Abstract
Background
A new prototype of bio-conditioner useful in rehabilitation of degraded soils was performed. In order to obtain this aim two stages were established: production of biomass of Microbacterium sp. CSB3 and formulation of this inoculum in a sediment supplemented with low-rank coal (LRC).
Materials and methods
The effect of agitation and pH on microbial growth was determined. As response variables, the final production of biomass (Xf) and yield (Yx/s) were determined. Growth dynamics of CSB3 in a 2-L reactor was also evaluated through Xf, Yx/s and the determination of kinetic parameters (specific growth rate [μ] and duplication time [Dt]). The formulation of CSB3 was evaluated; mixtures of several LRC proportions with a sediment from a municipal aqueduct were made. During 90 days, the viability of CSB3 was monitored by counting CFU.
Results
The optimal pH and agitation for Xf and Yx/x were 7.5 and 232 rpm, respectively; the values of Xf, Yx/s, μ and Dt in 2-L reactor were: 1.5 gL−1, 0.28 g/g, 0.0208 h−1, 33.3 h, respectively. Regarding the formulation, the most suitable combination to conserve the viability of CSB3 was LRC 25%–sediment 75%; the heavy metals content of LRC allow to infer that the prototype of bio-conditioner does not represent a pollution risk for environment soil.
Conclusions
It was possible to optimize the growth of CSB3 under laboratory conditions. The viability of CSB3 could be maintained by a formulation in a sediment supplemented with lignite; this formulation constitutes a new prototype of soil bio-conditioner.

Similar content being viewed by others
Background
Low-rank coals, which are a by-product of coal mining, have low calorific value and lack commercial interest; however, they are an important source of humic substances with potential applications at agricultural and industrial levels. Some research works have provided evidence of the ability of some bacteria to solubilize such coals and release humic substances during the process [1]. It has also been observed that the direct application of these coals, inoculated with coal-solubilizing bacteria, has a positive effect on the edaphic properties of post-mining soils [2]. Such background places the possibility of developing useful bioproducts within a rational context in the rehabilitation of degraded soils from the interaction of these bacteria and low-rank coals.
The production of microbial biomass, for the production of biological inoculants, requires the optimization, adjustment and control of variables related to microbial growth, such as pH, agitation, oxygen supply (if required), and temperature, among others [3, 4]; in this manner, each microbial species has specific requirements, which must be determined if future modeling and scaling processes are to be considered [3].
Formulation consists of mixing a cell suspension, at a known concentration of the selected microorganism, with a solid or liquid substrate, which allows it to retain its viability and activity for a defined timeframe [5]; thus, the way of supplying or formulating the inoculant in the soil–plant system is determined.
The substrate selected for the formulation must be inexpensive and easy to access, as well as easy to use, which keeps the viability and microbial activity for a reasonable time, features chemical and physical uniformity, it is non-toxic, with moisture retention capacity, easy to sterilize, and not causing any alterations in the soil [6]; in this way, several substrates have been evaluated: humic substances (HS) and chelates of iron and magnesium [5], peat [7], zeolite [8], alginate polymers [9], silty sediments rich in organic material from the pre-decantation of solids in drinking water treatment plants [6], among others; the physical and chemical properties of low-rank coal could be useful for the formulation of biological inoculants of agricultural interest. There are no reports on the formulation of coal-solubilizing bacterial strains (CSB) in low-rank coals (LRC).
The purpose of this work was to evaluate the effect of pH and agitation on the biomass production of Microbacteium sp. CSB3. In laboratory conditions, additionally, a 2-L bioreactor test was also carried out to determine the behavior of biomass production with previously optimized factors; as response variables, biomass yield was quantified with respect to the consumption of substrate (Yx/s), specific speed of growth (μ) and duplication time (Dt). Furthermore, the formulation of the strain was evaluated in a mixture of lignite-like LRC and silty solids from a drinking water treatment plant; such sediments have previously demonstrated the ability to maintain the viability of bacterial strains with biotechnological potential for agricultural purpose, given their content of organic matter and their capacity to maintain humidity for long periods of time. It was hypothesized that the chemical composition of coal and its porous structure will contribute to maintaining the viability of the CSB3 strain.
Previous work provided evidence of improvement in edaphic indices of soils degraded by mining due to the joint application of CSB and LRC strains; these results were attributed to the in situ solubilization of LRC by the strains with a subsequent release of humic substances into the edaphic environment and changes in soil structure induced by the application of coal [2]; on the other hand, in vitro studies allowed for the inference that the CSB3 strain has a superior ability to solubilize LRC with respect to other strains; therefore, it is more efficient in the biological production of HS [1]. In this sense, this work seeks to capitalize the potential of the CSB3–LRC interaction to develop the preliminary formulation of a bio-conditioner prototype that is potentially useful in the treatment of degraded soils.
