Abstract
Background
The efficient removal of toxic inhibitors from pretreated lignocellulose biomass is crucially important for consequent cellulosic ethanol fermentation. A. resinae ZN1 biodetoxifies all toxic inhibitors at the neutral pH of 4–6, and the neutralization of acid catalyst in the pretreated lignocellulose is required. However, aqueous alkaline solutions such as sodium hydroxide solution and calcium hydroxide slurry are used which generate several difficulties.
Results
In this study, a dry biodetoxification method was investigated using dry calcium carbonate (CaCO3) powder as an acid-neutralizing reagent to avoid the use of an aqueous alkaline solution. Dry biodetoxification provides a mild and stable pH without the generation of phenolic compounds. The acid pretreated and dry biodetoxified wheat straw was used as the feedstock of ethanol fermentation and the same performance with the wet biodetoxification using aqueous Ca(OH)2 slurry. The 72 g/L or 9.1% (v/v) of ethanol produced from wheat straw was very close to that of ethanol from corn starch.
Conclusions
Dry biodetoxification provided a practical method to simplify the process of conventional wet biodetoxification to reduce the time, cost and labor.
Similar content being viewed by others
Introduction
Pretreatment is an essential step to overcome the recalcitrance of lignocellulosic biomass to facilitate enzymatic hydrolysis. Pretreatment of lignocellulosic biomass generates various toxic inhibitors such as furfural, 5-hydroxymethylfurfural (HMF), acetic acid and phenolic aldehydes, which required to be removed before bioconversion of enzymatic hydrolysis and fermentation process (Klinke et al. 2014; Jing et al. 2009; Palmqvist and Hahn-Hagerdal 2000; Dong and Bao 2010). A kerosene fungus Amorphotheca resinae ZN1 was isolated for complete removal of inhibitors from acid pretreated lignocellulose biomass in our previous studies (Zhang et al. 2010). The biodetoxified biomass has been applied to produce ethanol (Liu et al. 2018), chiral lactic acid (Qiu et al. 2017, 2018), glutamic acid (Wen et al. 2018), citric acid (Zhou et al. 2017), and gluconic acid (Zhang et al. 2016).
Amorphotheca resinae ZN1 grows and metabolizes at the neutral pH range of 4–6, thus the acid catalyst in the pretreated biomass has to be neutralized before biodetoxification (Zhang et al. 2010). Generally, aqueous calcium hydroxide slurry was used to neutralize the acid to pH 5 before biodetoxification. However, the use of calcium oxide (CaO) and calcium hydroxide slurry and mixing of small portion of them with a large amount of solid biomass generate several difficulties including: (1) the reduced solid contents of lignocellulosic biomass; (2) incomplete mixing between the solid particles and liquid slurry; (3) generation of phenolic compounds; (4) expensive and unsafe to handle. Furthermore, a high amount of carbon dioxide is generated and the high energy consumption is required during calcium oxide (CaO) manufacture. Here, we proposed a complete dry biodetoxification using inert calcium carbonate (CaCO3) powder to neutralize the acid catalyst in lignocellulose biomass to avoid the use of the aqueous alkaline solution. In the dry biodetoxification, pH changes slowly and complete mixing could be realized during the mild neutralization period. CaCO3 neutralized acid pretreated biomass can be kept in an open environment because of its stable nature and convenient onsite biodetoxification can be performed after pretreatment. This study tested the reaction balance of the biodetoxification, metabolism, and neutralization, as well as cellulosic ethanol fermentation performance.
Methods
Lignocellulosic biomass and reagents
Wheat straw was obtained from Nanyang, Henan, China, in the year 2018. The collected wheat straw was washed, dried and milled using a hammer crusher to pass through the 10 mm (diameter) apertures, and stored in plastic bags at room temperature in dry condition. The raw wheat straw contained 34.59% of cellulose, 24.26% of xylan, 18% of lignin, and 9.6% of ash by weight percentage measured according to NREL protocols (Sluiter et al. 2008, 2012).
Cellulase enzyme Cellic CTec 2.0 was purchased from Novozymes (China), Beijing, China. Yeast extract and peptone were purchased from Oxoid, Basingstoke, Hampshire, UK. CaCO3 and Ca(OH)2 were purchased from Shanghai Titanchem Co. Other chemicals and reagents included glucose, xylose, KH2PO4, MgSO4·7H2O, NaOH, and H2SO4 were purchased from Lingfeng Chemical Reagent Co.
