Abstract
Background
To date, only scarce information is available about the diaptomid copepods of the Middle East despite the ecological and biogeographical importance of the family Diaptomidae in the inland waters of the Holarctic region. Moreover, the taxonomic status of some of the taxa occurring in the area is in need of revision. We studied crustaceans collected from temporary and permanent lentic water bodies in Israel with the aim of providing an updated census of the diaptomid copepods occurring in the country. Furthermore, we morphologically and genetically analysed samples of Arctodiaptomus similis s.l. to shed light on its taxonomy.
Results
Five diaptomid taxa were collected during this survey. Among these, Phyllodiaptomus blanci is new for the country and the whole circum-Mediterranean area and might be an allochthonous species of eastern origin. Within the collected samples, we singled out two parapatric groups of populations within A. similis s.l.; these consistently differ both based on morphology (chaetotaxy of the left male antennule) and molecular data (divergence over 17% at the mitochondrial gene for the cytochrome b). We thus attribute the full species rank to Arctodiaptomus irregularis Dimentman & Por, 1985 stat. rev., originally described as a subspecies of the widespread species Arctodiaptomus similis (Baird, 1859).
Conclusions
We critically evaluated all species hitherto reported for Israeli inland waters. Considering both the confirmed literature data and the new findings, Israeli diaptomid fauna is composed of at least seven species. However, the need for further surveys in the Middle East and for detailed systematic revisions of some controversial taxa is stressed. Our results on the systematics of A. similis s.l. illustrate the importance of implementing molecular analyses when investigating diversity patterns of groups which are difficult to resolve based on morphology alone.
Similar content being viewed by others
Background
Representatives of the copepod family Diaptomidae often dominate the lentic inland water bodies of the Holarctic, Oriental, and Afrotropical biogeographical regions, being a key taxon in many lacustrine and pond ecosystems (Dussart and Defaye [2001], [2002]). Species of this family are as a rule characterised by limited distributional ranges, which are significantly constrained by the legacies of historical biogeographical events (Leibold et al. [2010]). However, an ever-increasing corpus of molecular evidence suggests that our knowledge on diaptomid species distributions and biogeography is currently hampered by a gross underestimation of the actual diversity of the group (e.g., Marrone et al. [2013], and references therein). In the inland waters of the Mediterranean area, about 100 diaptomid species belonging to 14 genera are currently reported to occur (Dussart and Defaye [2002]), but the information available for certain regions is far from being satisfactory (cf. Marrone [2006]).
To date, uneven information is available on the inland water calanoid copepod fauna of the Middle East. While the diaptomid faunas of Turkey (e.g., Gündüz [1998]; Ustaoğlu [2004]; Ustaoğlu et al. [2005]) and Iran (e.g., Brehm [1937]; Löffler [1956], [1961]) are relatively well-known, only scarce and anecdotal records are currently available for other countries of this region (e.g., Gurney [1921]; Spandl [1923]; Kiefer [1978b]; Dumont [1979], [2009]; Khalaf [2008]; Mohamed and Salman [2009]). The Israeli diaptomid fauna is quite a peculiar case: although several papers and reviews are available (e.g., Baird [1859]; Richard [1893]; Kiefer [1930]; Yaron [1964]; Dimentman and Por [1985], Azoulay [2001], and references therein), only five diaptomid taxa are currently listed for the country; this seems quite a species-poor fauna when compared to other circum-Mediterranean regions of comparable area, where usually about 9 to 14 diaptomid species are present (e.g., Ruffo and Stoch [2005]; Marrone et al. [2005]; Marrone [2006]; Turki and Turki [2010]; Alfonso and Belmonte [2011]). Furthermore, the taxonomical validity of some ‘forms’ or ‘subspecies’ described in the past decades based on Israeli specimens is rather controversial, and the presence itself of some taxa in the country is in need of confirmation, thus casting further uncertainty on the actual composition of the Israeli diaptomid fauna.
We studied plankton samples collected in Israeli inland waters between 2003 and 2011 in order to update the available information on the composition and distribution of diaptomid copepods in the country. Furthermore, we genetically analysed several populations of Arctodiaptomus similis s.l. to test the hypothesis that two parapatric groups of populations differing in morphology, observed in our dataset, actually represent distinct evolutionary lineages of species rank.
Methods
Plankton samples were collected throughout the country, focusing on small- to medium-sized freshwater habitats; both permanent and temporary water bodies were investigated, and some sites were sampled multiple times on different dates. Altogether, we analysed samples from 60 different sites (Figure 1). Samples were collected using a 200-μm mesh hand net along the water bodies' shorelines; open waters were sampled using 80-to-200-μm mesh-sized towing nets. Collected crustaceans were fixed in situ with 96% ethanol. They were then sorted in the laboratory under a dissecting microscope, and diaptomid specimens were prepared according to Dussart and Defaye ([2001]). Morphological identification was performed according to Kiefer ([1974], [1978a]), Borutzky et al. ([1991]), and Ranga-Reddy ([1994]). Line drawings were prepared using a compound microscope equipped with a camera lucida.