Materials and methods
Effect of pH and agitation on biomass production and yield of Microbacterium sp. CSB3 in MeLac (molasses–whey) medium
Microbacterium sp. CSB3 was isolated from mining area of the Colombian Caribbean, this strain was previously reported for its high capacity to solubilize coal and produce humic substances through this process [1, 10].
The evaluation, under culture conditions, of CSB3, was made in MeLac medium [11], which was previously optimized for biomass production of CSB3; MeLac medium was optimized from molasses and whey. In order to know the effect of agitation and pH on the production of CSB3 biomass, a surface response design of 23 was used [12], with five repetitions of the central point (Table 1). The experiment was carried out in glass containers with a capacity of 1 L, with 450 mL of MeLac; the inoculum was constituted by 50 mL of CSB3 culture in a 50:50 mixture of MeLac and nutritive broth (Oxoid®), at a concentration of 0.8 g L−1 (dry weight of biomass).
Bottles with 500 mL of working volume were incubated at 30 ± 2 °C; pH was modified with 0.5 N HCl and 0.5 N NaOH, depending on the need. An orbital shaker was used to change the agitation as required by the experiment.
Microbial growth and substrate consumption curves were made for each combination with the purpose of obtaining the final production and yield of biomass in order to establish the optimum agitation and pH. Samples and measurements were taken every 4 h for 72 h, the samples were not stored in refrigeration; the measurements were made in real time.
Biomass was determined by dry weight, and yield by using the following mathematical model:
Yx/s = (Xf−Xo)/(So−Sf), grams of biomass formed/gram substrate consumed. where Xf is final biomass; Xo is initial biomass; So is initial substrate; Sf is final substrate [13].
The monitoring of substrate consumption during the growth curve was made by determining the reducing sugars in the previously obtained and refrigerated samples [14].
Sucrose hydrolysis
Sucrose, the main source of carbon in molasses, is not a reducing sugar; therefore, it must be hydrolyzed for determination purposes. For this, a sucrose standard curve was constructed, with a concentration range from 0 to 6 g L−1. Then, 2 mL of each of the concentrations was taken and 2 mL of 18.5% HCl was added; this mixture was made in glass tubes, which were vigorously stirred and heated at 92° C for 10 min. The reaction was held on ice. Subsequently, three drops of phenolphthalein were added to each of the samples, followed by 25% NaOH until a light pink tone was observed. Finally, 5% HCl was added and stirred again [14]. The same procedure was practiced on the samples obtained from the growth curve, both in the designed medium and in the control medium.
Determination of reducing sugars
Reducing sugars were determined in hydrolyzed samples, using the dinitrosalicylic acid (DNS) technique [14]. 0.25 mL of the hydrolyzed medium was taken, which was mixed in glass tubes with 0.25 mL of the DNS reagent; then, the tubes were heated in a boiling water bath for 5 min. The reaction was held with ice. 2.5 mL of distilled water was added to each tube, then stirred, and an absorbance reading was performed at 540 nm [14]. Absorbance values were interpolated with the sucrose pattern curve to determine substrate consumption during the growth curve, when the absorbance values exceeded the maximum value of the curve, the samples were diluted and the concentration was subsequently corrected.
CSB3 biomass production test in a 2-L fermenter
After obtaining pH and agitation values that allowed for an optimization of Xf and Yx/s, under the experimental conditions evaluated, a fermentation test was performed in duplicate in a 2-L reactor equipped to measure and control temperature, agitation and pH. A working volume of 75% of the reactor capacity (1.5 L) was used and the inoculum made up for 10% of the work volume; the inoculum consisted of a liquid culture of CSB3 at a concentration of 0.8 g L−1, in a 70:30 MeLac–nutrient broth mixture. As a response variable, Xf, Yx/s and μ were taken into account [specific growth rate (h−1)] [11].