Strains and media
Biodetoxification fungus Amorphotheca resinae ZN1 was isolated in our previous studies and stored in China General Microorganism Collection Center (CGMCC), Beijing, China. The potato dextrose agar (PDA) medium for A. resinae ZN1 included 200 g/L potato extract juice, 20 g/L glucose, and 20 g/L agar.
Ethanol fermentation strain Saccharomyces cerevisiae XH7 was kindly provided by Prof. XM Bao from Shandong University, Jinan, Shandong, China (Li et al. 2015). The medium used for S. cerevisiae XH7 included: (i) medium for strain activation; YPD included, 20 g/L glucose, 20 g/L peptone and 10 g/L yeast extract; (ii) for seed culture, 5% (w/w) of biodetoxified wheat straw, 2 g/L KH2PO4, 2 g/L (NH4)2SO4, 1 g/L MgSO4 and 10 g/L yeast extract; (iii) for adaptation, 10% (w/w) of biodetoxified wheat straw, 2 g/L KH2PO4, 2 g/L (NH4)2SO4, 1 g/L MgSO4 and 10 g/L yeast extract; (iv) for SSCF, 2 g/L KH2PO4, 2 g/L (NH4)2SO4, 1 g/L MgSO4 and 10 g/L yeast extract.
Dry acid pretreatment, dry biodetoxification and ethanol fermentation
Dry acid pretreated wheat straw was prepared according to Zhang et al. (2011) and He et al. (2014). The pretreated wheat straw contained 26.7 mg/g DM of glucose and 153.3 mg/g DM of xylose, 3.14 mg/g DM of furfural, 1.46 mg/g DM of HMF, 12.52 mg/g DM of acetic acid, 0.43 mg/g DM of vanillin, 0.51 mg/g DM of syringaldehyde and 0.36 mg/g DM of 4-HBA, determined according to NREL protocols (Sluiter et al. 2008, 2012).
The dry acid pretreated wheat straw was neutralized to pH 5.5 with dry CaCO3 powder and 20% of Ca(OH)2 slurry, respectively, and disk milled to remove the long cellulosic fibers. Then, inoculated with 10% (v/v) solid seed of A. resinae ZN1 for biodetoxification at ambient temperature for 60 h. Ethanol fermentation was performed in 5 L bioreactor. The biodetoxified wheat straw was pre-hydrolyzed into liquid hydrolysate slurry at 50 °C, pH 5 for 12 h. Then, the simultaneous saccharification and co-fermentation (SSCF) was performed by inoculating shortly adapted fermentation yeast Saccharomyces cerevisiae XH7 into the hydrolysate at 10% (v/v) in the same bioreactor. The nutrients addition included 2 g/L of KH2PO4, 2 g/L of (NH4)2SO4, 1 g/L of MgSO4 and 10 g/L of yeast extract. The SSCF was carried out at 30 °C, pH 5.5 for 120 h.
Analysis
Glucose, xylose, ethanol, and inhibitors such as acetic acid furfural and HMF were analyzed using HPLC (LC-20AD, refractive index detector RID-10A, Shimadzu, Japan) with Bio-Rad Aminex HPX-87H column at 65 °C. The mobile phase was 5 mM H2SO4 at the flow rate of 0.6 mL/min. Syringaldehyde, vanillin, and hydroxybenzaldehyde (4-HBA) were analyzed using the reversed-phase HPLC (LC-20AT, UV/VIS detector SPD-20A, Shimadzu, Japan) with a YMC-Pack ODS-A column at ambient temperature (Liu et al. 2018). The mobile phase was 100% acetonitrile with 0.1% formic acid at the flow rate of 1.0 mL/min. The yeast cell viability during the simultaneous saccharification and co-fermentation (SSCF) was assayed on the YPD medium Petri dish by counting colony-forming units (CFU), when the 100 μL of the 105 diluted fermentation broth sample withdrawn after every 12 h was stretched and cultured at 30 °C for 48 h (Gu et al. 2015).