Map of the sampling sites. Samples collected between 2003 and 2011 from both temporary and permanent water bodies were analysed to improve knowledge on diversity and distribution of diaptomid copepods of Israel. Black dots represent sites where at least one diaptomid species was collected; white dots are sites without diaptomids.
Samples are stored in the crustacean collection of Federico Marrone and are available for loan on request. Voucher specimens from the type localities of Arctodiaptomus similis (Baird, [1859]) and Arctodiaptomus irregularis Dimentman & Por, [1985] stat. rev. have been deposited in the collection of the Smithsonian Institution (National Museum of Natural History) in Washington DC, USA, with the catalogue numbers USNM #1226919 and USNM #1226920.
Thirteen specimens of A. similis s.l. from various regions of Israel and two congeneric species to be used as out-groups (Arctodiaptomus cf. stephanidesi (Pesta, 1935) and Arctodiaptomus alpinus (Imhof, 1885), both collected in Italy) were analysed genetically by amplifying and sequencing a 329-bp long fragment of the mitochondrial gene for the cytochrome b (Cyt-b), a molecular marker which is known to be informative on the phylogeography and the molecular systematics of closely related diaptomid taxa (e.g., Staton et al. [2003]; Thum and Derry [2008]; Thum and Harrison [2009]; Marrone et al. [2010], [2013]). Genetic analyses were performed following the protocols and procedures described in Marrone et al. ([2010], [2013]), with the aim of investigating the molecular structuring of the taxon and to compare it with the observed patterns of morphological variation. Chromatograms were imported and edited with Chromas Lite 2.01 (Technelysium Pty. Ltd., South Brisbane, Australia) and aligned with BioEdit (Ibis Biosciences, Carlsbad, CA, USA) (Hall [1999]). The quality of the obtained sequences was checked with Sequence Scanner v1.0. Only sequences with continuous reads of high quality bases were used; when the sequences were not of sufficient quality, the reverse complement sequences were also obtained. MEGA 5 (Tamura et al. [2011]) was used to translate the Cyt-b sequences to amino acids in order to check for the possible presence of frameshifts or stop codons, which would indicate the presence of sequencing errors or pseudogenes. The sequences were deposited in GenBank under Accession Numbers KM488608 to KM488622.
Bayesian inference (BI) of phylogeny and maximum likelihood (ML) analyses were performed on the Cyt-b dataset as implemented by MrBayes 3.2.1 (Ronquist et al. [2012]) and PhyMl v.3 (Guindon and Gascuel [2003]), respectively. Both analyses were performed using a Hasegawa, Kishino, and Yano model of sequence evolution for molecular data with a proportion of invariable sites (HKY + I), as selected by the Akaike information criterion in MrModeltest 2.2 (Nylander [2004]). Node supports were evaluated by their posterior probabilities in the BI tree and with 1,000 bootstrap replicates in the ML analysis. The BI analysis was performed with two independent runs of 2,000,000 generations and four Markov chains using default heating values. Trees and parameter values were sampled every 100 generations resulting in 20,000 saved trees per analysis. An initial fraction of 5,000 trees (20%) was conservatively discarded as ‘burn-in’. For all analyses, standard deviation of split frequencies reached values lower than 0.0065, and values of the potential scale reduction factor (PSRF) were between 1.0 and 1.004 for all parameters, indicating convergence of the runs.
In order to compare the observed molecular distances with those available in literature for other diaptomid taxa, uncorrected molecular distances among specimens and between groups were calculated in PAUP 4.0b10 (Swofford [1998]).
Results
Morphological identification
We analysed samples originating from 60 different sampling sites. In 18 sites, diaptomid copepods were present (Figure 1, Table 1). Most of these habitats were temporary rain pools; diaptomids were only rarely encountered in permanent water bodies such as reservoirs and fishponds, although these were well-represented in our sample set. Altogether, five diaptomid taxa were collected (Table 2). Among these, Hemidiaptomus gurneyi canaanita, two ‘forms’ of A. similis s.l., and Neolovenula alluaudi were already known for the fauna of the country. Conversely, the finding of Phyllodiaptomus blanci in a reservoir of a city park in Tel Aviv is the first one for Israel and for the whole circum-Mediterranean area. In spite of previously published findings, no Eudiaptomus species were collected during the present survey.
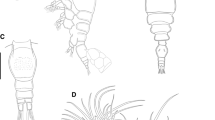
Studied A. similis s.l. populations differed in the antennular chaetotaxy, which consistently presents one versus two setae on the 15th and 17th segments of the left male antennule (Figure 2). Based on this character, these could be ascribed to two parapatric groups, one restricted to water bodies of the Golan Heights only, the other found in other regions of the country (Table 3).
Arctodiaptomus similis (Baird,[1859]) and Arctodiaptomus irregularis Dimentman & Por,[1985]. Anatomical features of A. irregularis collected in Bab El Hawa (ISR03) on 9 March 2010 (A- D) and A. similis collected in Birket Mamilla pool (ISR16) on 4 March 2011 (E- H). A and E: male fifth pair of the legs (posterior view). B and F: antepenultimate article of the male right antennula. C and G: detail of the chaetotaxy on the articles 13 to 17 of the male left antennula. D and H: articles 12 to 15 of the male right antennula. Scale bar 100 μm.