Formulation of inoculant in lignite-like LRC as vehicle
A lignite-type LRC was used as a substrate, this was obtained from the mining area of the Colombian Caribbean (La Guajira) and typically has high content of humified organic matter [1]. The lignite was crushed and sieved to a particle size of less than 2 mm and was elementally characterized [C, N, O, S (ASTM protocol D5373 in an EAI CE-440 elementary analyzer)], available phosphorus (Olsen method) [15], humidity, volatile material, fixed carbon and ash (by the thermogravimetric method), heavy metals (As, Cd, Cu, Pb, Hg and Zn, by the digestion method in nitric acid, hydrochloric acid, hydrogen peroxide and atomic absorption spectrophotometry according to EPA 3050B protocol) [16]. The above was done to rule out levels of toxicity that may affect soil, plants or microbial activity. In addition, in conjunction with the LRC, a silty sediment with a high content of organic matter from the pre-decanters of the drinking water treatment plant in the city of Valledupar was used. This sediment was elementally characterized and has proved to be a useful support in the maintenance of the viability of bacterial and fungal strains that promote plant growth for 75 days [6].
LRC was mixed with the sediment in different proportions (treatments) and distributed in sealed glass bottles at a ratio of 300 g (experimental units) (Table 2). Once the mixtures were obtained, they were sterilized in an autoclave at 15 psi, 121° C for 1 h. An inoculum of 3 × 108 cells mL−1 of CSB3 was prepared in MeLac medium, which was permeated in the substrate at a concentration of 42% v/p. The bottles with inoculated substrate were stored at a temperature of 25 °C ± 2; the cell viability of the inoculum was determined by plating and counting typical colony forming units (CFU) of CSB3 at 0, 30, 60 and 90 days [6].
To evaluate the viability of CSB3, a completely randomized experimental design was used with 5 treatments and 5 repetitions for a total of 25 experimental units (Table 2). The most suitable substrate mixture was selected for the conservation of the strain’s viability. In order to obtain evidence of the adherence of CSB3 bacterial cells to the selected treatment, the procedure was repeated with this treatment, and after 30 days of storage under the conditions previously described, bacterial structures were observed on the substrate using scanning electron microscopy (SEM) with a JEOL electron microscope, Reference JSM-5910LV.
Statistical analysis
To study the joint effect of agitation and pH, a surface optimization design of 23 response was used with the Statgraphics Centurion package. Regarding the formulation, data were analyzed by the Mann–Whitney test, with a 95% confidence interval, due to lack of fit to generalized linear models.
Results
Effect of pH and agitation on biomass production and yield of Microbacterium sp. CSB3
To obtain maximum yield and maximum biomass production, the optimum agitation values are similar (242.8 and 215.5 rpm, respectively); however, the optimum pH for biomass production was 6.5 (Table 3, Fig. 1b), and 7.5 for the yield (Table 4, Fig. 2b). When doing the multiple response analysis, which seeks to obtain an optimal joint response for both variables, optimal agitation was 232 rpm, while the pH was 7.5 (Table 5, Fig. 3).
Optimization of biomass production as a function of agitation and pH. a Pareto diagram for the analysis of the statistical significance of factors; factors: a pH and B: agitation. b Response surface of CSB3 biomass production, X axis: pH ranges in codes units; Y axis: bacterial biomass (g L−1); Z axis: agitation in codes units
CSB3 yield optimization (Yx/s) in the molasses–whey culture medium as a function of agitation and pH. a Pareto diagram for the analysis of the statistical significance of factors; factors: a pH and b agitation. b Response surface CSB3 yield with respect to substrate consumption, X axis: pH ranges in codes units; Y axis: yield Yx/s (g/g); Z axis: agitation in codes units
This result can be explained by the dynamics observed in the graphs of main effect for both variables; although for biomass production the optimum value was the lowest level (6.5), the curve that defines pH behavior in the experiment is observed on an almost linear basis (Fig. 4), which indicates a non-significative difference between pH values; nevertheless, regarding to the yield, the main effect chart shows that the optimum pH for obtaining the maximum possible yield was 7.5 (Fig. 5). Therefore, the multiple response is the result of the integration of the optimization of factors for both response variables.
CSB3 biomass production test in 2-L bioreactor
The growth curve of CSB3 indicates an adaptation phase of 16 h with diauxic growth during this period (Fig. 6a); the exponential phase occurred between 16 and 36 h, while the stationary phase occurred between 36 and 48 h. During this period, the highest biomass production was obtained (1.6 g L−1). The highest consumption of substrate was presented at 48 h (17.6 g L−1, starting from an initial concentration of 21.3 g L−1 of reducing sugars as a product of hydrolysis) and remained constant until the end of the process (Fig. 6 b). The final production and yield of biomass (56 h) featured lower values than those obtained in the previous test (Table 6), in which the work volume utilized was 500 mL. The values for μ, Yx/s and Dt were 0.0208 h−1, 0.28 g/g and 33.3 h, respectively (Table 6).
