hierarchy is accompanied by the first evidence of FSH-induced cAMP accumulation [23] and increased basal levels of LH receptor (LHR) mRNA [28] within the rapidly differentiating granulosa cell layer.
CART treatment of ovine granulosa cells had pronounced inhibitory effects on FSH-induced E2 production and blocked the FSH-induced increase in granulosa cells numbers observed over 7 day culture period [29], and results of studies demonstrate a similar yet distinct response of ovine granulosa cells to CART treatment as observed for the bovine system. Furthermore, recent studies support a prominent requirement of Wnt signaling for mediating stimulatory effects of FSH on E2 production and granulosa cell proliferation [30]. Investigation of direct effects of CART stimulation on Wnt signaling linked to E2 production and proliferation of hen granulosa cells is a focus of future studies.
In summary, results of present studies demonstrated that CART is expressed in granulosa and thecal layers of hen follicles, differential expression of CART based on follicular size and cellular layer in hen ovary, and the follicles (6–8 mm in diameter) at this time is the key turning point to continue to develop into the dominant follicle or atresia, results support a potential role for CART in regulation of follicular development in the hen. However, it is important to note that follicular dynamics and regulation in hen are distinct from that noted for cattle and ovine. It is acknowledged that study design was not optimal due to limited sample collection and test maneuverability,because the big follicles (>6 mm in diameter) could not dehydration for immunohistochemical localization, and small white follicles (1–2 mm in diameter) could not isolation of granulosa cells and theca cells for qRT-PCR. Despite such limitations, results have significantly enhanced understanding of hen ovary potential differences in CART expression associated with follicular development that are foundational to further study in the future. Hence, further study of CART potential Wnt signaling linked to regulation of atresia are necessary to dissect its potential species-specific role in regulation of follicular development.
Conclusion
CART mRNA and CART peptide were expressed in granulosa cells and theca cells of follicles in different sizes, this could affect steroidogenesis to further influence the hen follicular development, suggesting CART plays a potential role in developmental regulation of chicken follicles.
References
Onagbesan O, Bruggeman V, Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim Reprod Sci. 2009;111(2–4):121–40.
Lovell TM, Gladwell R, Groome NP, Knight PG. Ovarian follicle development in the laying hen is accompanied by divergent changes in inhibin A, inhibin B, activin A and follistatin production in granulosa and theca layers. J Endoerinol. 2003;177(1):45–55.
Gupta SK, Gilbert AB, Walker MA. Histological study of follicular atresia in the ovary of the domestic hen (Gallus domesticus). J Reprod Fertil. 1988;82(1):219–25.
Kobayashi Y, Jimenez-krassel F, Li Q, Yao J, Huang R, Ireland JJ, et al. Evidence that cocaine- and amphetamine-regulated transcript is a novel intra-ovarian regulator of follicular atresia. Endocrinology. 2004;145(11):5373–83.
Kobayashi Y, Jimenez-Krassel F, Ireland JJ. Evidence of a local negative role for cocaine and amphetamine regulated transcript (CART), inhibins and low molecular weight insulin like growth factor binding proteins in regulation of granulosa cell estradiol production during follicular waves in cattle. Reprod Biol Endocrinol. 2006;4(1):22–33.
Lv LH, Jimenez-krassel F, Sen A, Bettegowda A, Mondal M, Folger JK. Evidence supporting a role for cocaine and amphetamine regulated transcript (CART) in control of granulosa cell estradiol production associated with dominant follicle selection in cattle. Biol Reprod. 2009;81(3):580–6.
Nitta H, Osawa Y, Bahr JM. Multiple steroidogenic cell population in the thecal layer of pre-ovulatory follicles of the chicken ovary. Endocrinology. 1991;129(4):2033–40.
Johnson PA, Stoklosowa S, Bahr JM. Interaction of granulosa and theca layers in the control of progesterone secretion in the domestic hen. Biol Reprod. 1987;37(5):1149–55.
Johnson AL. Intracellular mechanisms regulating cell survival in ovarian follicles. Anim Reprod Sci. 2003;78(3–4):185–201.
Johnson AL, Woods DC. Dynamics of avian ovarian follicle development: cellular mechanisms of granulosa cell differentiation. Gen Comp Endocrinol. 2009;163(1–2):12–7.
Sen A, Lv LH, Bello N, Ireland JJ, Smith GW. Cocaine- and amphetamine-regulated transcript (CART) accelerates the termination of FSH induced ERK1/2 and AKT activation by regulating the expression and degradation of specific map kinase phosphatases. Mol Endocrinol. 2008;22(12):2655–76.
Li PF, Yue WB, Li FL, Huang Y, Sun JY, Zhu ZW, Yu XJ, He JP, Fan RW, Ren YS, Lv LH. Effects of CART on estradiol production of pig ovarian follicular granulosa cells in vitro culture. Acta Vet et Zootech Sin. 2012;43(12):1879–86.
Ginther OJ, Wiltbank MC, Fricke PM. Selection of the dominant follicle in cattle. Biol Reprod. 1996;55(3):1187–94.
Sen A, Bettegowda A, Jimenez-krassel F, Ireland JJ, Smith GW. Cocaine- and amphetamine-regulated transcript (CART) regulation of follicle stimulating hormone signal transduction in bovine granulosa cells. Endocrinology. 2007;33(2):1–18.