Molecular analyses
The BI and ML trees based on a 329-bp long fragment of the Cyt-b showed a concordant topology, with a clear division of the investigated A. similis s.l. samples into two groups, hereafter referred to as the ‘Golan’ and the ‘Israeli’ clades (Figure 3). These clades are in good accordance with the two groups singled out based on morphology (Table 3) and show a sharp geographical segregation.
Bayesian phylogram based on a 329-bp long fragment of the mitochondrial gene for the cytochrome b. Node support is reported as nodal posterior probabilities/ML bootstrap; values below 50 are indicated by a dash. Asterisk (*): type locality of A. similis (Baird, [1859]); Section sign (§): type locality of A. similis irregularis Dimentman & Por, [1985] (now A. irregularis).
The mean intra-clade uncorrected molecular distance was 1.6% (ranging from 0.9% to 3%) within the Israeli clade and 0.7% (ranging from 0% to 0.9%) within the Golan clade. The average uncorrected molecular distance between the two clades was 17.3%.
Discussion
Checklist and distribution
Five diaptomid taxa were collected in the present survey; in addition to these, Eudiaptomus drieschi and Arctodiaptomus (Rhabdodiaptomus) salinus have to be considered parts of the Israeli diaptomid checklist based on bibliographical evidence. The reports of Arctodiaptomus (Arctodiaptomus) wierzejskii and Eudiaptomus gracilis in the country are in need of being substantiated and are possibly erroneous (see comments below). At the present state of knowledge, the checklist of Israeli diaptomid copepods is thus composed of seven taxa (Table 2).
The paradiaptomid N. alluaudi, which was previously known in the country only from the Sinai and Negev deserts (Dimentman and Por [1985]), was collected in our survey both in the Negev desert and on the Golan Heights, thus widening to the north the distribution of this species in the country. This finding was recently anticipated by Alfonso and Belmonte ([2013]) in a review on N. alluaudi distribution.
Hemidiaptomus (Hemidiaptomus) gurneyi canaanita is an endemic taxon whose distinct status was confirmed by molecular analyses, in spite of the morphological identity of Israeli populations with those collected in the central Mediterranean, including the type locality of the species (Marrone et al. [2010]). Hemidiaptomus gurneyi s.l. is characterised by a sharply disjointed distribution, with the populations belonging to Hemidiaptomus gurneyi gurneyi occurring in the central Mediterranean area (from eastern Algeria to the Balkan Peninsula), and those belonging to the subspecies Hemidiaptomus gurneyi canaanita occurring in Israel (Marrone et al. [2010]). The report of the presence of the species in Hungary (Dussart and Defaye [2002]) is not supported by any reference or sample (cf. Kiefer [1978a]; Petkovski [1983]; Stella [1984]; Dussart [1989]; Borutzky et al. [1991]; Marrone et al. [2010]); it has thus to be considered a lapsus calami of the authors. In our survey, H. gurneyi canaanita was collected in water bodies of central and northern areas of Israel, which is in good accordance with the distribution pattern described for the species by Dimentman and Por ([1985]). Based on the available drawing, the report of the occurrence of a female Hemidiaptomus specimen in ‘Birket de Banias’ (Richard [1893]), originally attributed to Hemidiaptomus amblyodon (Marenzeller, 1873) by the author, can in fact be unequivocally ascribed to H. gurneyi canaanita.
Two species belonging to the genus Eudiaptomus are to date reported for the country: Eudiaptomus gracilis (G.O. Sars, 1863) and Eudiaptomus drieschi (Poppe & Mrázek, 1895), both of them for Lake Kinneret (see Azoulay [2001], and references therein). As it has already been stressed (e.g., Dimentman and Por [1985]; Azoulay [2001]), it is possible that the early reports of E. gracilis for Lake Kinneret should be in fact ascribed to a misidentification of E. drieschi, and that E. gracilis should thus be excluded from Israeli diaptomid fauna. However, conclusive evidence is not available, and the report of occurrence of this euryecious diaptomid species in the lake cannot be excluded. Eudiaptomus species were observed in Lake Kinneret only since the 1960s onwards, although the Lake Kinneret zooplankton was investigated by frequent sampling since the early 20th century. This suggests a relatively recent colonization of the lake from an unknown source area, possibly corresponding with various anthropogenic changes of the Lake Kinneret system which took place since the 1950s, including intensive stocking of fish exotic to the lake (e.g., Gophen [1979]). No Eudiaptomus species were collected during our survey; however, we did not sample this large freshwater lake.
A thriving population of the diaptomid P. blanci was observed in a permanent reservoir within a city park in Tel Aviv (Figure 4). This species is new for Israel and the whole circum-Mediterranean area. Phyllodiaptomus is a diaptomid genus whose distribution area encompasses Central and Eastern Asia, from India to Iraq (Borutzky et al. [1991]; Ranga-Reddy [1994]; Sanoamuang and Teeramaethee [2006]; Khalaf [2008]). P. blanci and Phyllodiaptomus irakiensis are the westernmost species of the genus, both of them being known to occur in Iraq (Khalaf [2008], and references therein). It is not clear whether the presence of a P. blanci population in Israel is to be ascribed to an anthropogenic introduction to the country or whether the species has to be considered autochthonous. However, the man-made origin of the single known Israeli locality of this species suggests that, pending further findings in the area, P. blanci is an alien copepod species in Israel.