Formulation of inoculant in lignite-type LRC as carrier
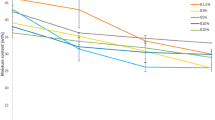
All treatments evaluated in order to optimize the formulation of CSB3 in sediments proposed showed a gradual reduction with cell concentration, expressed in CFU (Fig. 7). The most suitable treatment for the conservation of CSB3 viability was LRC 25%:sediment 75%, since it featured the highest R2 value (0.85), which indicates that the statistical model that gave rise to the trend line observed in Fig. 7 can explain in up to 85% the result obtained; in addition, this treatment presented a higher average during the 90 days of the trial (Table 7). It was also observed that the highest coal concentrations reduced the viability of CSB3 during the shelf life. The analysis by SEM (Fig. 8), provides evidence of the colonization of bacterial structures on the surface of the carrier after 30 days of inoculation and conservation.
The elemental composition of LRC showed a high carbon content and the presence of N and S. The determination of P (Table 8) refers to the available element; it must be taken into account that the content of P in LRC (0.009 mg kg−1) is lower than that obtained in the sediment (0.051 mg kg−1). Table 9 shows the content of heavy metals in the LRC used as one of the supports in the formulation; the content of heavy metals is within the normal allowable range for soil according to the EPA and other entities such as the UN Economic Commission for Europe. The registered value of lead is below the limit of toxicity established by the EPA.
Discussion
Agitation and pH are important variables in the optimization of bioprocesses aimed at the production of microbial biomass; in the results presented in this work we observe the effect of the variation of these variables in a volume of 500 mL. In order to optimize the biomass production of the CSB3 strain, the behavior of biomass production in 2-L bioreactor is also described, using the optimized values. High agitation values negatively influenced CSB3 biomass production (Pareto diagrams—Figs. 1a, 2a). Agitation is an important factor in fermentative processes, since it increases dissolved oxygen in the medium [17]; it is the most practical and economic way to meet the oxygen needs for fermentation in laboratory conditions (50–500 cm3 of work volume) [18].
Microbacterium sp. is an aerobic bacterial genus, therefore it requires high oxygen availability [19]. There are few reports of fermentative processes with this genus for biomass production purposes; however, Thys et al. [20] described the production of proteases with Microbacterium sp. using an orbital agitation of 125 rpm in a work volume of 50 mL, while Shivakumar [21] described the production of polyhydroxybutyrate by Microbacterium barkeri in the same volume, with an agitation of 120 rpm. The optimum agitation speed described in this work for Xf and Yx/s of Microbacterium sp. was 232 rpm; this data differs from those described above due to the increase in the work volume, since Stanbury et al. [18] established that increasing the volume makes it difficult to transfer the dissolved oxygen to the microbial cell. Therefore, the increase in agitation or the injection of air must be resorted to. Results show that the increase in agitation has a negative effect on the optimization of response variables; this coincides with what was described by Purwanto et al. [17], who determined that excessive agitation can cause mechanical damage at the bioprocess level, such as rupture of the cell wall, variations in the efficiency of biomass or metabolite production, reduction in the generation of enzymes and destruction of biofilms and/or cell clusters in the bioreactor.
On the other hand, the increase in pH positively and significantly affects biomass production and yield (Pareto diagrams—Figs. 1a, 2a); the optimum pH described in this work for the growth of CSB3 was 7.5, which is a value close to neutrality, with a tendency to alkalinity. These results agree with those obtained by Chen et al. [22], who by means of a response surface design evaluated the growth and production of cyclic adenosine-3′,5′-monophosphate (cAMP) by Microbacterium sp.-205 in 500-mL flasks with 50 mL of effective volume; they found that the initial pH value required for maximum cAMP production was 7.5 (optimal pH). Optimal growth of Microbacterium sp., has been described within a pH range between 5 and 9 [23]; however, this microorganism has been shown to have tolerance to alkalinity, even at pH values of 11 [24, 25].
There is little background data on the development of growth curves for the genus Microbacterium sp. However, Mounier et al. [26] described the dynamics of a growth curve for the strain Microbacterium gubbeenense DPC 5286 T by using a culture medium with glucose as a carbon source without observing any diauxic growth during the adaptation phase of the culture. Diauxic behavior is caused in batch conditions through the simultaneous use of different carbon sources by microorganisms in complex culture media [27]; in this way, cane molasses (the main source of carbon in the MeLac medium) has, in its composition, residues of several easily assimilable sugars, such as glucose, fructose, sucrose and raffinose [14]. Besides, whey has lactose within its composition [28]; this could explain the diauxic effect of the growth curve during the first hours of the culture, due to several sugar types in the medium allow a diauxic growth. The inoculum culture medium contained 30% nutrient broth, which served as an inducer of microbial growth. However, the MeLac medium had a higher concentration of sugars; this transition generates irregular growth during the first hours of the culture, which is attributed to catabolic repression processes. This behavior, nonetheless, is usually expected in culture media designed from agroindustry waste and is of less importance as long as it does not affect the beginning of the logarithmic phase nor the final production of biomass [29].