Bakke LJ, Li Q, Cassar CA, Dow MP, Pursley JR, Smith GW. Gonadotropin surge-induced differential up-regulation of collagenase-1 (MMP-1) and collagenase-3 (MMP-13) messenger RNA and protein in bovine pre-ovulatory follicles. Biol Reprod. 2004;71(2):605–12.
Shi L, Zhao H, Ren Y, Yao X, Song R, Yue W. Effects of different levels of dietary selenium on the proliferation of spermatogonial stem cells and antioxidant status in testis of roosters. Anim Reprod Sci. 2014;149(3–4):266–72.
Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science. 2002;296(5576):2178–80.
Jones DC, Kuhar MJ. Cocaine–amphetamine-regulated transcript expression in the rat nucleus accumbens is regulated by adenylyl cyclase and the cyclic adenosine 50-monophosphate/protein kinase a second messenger system. J Pharmacol Exp Ther. 2006;317(1):454–61.
Li PF, Yue WB, Huang Y, Sun JY, Li XM, Pang YY, Yu XJ, He JP, Meng JZ, Ren YS, Lv LH. Effects of CART on estradiol production in vitro in follicular granulose cells of sheep ovarian. Acta Vet et Zootech Sin. 2013;44(6):853–7.
Smith GW, Sen A, Folger JK, Ireland JJ. Putative role of cocaine- and amphetamine-regulated transcript (CARTPT) in dominant follicle selection in cattle. Soc Reprod Fertil Suppl. 2010;67:105–17.
Moore BC, Forouhar S, Kohno S, Botteri NL, Hamlin HJ, Jr Guillette L J. Gonadotropin-induced changes in oviducal mRNA expression levels of sex steroid hormone receptors and activin-related signaling factors in the alligator. Gen Comp Endocrinol. 2012;175(2):251–8.
Hernandez AG, Bahr JM. Role of FSH and epidermal growth factor (EGF) in the initiation of steroidogenesis in granulosa cells associated with follicular selection in chicken ovaries. Reproduction. 2003;125(5):683–91.
Tilly JL, Kowaiski KI, Johnson AL. Stage of follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biol Reprod. 1991;44(2):305–14.
Marrone BL, Hertelendy F. Decreased androstenedione production with increased follicular maturation in theca cells from the domestic hen. J Reprod Fertil. 1985;74(2):543–50.
Porter TE, Hargis BE, Halawani ME, el Halawani ME. Differential steroid production between theca interina and theca externa cells: a three-cell model for follicular steroidogenesis in avian species. Endocrinology. 1989;125(1):109–16.
Mahon MG, Lindstedt KA, Hermann M, Nimpf J, Schneider WJ. Multiple involvement of clusterin in chicken ovarian follicle development binding to two oocyte-specific members of the low density lipoprotein receptor gene family. J Biol Chem. 1999;274(7):4036–44.
Woods DC, Johnson AL. Regulation of follicular-stimulating hormone-receptor message rRNA in hen granulosa cells relative to follicle selection. Biol Reprod. 2005;72(3):643–50.
Johnson AL, Bridgham JT, Wagner B. Characterization of a chicken luteinizing hormone receptor (cLH-R) cDNA, and expression of cLH-R mRNA in the ovary. Biol Reprod. 1996;55(2):304–9.
Huang Y, Yao XL, Meng JZ, Liu Y, Jiang XL, Chen JW, Li PF, Ren YS, Liu WZ, Yao JB, Folger JK, Smith GW, Lv LH. Intrafollicular expression and potential regulatory role of cocaine- and amphetamine-regulated transcript in the ovine ovary. Domest Anim Endocrinol. 2016;54(1):30–6.
Gupta PS, Folger JK, Rajput SK, Lv L, Yao J, Ireland JJ, Smith GW. Regulation and regulatory role of WNT signaling in potentiating FSH action during bovine dominant follicle selection. PLoS ONE. 2014;9(6):e100201.
Authors’ contributions
PF carried out the present research by assembling and verifying the datasets for analysis and writing the initial draft of the manuscript. XJ and JS carried out the molecular clone studies and the sequence alignment. XL carried out the immunoassays. ZW carried out the qRT-PCR. WZ and LH designed the study and performed the statistical analysis. JB helped to modify the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Authors are grateful of Prof. George W Smith in Michigan State University in the USA for his directions of sample collection and research designs in this study.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
We confirm that this study did not involve relevant clause of the Ethics Committee, and all animal procedures were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Funding
This study was supported by Shanxi Scholarship Council of China Grant No. 2014-key 5, Shanxi Sci-technological Collaboration Grant No. 201603D421006, Shanxi Talent Introduction and Sanjin Talent Program, Shanxi Provincial Talent Introduction and SXAU (Shanxi Agricultural University) Major Research Achievement Cultivation Grant No. zdpy 201403/201503 to Lyu; SXAU Introduction of Doctor Research Startup Fund Grant No. 2014ZZ04 to Li; Chinese Natural Science Foundation Grant No. 31402156 to Zhu.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, P., Yu, X., Xie, J. et al. Expression of cocaine- and amphetamine-regulated transcript (CART) in hen ovary. Biol Res 50, 18 (2017). https://doi.org/10.1186/s40659-017-0123-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40659-017-0123-x