Phyllodiaptomus blanci . Specimens collected in Tel Aviv on 29 January 2003 (ISR12). (A) Female habitus, dorsal view. (B) Male habitus, dorsal view. (C) Female urosome, dorsal view. (D) Female fifth pair of legs, posterior view. (E) Male urosome, dorsal view. (F) Male fifth pair of legs, posterior view. (G) Male right leg, outer lateral view. (H) Male right leg, inner lateral view. (I) Male right antennula. (L- O) Variability of the antepenultimate segment of the male right antennula. (P- R) Variability of 13° and 14° articles of the male right antennula. Scale bar 100 μm.
Richard ([1893]) reported the occurrence of Arctodiaptomus (Rhabdodiaptomus) salinus from ‘Birket Abo-Zeineh’, a brackish marsh close to the northern coast of Lake Kinneret. The species is considered part of the Israeli fauna by Ranga-Reddy ([1994]) and Dussart and Defaye ([2002]). Dimentman and Por ([1985]) considered that this finding needed to be substantiated; however the presence of A. salinus in Israel is rather likely as this halophilous taxon is characterised by a broad circum-Mediterranean and Palaearctic distribution, including several countries in the Middle East (Dussart and Defaye [2002]; Dumont [2009]). Inland endorheic water bodies and salty coastal marshes and lagoons are the habitats to be sampled in order to find new evidence for the presence of this taxon in Israel.
Arctodiaptomus (Arctodiaptomus) wierzejskii was xerroneously cited to be present in Israel by Yaron ([1964]), who misidentified A. similis s.l. for this species (this erroneous datum was then unfortunately perpetuated by Dussart and Defaye [2002]). The mistake was possibly due to the use of the trinomen Diaptomus wierzejskii palaestinensis by Kiefer ([1930]) for the diaptomid population inhabiting ‘Birket Mamilla’ and by Fischer ([1953]) for the populations inhabiting some fish ponds throughout the country. D. wierzejskii palaestinensis is in fact a synonym of Arctodiaptomus similis (Kiefer [1932], [1974]).
The diaptomin taxon Arctodiaptomus (Arctodiaptomus) similis s.l. (Baird, [1859]) inhabits both permanent and temporary water bodies of central and northern parts of the country, proving to be the commonest calanoid copepod occurring in Israeli inland waters. In spite of its abundance, the taxonomy of A. similis s.l. is to date controversial. Currently, two taxa of subspecific rank are ascribed to A. similis s.l., but the taxonomical arrangement of the species is in need of revision (cf. Ranga-Reddy [1994]; Dussart and Defaye [2002]). The species was described from Israel by Baird ([1859]) and later reported to occur throughout Southwestern Asia and Eastern Europe (e.g., Richard [1893]; Petkovski [1961]; Kiefer [1930], [1974], [1978]; Dimentman and Por [1985], Azoulay [2001], Dussart and Defaye [2002]). Some authors reported the presence of the species in Sardinia (Kiefer [1978a]; Dussart and Defaye, [2002]) without providing any drawing or precise locality data; furthermore, extensive limnological surveys carried out in the island (e.g., Stella [1970]; Stella et al. [1972]; Stella and Margaritora [1975/1976]; Fadda et al. [2011]; Marrone and Stoch, unpublished data) only recorded the presence of the congeneric species A. salinus and A. wierzejskii, and no evidence of the presence of A. similis s.l. was ever collected. Thus, the report of the presence of the species in Sardinia is almost certainly erroneous, and the species is currently not considered part of the Italian copepod fauna (Stella [1984]; Ruffo and Stoch [2005]). The Israeli populations of A. similis s.l. studied by us could be split into two parapatric groups (Table 3), differing in the chaetotaxy of the left male antennule (Figure 2C,G) and in the mtDNA Cyt-b sequences (Figure 3).
Taxonomical notes on Arctodiaptomus(Arctodiaptomus) similis(Baird, [1859]) and Arctodiaptomus(Arctodiaptomus) irregularis Dimentman & Por, [1985] stat. rev
A. similis was described by Baird ([1859]) based on specimens raised from dried mud collected in the pool of Gihon in Jerusalem (now Birket Mamilla pond). Later on, Kiefer ([1930]) described D. wierzejskii palaestinensis based on specimens collected in Birket Mamilla, but this taxon was later synonymised, by the author himself, with A. similis (see Kiefer [1974] for an annotated list of the synonyms of the species). In the same work, Kiefer ([1974]) stressed the variability of the ornamentation and chaetotaxy of the male antennule and established two infrasubspecific formae based on the presence of two versus one setae on the 13th, 15th, and 17th segments of the left male antennule (forma saetosior) and on the presence of a rod-like instead of claw-like process of the antepenultimate segment of the right male antennule (forma irregularis). Based on this last character, and on the presence versus absence of a tooth on the 14th segment of the right male second antenna, Dimentman and Por ([1985]) established the subspecies Arctodiaptomus similis irregularis, whose type locality lies in Birket Bab el Hawa, on the Golan Heights. However, it has to be stressed that several authors (Richard [1893]; Petkovski [1961]; Kiefer [1974], [1978a]) reported the presence of a pronounced intra-populational morphological variability for the morphological characters which, according to Dimentman and Por ([1985]), should allow unequivocal assignment of each population to one or the other subspecies.