The start of the exponential phase and the maximum production of biomass in a 2-L bioreactor coincide with those reported by Mounier et al. [26], who described the exponential phase between 10 and 40 h, on the basis of which the stationary phase initiated in a growth curve of Microbacterium gubbeenense. It should be taken into account that the genus Microbacterium sp., is of slow growth and, when cultivated in solid medium, a period of 3–4 days is recommended for the observation of well-differentiated colonies [30]. This fact is evidenced by the time of duplication obtained of 33.3 h (Table 6). On the other hand, the reduction in biomass production in a 2-L reactor, compared to 500-ml bottles is a regular phenomenon with an increase in volume; the oxygen dissolved in the medium decreases when the work volume increases, which affects the reduction of biomass production [3]; however, the values of μ and Dt (Table 6) agree with those previously reported during the optimization of the culture medium [11], which indicates that the results obtained in some growth variables tend to be reproducible and these were not affected by the change in work volume.
The value of μ obtained in 2-L reactor (0.0208 h−1) is similar to values previously reported by other authors for Microbacterium sp., and for other actinobacteria. Chen et al. [31] described values of μ between 0.036 and 0.318 for the Microbacterium sp., ZD-M2 strain, at a work volume of 100 mL; [32] reported values of μmax between 0.09 and 0.12 for a strain of Microbacterium sp., which expressed the activity of aryl sulfatase. μ values have also been described for several species of the genus Streptomyces (actinobacteria) between 0.16 and 0.55 in different work volumes and carbon sources [32,33,34]. The differences between such reports and this work may be the product of changes in work volumes and composition of culture media.
In the formulation a gradual reduction of CFUs over time was observed, Rey et al. [35] describe this behavior in a formula of the actinobacteria Frankia sp., with a mixture of peat and rice husk, and attribute this result to the reduction of substrate moisture and pH decrease. Due to the similarity between peat and lignite, such phenomena could also explain the results obtained in this work. The solid and liquid substrates utilized in the formulation of inoculants act by reducing the microbial metabolic rate, with a carbon source of slow assimilation through the conserved strain [5]. However, the microbial activity continues, which causes the gradual reduction of the microbial viability [35].
Previously the Microbacterium sp. CSB3 strain could be cultivated in media with LRC 5% as the sole carbon source and showed the ability to solubilize carbon in liquid culture media supplemented with 1% LRC [1]. For this reason, an initial hypothesis that suggested the ability of LRC to act as an adequate support to maintain the viability of this strain had previously shown to be a potential source of HS for the soil; however, excess coal in the medium could generate catabolic repression phenomena expressed in the reduction in the strain’s viability [29]. However, coals are highly adsorbent materials and tend to decrease the bioavailability of some ions and molecules [36, 37], which could limit the access of bacterial cells to other elements necessary for their nutrition. This could explain the reduction in the availability of phosphorus in the coal compared to the sediment and the subsequent decrease of CFUs in treatments with high carbon concentration.
Table 8 shows that approximately half of the carbon contained in LRC and all the content in the sediment were in an available state, which facilitates its use as a carbon source for microbial metabolism. In this manner, the LRC 25%:sediment 75% mixture was the most appropriate combination of nutrients and other factors to conserve the viability of CSB3 during the test, especially in the first 60 days. Furthermore, for the development of the bio-conditioner prototype proposed in this work, the content of LRC in the formulation is necessary, since this will guarantee a humified source of organic matter for the soil. Valero et al. [6] showed that the sediment used in this test conserved the viability of a bacterial plant promoter strain for 75 days; this effect was attributed to the ability of the sediment to conserve moisture, in addition to having a pH close to neutrality (6.5) and a content of organic matter that allowed for the strain to reduce its metabolic activity without losing its viability.