Results and discussion
Dry neutralization of acid catalyst using calcium carbonate and the biodetoxification assay
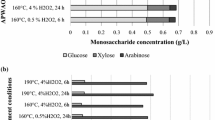
Dry biodetoxification of acid pretreated wheat straw for cellulosic ethanol fermentation was investigated using CaCO3 powder as a pH neutralizing agent. Figure 1 shows the biodetoxification profiles of the major inhibitors and sugar consumption using dry neutralization by CaCO3 powder. Furfural, HMF, acetic acid, and the three phenolic aldehydes were degraded quickly by A. resinae ZN1 (Fig. 1a–d). The fermentable sugars including glucose and xylose maintained approximately constant before and after the biodetoxification (Fig. 1e). The results indicate that the biodetoxification of the inhibitors in the acid pretreated wheat straw was fast and complete with the use of both CaCO3 powder and aqueous Ca(OH)2 slurry and the fermentable sugars were almost untouched. This study proves that dry biodetoxification is a simple and an efficient method to remove all inhibitors compares to conventional wet biodetoxification within 24–48 h (Zhang et al. 2010), that is enough for performing the consequent ethanol production by the SSCF process.
Inhibitors and sugars consumption profiles in dry biodetoxification using CaCO3 powder and wet biodetoxification using Ca(OH)2 slurry. Biodetoxification was performed at ambient temperature. a Furfural degradation; b HMF degradation; c acetic acid degradation; d phenolics (syringaldehyde, vanillin and HBA) degradation and e sugar (glucose and xylose) consumption. Conditions: 10% solid seed of A. resinae ZN1, biodetoxified for 60 h
Evaluation of ethanol fermentation using dry biodetoxified wheat straw
The ethanol production through simultaneous saccharification and co-fermentation (SSCF) of the acid pretreated and dry biodetoxified wheat straw was investigated using S. cerevisiae XH7. Figure 2 shows the ethanol fermentation at 30% (w/w) solids loading using dry and wet biodetoxified feedstocks. The results show that glucose was quickly consumed after inoculation of S. cerevisiae XH7 and converted into ethanol and xylose was consumed continuously till the end of fermentation by S. cerevisiae XH7 in both (dry and wet) biodetoxified wheat straw (Fig. 2a, b). The 72 g/L or 9.1% (v/v) of ethanol was obtained from dry biodetoxified wheat straw, very close to that of ethanol from corn starch (Liu and Bao 2017). This may be economically viable to produce in large scale.
Evaluation of dry and wet biodetoxified wheat straw feedstocks on simultaneous saccharification and ethanol co-fermentation (SSCF). a SSCF using dry biodetoxified wheat straw with CaCO3 powder; b SSCF using wet biodetoxified wheat straw with 20% of Ca(OH)2 slurry. Conditions: 30% solids loading, cellulase dosage of 10 mg total protein/g cellulose, 10% (v/v) inoculum ratio of S. cerevisiae XH7. Pre-hydrolysis step, 50 °C, pH 5.0 for 12 h; SSCF step, 30 °C, pH 5.5, for 120 h
Amorphotheca resinae ZN1 is a biodetoxification strain and used on the pretreated wheat straw to remove the inhibitors. After the biodetoxification, wheat straw was enzymatically hydrolyzed into fermentable sugars such as glucose and xylose. The fermentation microorganism S. cerevisiae XH7 was used to convert the glucose and xylose sugars to ethanol. The aerobic fungus Amorphotheca resinae ZN1 was no long alive because of the anaerobic condition in hydrolysis and ethanol fermentation.
The dry biodetoxification method performs excellent cellulosic ethanol production and provides mild and stable pH with no reduction of fermentable sugars and solid contents and without the generation of phenolic compounds. Dry biodetoxification is a practical method to improve biodetoxification efficiency for cellulosic ethanol production from acid pretreated lignocellulose feedstock.
Conclusion
Dry biodetoxification in this study realized complete mixing of solid–solid particles without loss of solid content and generation of phenolic derivatives which is more safe and costly effective than that of conventional wet biodetoxification. Consequently, use of dry biodetoxified biomass achieved high cellulosic ethanol production [72 g/L or 9.1% (v/v)] and all advantages of this method make it possible for further industrial applications.
Availability of data and materials
The datasets supporting the conclusions of this article are included in the main manuscript file.