In good accordance with Richard ([1893]) and Petkovski ([1961]), the A. similis s.l. populations studied by us showed a noteworthy variability in the morphology of the right male antennule; although the populations from the Golan Heights show, as a rule, a smaller tooth on the 14th segment (Figure 2D) and a blunter process on the antepenultimate segment of the antennule (Figure 2B), exceptions and intermediate forms were observed. Conversely, the number of setae on the 15th and 17th segments of the left male antennule allowed us to soundly distinguish the specimens collected throughout the country in two groups (Table 3). This observation is in sharp contrast with Kiefer ([1974], [1978a]), who stressed a significant variability for the chaetotaxy of the segments 13th, 15th, and 17th both at intra- and inter-population level.
The two groups of populations singled out based on the number of setae present on the 15th and 17th segments of the left male antennule (Figure 2C,G) are parapatric and in good accordance with the observed molecular clustering in two clades of the studied populations (Figure 3). The molecular distance observed between the two groups is of the same order of magnitude of those observed among different lineages deserving the status of cryptic species within other diaptomid species, like Skistodiaptomus pallidus (14.3% to 17.2%, Thum and Harrison [2009]), Hemidiaptomus ingens (18%, Marrone et al. [2010]), and Onychodiaptomus sanguineus (22%, Thum and Derry [2008]); furthermore, a high sequence similarity was observed within each of the two clades.
Our morphological and genetic data thus support a classification of Israeli A. similis s.l. into two main lineages of species rank. Accordingly, two species are present in Israel: A. similis (Baird, [1859]) (Figure 2E,F,G,H), inhabiting the whole country with the exception of the Golan Heights, and A. irregularis Dimentman & Por, [1985] stat. rev. (Figure 2A,B,C,D), apparently confined to the Golan Heights.
Arctodiaptomus similis (Baird, [1859])
Type locality: Mamilla pool (Jerusalem District; ISR16)
syn.: Diaptomus wierzejskii palaestinensis Kiefer, [1930]
Arctodiaptomus similis similis Dimentman & Por, [1985]
A. irregularis Dimentman & Por, [1985] stat. rev.
Type locality: Bab el Hawa pool (Golan Heights; ISR03)
syn.: Arctodiaptomus similis forma irregularis Kiefer, [1974]
Arctodiaptomus similis irregularis Dimentman & Por, [1985]
The two taxa Arctodiaptomus similis var. smirnovi Brehm, 1938 and Arctodiaptomus spectabilis Mann, 1940 are junior synonyms of A. similis s.l. (cf. Kiefer, [1974]), but at the current state of knowledge, it is impossible to soundly ascribe them to, or differentiate from, either A. similis s.s. or A. irregularis. The distribution of these two taxa in the Middle East and Eastern Europe is in fact to date unknown. Interestingly, a parapatric distribution of two distinct lineages similar to that within A. similis has also been observed in Israeli populations of the cladoceran Daphnia (Ctenodaphnia) chevreuxi Richard, 1896: one lineage was only detected in lowland pools in the Mediterranean coastal plain, while a genetically distinct lineage was widespread in the Golan Heights but not found elsewhere (A. Petrusek, unpublished data).
Conclusions
In the light of a critical review of the existing and new data, the calanoid family Diaptomidae proved to be better represented in Israel than previous literature data suggested, with seven species certainly occurring in the country. This value is close to that observed in other circum-Mediterranean countries of comparable size (e.g., Marrone [2006]). Furthermore, it also needs to be stressed that representatives of the genus Metadiaptomus, known to occur throughout the arid regions of the Mediterranean and Black Sea (e.g., Kiefer [1978b]; Jaume [1989]; Rayner [1999]; Marrone and Naselli-Flores [2005]: Samchyshyna [2011], and references therein), although never recorded to date in Israel, are likely to also occur in the country; for instance, Metadiaptomus chevreuxi (de Guerne & Richard, 1894) is known from Jordan, and Metadiaptomus mauretanicus Kiefer & Roy, 1942 from Egypt (Dumont [1979], [2009]).
Based on the updated checklist of Israeli diaptomids (Table 2), counts of endemic (i.e., H. gurneyi canaanita, A. irregularis) and eastern taxa (A. similis, E. drieschi, P. blanci) in this country are comparable to counts of taxa with wider distribution areas (i.e., Arctodiaptomus salinus and Neolovenula alluaudi). Unfortunately, due to a lack of sound checklists for most of the other Middle East countries, it is currently difficult to understand the biogeographic affinities of the Israeli calanoid copepod fauna.