The content of heavy metals in the LRC does not represent contamination risks for the soil ecosystem (Table 9) [16, 38, 39]. It should be taken into account that the concentrations of heavy metals recorded in Table 9 do not refer to the concentration in the final formulation, but to the content in LRC, which only constitutes 25% of the formulated content. In this manner, the concentration in the final formulation is lower than that recorded in the table. In that sense, heavy metal content does not represent a risk of soil contamination when used as a support for a microbial inoculant and does not represent any degree of toxicity for plants. The analysis by SEM (Fig. 8) provides evidence of the colonization of bacterial structures on the surface of the carrier after 30 days of inoculation and conservation. The logical inference is that these structures belong to CSB3, because the substrate was sterilized prior to its inoculation and the CFU count at 30 days does not show any other type of bacterial growth.
Electron microscopy images of the prototype designed. a Block-like structures corresponding to LRC and amorphous particles from sediment X100/scale: 100 μm; b laminar structures of LRC and presence of bacteria X2000/scale: 10 μm; c coal particle covered with sediment and bacteria on the surface X4000/scale 5 μm; d Laminar structure of LRC with bacteria in interstitial spaces X2000/scale: 10 μm. BLOC: block-like particle. LAM: laminar particle. SEDIM: sediment. BSC3: bacterial structures attributed to colonization of Microbacterium sp. CSB3
The final version of the bio-conditioner prototype was evaluated under greenhouse conditions, showing promising results in the rehabilitation of degraded soils by open-pit coal mining. These evidences show the effect of the product on the biological, chemical and physical properties of these edaphic materials, as well as on the growth and adaptation of grasses [40]. Field experiments are currently being developed to evaluate the effectiveness of the product in these conditions.
Conclusions
The most suitable pH and agitation values in the optimization of CSB3 biomass production and yield (Yx/s) were 7.5 and 232 rpm, respectively.
CSB3 growth at the of 2-L reactor level was diauxic during the first hours of the culture; however, this did not affect the start of the exponential phase nor the final production of biomass. The growth curve featured a behavior similar to that obtained by other authors for Microbacterium sp., and for other actinobacteria; in the same manner, the evaluated kinetic parameters showed values similar to those previously described for this bacterial genus.
With respect to the formulation, the LRC 25%–sediment 75% mixture was the most appropriate combination to maintain the viability of CSB3 during the shelf life (90 days). The elemental composition of both supports showed that they have the ability to preserve the viability of the strains from the nutritional point of view and LRC does not represent a risk of contamination to the soil or of toxicity to the plants by means of heavy metals.
Availability of data and materials
The authors declare the availability of data used in order to write this document when these will be required.
Abbreviations
- S:
-
humic substances
- CSB:
-
coal-solubilizing bacteria;
- CSB3:
-
strain Microbacterium sp. CSB3
- LRC:
-
low-rank coal
- Yx/s:
-
biomass yield with respect to consumption of substrate
- μ :
-
specific speed of growth
- DT:
-
duplication time
- MeLac:
-
molasses–whey medium
- rpm:
-
revolutions per minute
- Xf:
-
final biomass
- Xo:
-
initial biomass
- So:
-
initial substrate
- Sf:
-
final substrate
- DNS:
-
dinitrosalicylic acid
- ASTM:
-
American Society for Tests and Materials
- CFU:
-
colony forming units
- SEM:
-
scanning electronic microscopy
- cAMP:
-
adenosine monophosphate
References
Valero N, Gómez L, Pantoja M, Ramírez R. Production of humic substances through coal-solubilizing bacteria. Braz J Microbiol. 2014;45:911–8.
Valero N, Melgarejo LM, Ramírez R. Effect of low-rank coal inoculated with coal solubilizing bacteria on edaphic materials used in post-coal-mining land reclamation: a greenhouse trial. Chem Biol Technol Agric. 2016;3:1–10.
Boze H, Moulin G, Galzy P. Production of Microbial biomass. In: Rehm H, editor. Biotechnology set. New Jersey: Wiley; 2001. p. 165–220.
Doran P. Bioprocess engineering principles. London: Elsevier; 2013.
Rojas J, Moreno-Sarmiento N. Producción y formulación de prototipos de un biofertilizante a partir de bacterias nativas asociadas al cultivo de arroz (Oryza sativa). Revista Colombiana de Biotecnología. 2008;10:50–62.
Valero N, Araujo H, Pantoja M. Viabilidad y actividad de B cepacia YP lilacinus (promotores del crecimiento vegetal) conservados en sedimentos generados en el sistema de potabilización de agua de la ciudad de Valledupar. para su aprovechamiento en el diseño de un biofertilizante. In: Castilla LA, ed. Biofertilización: Avances en Investigación. Ibagué: Sociedad Colombiana de la Ciencia del Suelo; 2012. p. 151–61.