Abbreviations
- CaCO3 :
-
calcium carbonate
- Ca(OH)2 :
-
calcium hydroxide
- HMF:
-
5-hydroxymethylfurfural
- A. resinae ZN1:
-
Amorphotheca resinae ZN1
- CaO:
-
calcium oxide
- NREL:
-
National Renewable Energy Laboratory
- KH2PO4 :
-
potassium dihydrogenphosphate
- MgSO4·7H2O:
-
magnesium sulfate heptahydrate
- NaOH:
-
sodium hydroxide
- H2SO4 :
-
sulfuric acid
- (NH4)2SO4 :
-
ammonium sulfate
- PDA:
-
potato dextrose agar
References
Dong H, Bao J (2010) Biofuel via biodetoxification. Nat Chem Biol 6:316–318
Gu HQ, Zhang J, Bao J (2015) High tolerance and physiological mechanism of Zymomonas mobilis to phenolic inhibitors in ethanol fermentation of corncob residue. Biotechnol Bioeng 112:1770–1782
He YQ, Zhang LP, Zhang J, Bao J (2014) Helically agitated mixing in dry dilute acid pretreatment enhances the bioconversion of corn stover into ethanol. Biotechnol Biofuels 7:1
Jing X, Zhang X, Bao J (2009) Inhibition performance of lignocellulose degradation products on industrial cellulase enzymes during cellulose hydrolysis. Appl Biochem Biotechnol 159:696–707
Klinke HB, Thomsen AB, Ahring BK (2014) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26
Li H, Wu M, Xu L, Jin H, Guo T, Bao X (2015) Evaluation of industrial Saccharomyces cerevisiae strains as the chassis cell for second-generation bioethanol production. Microb Biotechnol 82:6
Liu G, Bao J (2017) Maximizing cellulosic ethanol potentials by minimizing wastewater generation and energy consumption: competing with corn ethanol. Bioresour Technol 245:18–26
Liu G, Zhang Q, Li H, Qureshi AS, Zhang J, Bao X, Bao J (2018) Dry biorefining maximizes the potentials of simultaneous saccharification and co-fermentation for cellulosic ethanol production. Biotechnol Bioeng 115:60–69
Palmqvist E, Hahn-Hagerdal B (2000) Fermentation of lignocellulosic hydrolysates. I: inhibition and detoxification. Bioresour Technol 74:17–24
Qiu Z, Gao Q, Bao J (2017) Constructing xylose-assimilating pathways in Pediococcus acidilactici for high titer D-lactic acid fermentation from corn stover feedstock. Bioresour Technol 245:1369–1376
Qiu Z, Gao Q, Bao J (2018) Engineering Pediococcus acidilactici with xylose assimilation pathway for high titer cellulosic L-lactic acid fermentation. Bioresour Technol 249:9–15
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008) Determination of sugars, byproducts and degradation products in liquid fraction process samples. NREL/TP-510-42623. National Renewable Energy Laboratory, Golden
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass. NREL/TP-510-42618. National Renewable Energy Laboratory, Golden
Wen J, Xiao Y, Liu T, Gao Q, Bao J (2018) Rich biotin content in lignocellulose biomass plays the key role in determining cellulosic glutamic acid accumulation by Corynebacterium glutamicum. Biotechnol Biofuels 11:132
Zhang J, Zhu Z, Wang X, Wang N, Wang W, Bao J (2010) Biodetoxification of toxins generated from lignocellulose pretreatment using a newly isolated fungus, Amorphothec resinae ZN1 and the consequent ethanol fermentation. Biotechnol Biofuels 3:26
Zhang J, Wang X, Chu D, He Y, Bao J (2011) Dry pretreatment of lignocellulose with extremely low steam and water usage for bioethanol production. Bioresour Technol 102:4480–4488
Zhang H, Zhang J, Bao J (2016) High titer gluconic acid fermentation by Aspergillus niger from dry dilute acid pretreated corn stover without detoxification. Bioresour Technol 203:211–219
Zhou PP, Meng J, Bao J (2017) Fermentative production of high titer citric acid from corn stover feedstock after dry dilute acid pretreatment and biodetoxification. Bioresour Technol 224:563–572
Acknowledgements
The authors are grateful for the financial support received from the National Natural Science Foundation of China (31961133006).
Funding
This work was supported by National Natural Science Foundation of China (31961133006).
Author information
Authors and Affiliations
Contributions
FA, YZ and JB designed and wrote the experiment; FA and YZ conducted the experiment; JB conceived the research. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the authors have read and agreed the ethics for publishing the manuscript.
Consent for publication
The authors approved the consent for publishing the manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ahmed, F., Yan, Z. & Bao, J. Dry biodetoxification of acid pretreated wheat straw for cellulosic ethanol fermentation. Bioresour. Bioprocess. 6, 24 (2019). https://doi.org/10.1186/s40643-019-0260-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40643-019-0260-x