The possible presence of an allochthonous species in the country is in accordance with the ever-growing number of successful biological invasions affecting the freshwater zooplankton in the last decades. Such phenomenon is becoming increasingly important in the Western Palaearctic region, where the records of allochthonous calanoid copepods are increasing at a fast pace (e.g., Ferrari et al.[1991]; Rossetti et al. [1996]; Alfonso and Belmonte [2008]; Brandorff [2011]; Alfonso et al. [2014]).
References
Alfonso G, Belmonte G: Expanding distribution of Boeckella triarticulata (Thomson, 1883) (Copepoda: Calanoida: Centropagidae) in Southern Italy. Aquat Invasions 2008, 3: 247–251. 10.3391/ai.2008.3.2.17
Alfonso G, Belmonte G: Calanoida (Crustacea Copepoda) from the inland waters of Apulia (south-eastern Italy). J Limnol 2011, 70: 57–68. 10.4081/jlimnol.2011.57
Alfonso G, Belmonte G: Neolovenula alluaudi (Guerne and Richard, 1890) (Calanoida: Diaptomidae: Paradiaptominae): first record in Italy and review of geographical distribution. J Limnol 2013, 72: 251–261. 10.4081/jlimnol.2013.e20
Alfonso G, Russo R, Belmonte G: First record of Neodiaptomus schmackeri (Poppe & Richard, 1892) (Copepoda: Calanoida: Diaptomidae) in Europe: evidences of a successful invasion from Asia. J Limnol 2014, 73: 584–592. 10.4081/jlimnol.2014.972
Azoulay BR (2001) The Autoecology of Eudiaptomus cf. drieschi (Poppe & Mrazek 1895) (Copepoda, Calanoida) in Lake Kinneret. PhD thesis. The Hebrew University of Jerusalem, Israel
Baird W: Description of several species of Entomostracous Crustacea from Jerusalem. Ann Mag nat Hist (ser III) 1859, 4: 280–293.
Borutzky EB, Stepanova LA, Koss MS: Opredelitel' Calanoida presnykh vod SSSR. Nauka, St. Petersburg; 1991.
Brandorff GO: The copepod invader Skistodiaptomus pallidus (Herrick, 1879) (Crustacea, Copepoda, Diaptomidae) from North America in water bodies of Bremen, northern Germany. Aquat Invasions 2011,6(Suppl 1):S1-S5. 10.3391/ai.2011.6.S1.001
Brehm V: Süsswasserorganismen aus dem Elbursgebirge (Persien). Zool Anz 1937, 118: 214–223.
Dimentman C, Por FD: Diaptomidae (Copepoda, Calanoida) of Israel and Northern Sinai. Hydrobiologia 1985, 127: 89–95. 10.1007/BF00004666
Dumont HJ (1979) Limnologie van Sahara en Sahel. Thesis. Rijksuniversiteit Gent
Dumont HJ:Dumont HJ (Ed): The crustacean zooplankton (Copepoda, Branchiopoda), Atyid Decapoda, and Syncarida of the Nile basin In The Nile: Origin, Environments, Limnology and Human Use. Springer, The Netherlands; 2009:521–545. 10.1007/978-1-4020-9726-3_26
Dussart B: Crustacés Copépodes Calanoides des eaux intérieures africaines. Crustaceana 1989,15(Suppl):1–2015.
Dussart B, Defaye D: Introduction to Copepods. 2nd edition. Backhuys Publishers, Leiden; 2001.
Dussart B, Defaye D: World directory of Crustacea Copepoda of inland waters. Backhuys Publishers, Leiden, I Calaniformes; 2002.
Fadda A, Marková S, Kotlík P, Lugliè A, Padedda B, Buscarinu P, Sechi N, Manca M: First record of planktonic crustaceans in Sardinian reservoirs. Biologia 2011, 66: 856–865. 10.2478/s11756-011-0092-4
Ferrari I, Farabegoli A, Pugnetti A, Stella E: The occurrence of a Calanoid Australasian species, Boeckella triarticulata (Thomson), in fish ponds of Northern Italy. Verh Int Verein Limnol 1991, 24: 2822–2827.
Fischer O: Notes on the zooplankton of Israel fish ponds. Bull Res Counc Israel 1953, 2: 441–442.
Gophen M: Extinction of Daphnia lumholtzi (Sars) in Lake Kinneret (Israel). Aquaculture 1979, 16: 67–71. 10.1016/0044-8486(79)90173-X
Gündüz E: Eudiaptomus anatolicus n.sp. (Copepoda: Calanoida) from Turkey. Hydrobiologia 1998, 368: 193–199. 10.1023/A:1003214619301
Guindon S, Gascuel O: A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 2003, 52: 696–704. 10.1080/10635150390235520
Gurney R: Freshwater Crustacea collected by Dr. P. A. Buxton in Mesopotamia and Persia. J Bombay Nat Hist Soc 1921, 27: 835–843.
Hall TA: BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999, 41: 95–98.