Estrada GA. Calidad de inoculantes almacenados a diferentes temperaturas: efecto sobre la población, humedad y pH del producto. In: Pontificia Universidad Javeriana. 2008. https://repository.javeriana.edu.co/bitstream/handle/10554/8235/tesis228.pdf?sequence=1. Accessed 8 Aug 2016.
Borges-Rodríguez D, San Juan-Rodríguez AN, Díaz-LLanes AO, Gómez-Santiesteban E, Hernández-Sanchez R. Evaluación de la zeolita como soporte sólido para la formulación del biofertilizante Azospirillum. ICIDCA Sobre los Derivados de la Caña de Azúcar. Instituto Cubano de Investigaciones de los Derivados de la Caña de Azúcar; 2012;46:12–8.
Rivera DM. Formulación de un prototipo de biofertilizante con base en Rhizobium sp. In: Universidad Nacional de Colombia. 2012. https://www.bdigital.unal.edu.co/7026/. Accesed 16 Jun 2016.
Valero N, Rodriguez LN, Mancilla S, Contreras L. Obtaining low rank coal biotransforming bacteria from microhabitats enriched with carbonaceous residues. Acta Biológica Colombiana. 2012;17:335–47.
Pantoja-Guerra M, Reyes-Mendoza S, Valero-Valero N. Diseño de un medio de cultivo para la producción de biomasa de Microbacterium sp (BSC3) para la generación de materia orgánica humificada a partir de lignito. Revista Colombiana de Biotecnología. 2018;20:31–41.
Kumar V, Bhalla A, Rathore AS. Design of experiments applications in bioprocessing: concepts and approach. Biotechnol Progr. 2014;30:86–99.
Castaño H, Mejia CE. Producción de etanol a partir de almidón de yuca utilizando la estrategia de proceso sacarificación-fermentación simultáneas (SSF). Vitae Scholasticae. 2008;15:251–8.
Fajardo-Castillo E, Sarmiento-Forero S. Evaluación de melaza de caña como sustrato para la producción de Saccharomyces cerevisiae. In: Pontificia Universidad Javeriana. 2007. https://www.javeriana.edu.co/biblos/tesis/ciencias/tesis26.pdf. Accessed 18 Jun 206.
do Carmo M, Torrent J. The Olsen P method as an agronomic and environmental test for predicting phosphate release from acid soils. Nutr Cycl Agroecosyst. 2007;77: 283–292.
Galán E, Romero A. Contaminación de suelos por metales pesados. Revista de la sociedad española de mineralogía. 2008;10:48–60.
Purwanto LA, Ibrahim D, Sudrajat H. Effect of agitation speed on morphological changes in Aspergillus niger hyphae during production of tannase. World J Chem. 2009;4:34–8.
Stanbury PF, Whitaker A, Hall SJ. Principles of fermentation technology. London: Elsevier; 2013.
Fidalgo C, Riesco R, Henriques I, Trujillo ME, Alves A. Microbacterium diaminobutyricum sp nov, isolated from Halimione portulacoides, which contains diaminobutyric acid in its cell wall, and emended description of the genus Microbacterium. Int J Syst Evol Microbiol. 2016;66:4492–500.
Thys RCS, Guzzon SO, Cladera-Olivera F, Brandelli A. Optimization of protease production by Microbacterium sp. in feather meal using response surface methodology. Process Biochem. 2006;41:67–73.
Shivakumar S. Accumulation of poly (3-hydroxybutyrate) by Microbacterium barkeri DSM 20145. Turk J Biol. 2012;36:225–32.
Chen X-C, Bai J-X, Cao J-M, Li Z-J, Xiong J, Zhang L, et al. Medium optimization for the production of cyclic adenosine 3′,5′-monophosphate by Microbacterium sp. no 205 using response surface methodology. Bioresour Technol. 2009;100:919–24.
Park M-J, Kim MK, Kim H, Im W, Yi T, Kim S, et al. Microbacterium ginsengisoli sp nov, a -glucosidase-producing bacterium isolated from soil of a ginseng field. Int J Syst Evol Microbiol. 2008;58:429–33.
Rashid S, Charles TC, Glick BR. Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl Soil Ecol. 2012;61:217–24.
Alves A, Correia A, Igual JM, Trujillo ME. Microbacterium endophyticum sp. nov. and Microbacterium halimionae sp. nov., endophytes isolated from the salt-marsh plant Halimione portulacoides and emended description of the genus Microbacterium. Syst Appl Microbiol. 2014;37:474–9.
Mounier J, Rea MC, Connor PM, Fitzgerald GF, Cogan TM. Growth characteristics of Brevibacterium, Corynebacterium, Microbacterium, and Staphylococcus spp. isolated from surface-ripened cheese. Appl Environ Microbiol. 2007;73:7732–9.
Egli T, Lendenmann U, Snozzi M. Kinetics of microbial growth with mixtures of carbon sources. Antonie Van Leeuwenhoek. 1993;63:289–98.
Loaiza Castillo MA. Aprovechamiento del suero de leche para la elaboración de una bebida funcional. In: Universidad de las Americas. 2011. https://dspace.udla.edu.ec/handle/33000/752. Accessed 5 Jul 2016.
Hall BG, Acar H, Nandipati A, Barlow M. Growth rates made easy. Mol Biol Evol. 2014;31:232–8.
Holt JG, Krieg NR, Sneath PHA, Others. Bergey’s manual of systematic bacteriology. Baltimore: Williams & Wilkins; 1984. p. 2784.
Chen H, Zhang W-J, Cai Y-B, Zhang Y, Li W. Elucidation of 2-hydroxybiphenyl effect on dibenzothiophene desulfurization by Microbacterium sp. strain ZD-M2. Bioresour Technol. 2008;99:6928–33.
Cregut M, Piutti S, Slezack-Deschaumes S, Benizri E. Compartmentalization and regulation of arylsulfatase activities in Streptomyces sp, Microbacterium sp. and Rhodococcus sp. soil isolates in response to inorganic sulfate limitation. Microbiol Res. 2013;168:12–211.
Shahab N, Flett F, Oliver SG, Butler PR. Growth rate control of protein and nucleic acid content in Streptomyces coelicolor A3(2) and Escherichia coli B/r. Microbiology. 1996;142(8):1927–35.
Van Wezel GP, Krabben P, Traag BA, Keijser BJF, Kerste R, Vijgenboom E, et al. Unlocking Streptomyces spp. for use as sustainable industrial production platforms by morphological engineering. Appl Environ Microbiol. 2006;72:5283–8.
Rey AM, Chamorro D, Barahona R. Efecto del medio de soporte en la estabilidad biológica de dos cepas de Frankia aisladas de Alnus acuminata HBK. Pastos y Forrajes. 2014;37:305–12.
Crosdale PJ, Moore TA, Mares TE. Influence of moisture content and temperature on methane adsorption isotherm analysis for coals from a low-rank, biogenically-sourced gas reservoir. Int J Coal Geol. 2008;76:166–74.
Yu J, Tahmasebi A, Han Y, Yin F, Li X. A review on water in low rank coals: The existence, interaction with coal structure and effects on coal utilization. Fuel Process Technol. 2013;106:9–20.
Camargo-García J, Arias-Morales J, Muñoz-Paredes D. Evaluación del contenido de mercurio en suelos y lechos de quebradas en la zona minera de Miraflores, Quinchía, Colombia. Acta Agron. 2015;64:165–77.
Mahecha-Pulido J, J GT-G, Torres-Mora M. Contenido de metales pesados en suelos agrícolas de la región del Ariari, Departamento del Meta. Orinoquia. Universidad de los Llanos; 2015;19:118–22.
Pantoja-Guerra M, Ramirez-Pisco R, Valero-Valero N. Improvement of mining soil properties through the use of a new bio-conditioner prototype: a greenhouse trial. J Soils Sediment. 2019;19:1850–65.
Acknowledgements
The authors would like to convey their gratitude to COLCIENCIAS for the funding of the project “Design of products based on the combined use of beneficial microorganisms and low ranking coal as a source of humified organic matter for the rehabilitation of degraded soils”—call 576 of 2012 (National Biotechnology Program), which was carried out within the framework of the Research Network for the Use of Natural Resources and Obtainment of Biotechnological Products for Soils Disturbed by Antropical Activity.
Funding
COLCIENCIAS (convocatoria 576 de 2012 Convenio 8790–4494-13).
Author information
Authors and Affiliations
Contributions
Manuel Pantoja-Guerra carried out the experiments and wrote the first version of this paper. Nelson Valero-Valero contributed to the experimental idea and advised the experiments execution. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This manuscript is an original paper and has not been published in other journals. The authors agreed to keep the copyright rule.
Consent for publication
The authors agreed to the publication of the manuscript in this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pantoja-Guerra, M., Valero-Valero, N. Design of a bio-conditioner prototype for the treatment of degraded soils: biomass production and lignite formulation for Microbacterium sp. CSB3. Chem. Biol. Technol. Agric. 7, 3 (2020). https://doi.org/10.1186/s40538-019-0167-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-019-0167-y