Jaume D: Metadiaptomus chevreuxi (Copepoda: Calanoida: Diaptomidae) and Leptestheria mayeti (Branchiopoda: Conchostraca: Leptestheriidae), two African freshwater crustaceans recorded in Majorca. Limnetica 1989, 5: 101–109.
Khalaf TA: A new species of Phyllodiaptomus Kiefer (Copepoda, Calanoida) from the Shatt Al-Arab river, southern Iraq. Crustaceana 2008, 81: 257–269. 10.1163/156854008783564028
Kiefer F: Zur Kenntnis der Copepodenfauna Palästinas. Zool Anz 1930, 92: 242–246.
Kiefer F: Versuch eines Systems der Diaptomiden (Copepoda Calanoida). Zool Jb Syst 1932, 63: 451–520.
Kiefer F: Zur Kenntnis von Morphologie und Systematik einiger Arten der Gattung Arctodiaptomus Kiefer (Crustacea, Copepoda). Zool Scripta 1974, 3: 11–22. 10.1111/j.1463-6409.1974.tb00799.x
Kiefer F (1978a) Das Zooplankton der Binnengewässer. Freilebende Copepoda. Die Binnengewässer 26:1–343, E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart
Kiefer F: Zur Kenntnis der Copepodenfauna ägyptischer Binnengewässer. Arch Hydrobiol 1978, 84: 480–499.
Leibold MA, Economo EP, Peres-Neto P: Metacommunity phylogenetics: separating the roles of environmental filters and historical biogeography. Ecol Lett 2010, 13: 1290–1299. 10.1111/j.1461-0248.2010.01523.x
Löffler H: Limnologische Beobachtungen an Iranischen Binnengewässern. Hydrobiologia 1956, 8: 239–278. 10.1007/BF00151755
Löffler H: Beiträge zur Kenntnis der Iranischen Binnengewässer II: regional-limnologische Studie mit besonderer Berücksichtigung der Crustaceenfauna. Int Revue Ges Hydrobiol 1961, 46: 309–406. 10.1002/iroh.19610460304
Marrone F: The microcrustacean fauna of Sicily and the Central Mediterranean Sea area – current knowledge and gaps to be filled. Pol J Ecol 2006, 54: 681–685.
Marrone F, Naselli-Flores L: First record of a representative of the subfamily Paradiaptominae (Copepoda Calanoida Diaptomidae) in Italy: Metadiaptomus chevreuxi (Guerne & Richard, 1894). J Limnol 2005, 64: 89–92. 10.4081/jlimnol.2005.89
Marrone F, Castelli G, Barone R, Naselli-Flores L: Ecology and distribution of calanoid copepods in Sicilian inland waters (Italy). Verh Internat Verein Limnol 2005, 29: 2150–2156.
Marrone F, Lo Brutto S, Arculeo M: Molecular evidence for the presence of cryptic evolutionary lineages in the freshwater copepod genus Hemidiaptomus G.O. Sars, 1903 (Calanoida, Diaptomidae). Hydrobiologia 2010, 644: 115–125. 10.1007/s10750-010-0101-6
Marrone F, Lo Brutto S, Hundsdoerfer AK, Arculeo M: Overlooked cryptic endemism in copepods: systematics and natural history of the calanoid subgenus Occidodiaptomus Borutzky 1991 (Copepoda, Calanoida, Diaptomidae). Mol Phylogenet Evol 2013, 66: 190–202. 10.1016/j.ympev.2012.09.016
Mohamed HH, Salman SD: Copepoda of the Southern Iraqi Marshes. 1. Calanoida. Marsh Bull 2009, 4: 148–161.
Nylander JAA (2004) Program Distributed by the Author. Evolutionary Biology Centre, Uppsala University
Petkovski TK: Über einige Diaptomiden aus Jugoslawien und Israel (Crustacea: Copepoda Calanoida). Acta Mus maced Sci nat Skopje 1961, 7: 175–201.
Petkovski TK: Calanoides - Calanoida (Crustacea -Copepoda). Faune de Macédoine. Musée Histoire Naturelle de Macedonie, Skopje; 1983.
Por FD: An outline of the distribution patterns of freshwater Copepoda of Israel and surroundings. Hydrobiologia 1984, 113: 151–154. 10.1007/BF00026602
Ranga-Reddy Y (1994) Copepoda: Calanoida: Diaptomidae. Key to the genera Heliodiaptomus, Allodiaptomus, Neodiaptomus, Phyllodiaptomus, Eodiaptomus, Arctodiaptomus and Sinodiaptomus. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World. SPB Academic Publishing, The Hague, p 5
Rayner NA: Copepoda: Calanoida (Diaptomidae: Paradiaptominae). Guides to the identification of the Microinvertebrates of the Continental Waters of the World. Backhuys Publishers, Leiden; 1999.
Richard J: Copépodes recueillis par M. le Dr. Th. Barrois en Egypte, en Syrie et en Palestine. Revue Biologique du Nord de la France 1893, 5: 3–36.
Ronquist F, Teslenko MP, van der Mark DL, Ayres A, Darling S, Höhna B, Larget L, Suchard MA, Huelsenbeck JP: MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 2012, 61: 539–542. 10.1093/sysbio/sys029
Rossetti G, Dussart B, Viaroli P: Finding of the calanoid Eudiaptomus gracilis (Sars) in perifluvial environments of the Po River. Mem Ist ital Idrobiol 1996, 54: 51–59.
Ruffo S, Stoch F: Checklist e distribuzione della fauna italiana. 10.000 specie terrestri e delle acque interne. Mem Mus Civ St Nat Verona, Sez Scienze della Vita 2005, 16: 1–307.
Samchyshyna L: Faunistical overview of calanoid copepods (Crustacea) from continental waters of Ukraine. Vestn zool 2011, 45: 299–305.
Sanoamuang L, Teeramaethee J: Phyllodiaptomus thailandicus , a new freshwater copepod (Copepoda, Calanoida, Diaptomidae) from Thailand. Crustaceana 2006, 79: 475–487. 10.1163/156854006777554802
Spandl H: Zur Kenntnis der Susswasser-Mikrofauna Vorderasiens. Ann Naturhist Mus Wien 1923, 36: 124–149.
Staton JL, Taylor BE, Schizas NV, Wetzer R, Glenn TC, Coull BC: Mitochondrial gene diversity of Skistodiaptomus mississippiensis in impoundments of the Upper Coastal Plain near Aiken, South Carolina, USA. Arch Hydrobiol 2003, 158: 215–231. 10.1127/0003-9136/2003/0158-0215
Stella E: Diaptomidi della Sardegna. Ist Lomb (Rend Sc) B 1970, 104: 69–87.
Stella E: Copepoda: Calanoida. XXI Fauna d'Italia, Calderini, Bologna; 1984.
Stella E, Margaritora FG, Cottarelli V: La fauna ad entomostraci di acque astatiche della Sardegna nord orientale. Ricerche biologiche ed ecologiche. Rendiconti Accademia Nazionale dei XL 1972, 4: 3–50.
Stella E, Margaritora G (1975/1976) Contributo alla conoscenza della fauna ad entomostraci di acque astatiche della Sardegna (zone Nord occidentale e Centrale). Considerazioni ecologiche e biogeografiche. Rendiconti Accademia Nazionale dei XL 5:1–11
Swofford DL: PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer Associates, Sunderland, Massachusetts; 1998.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S: MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011, 28: 2731–2739. 10.1093/molbev/msr121
Thum R, Derry AM: Taxonomic implications for diaptomid copepods based on contrasting patterns of mitochondrial DNA sequences in four morphospecies. Hydrobiologia 2008, 614: 197–207. 10.1007/s10750-008-9506-x
Thum RA, Harrison RG: Deep genetic divergences among morphologically similar and parapatric Skistodiaptomus (Copepoda: Calanoida: Diaptomidae) challenge the hypothesis of Pleistocene speciation. Biol J Linn Soc 2009, 96: 150–165. 10.1111/j.1095-8312.2008.01105.x
Turki S, Turki B: Copepoda and Branchiopoda from Tunisian temporary waters. Int J Biodivers Conserv 2010, 2: 86–97.
Ustaoğlu MR: A check-list for zooplankton of Turkish inland waters. E.U. J Fisheries Aquat Sci 2004, 21: 191–199.
Ustaoğlu MR, Balik S, Mis DO, Aygem C: The zooplankton of some mountain lakes in the Taurus Range (Turkey). Zool Middle East 2005, 34: 101–108. 10.1080/09397140.2005.10638089
Yaron Z: Notes on the ecology and entomostracan fauna of temporary rainpools of in Israel. Hydrobiologia 1964, 24: 489–513. 10.1007/BF00141999
Acknowledgements
Nicolas Rabet (National Museum of Natural History, Paris, and University Pierre and Marie Curie, Paris) kindly provided the diaptomid sample from Moshav Ishrash (Center District). Tereza Petrusková helped during the sampling campaigns. T. Chad Walter (National Museum of Natural History, Washington DC) kindly provided the catalogue numbers of the copepod specimens deposited in the collection of the Smithsonian Institution. We thank Fabio Stoch (University of L'Aquila, L'Aquila) for his help in the correct application of the International Code of Zoological Nomenclature and Jessica Richardson for language corrections.
The research was partially supported by the ‘Fondi di Ateneo’ (60%) of the University of Palermo.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests
Authors’ contributions
FM carried out the morphological identification of the samples, carried out the molecular analyses, and drafted the manuscript. AP carried out the samplings in Israel and helped to draft the manuscript. GA helped with the bibliographical research, realised the figures, and provided important comments to a first draft of the manuscript. MA supervised and coordinated the research and helped with the molecular analyses. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Marrone, F., Petrusek, A., Alfonso, G. et al. The diaptomid fauna of Israel (Copepoda, Calanoida, Diaptomidae), with notes on the systematics of Arctodiaptomus similis (Baird, 1859) and Arctodiaptomus irregularis Dimentman & Por, 1985 stat. rev. Zool. Stud. 53, 74 (2014). https://doi.org/10.1186/s40555-014-0074-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40555-014-0074-7