Abstract
Background
Human papillomavirus (HPV) has been identified as an etiopathogenetic factor in oropharyngeal squamous cell carcinoma. The HPV E6 and E7 oncogenes are instrumental in promoting proliferation and blocking differentiation leading to tumorigenesis. Although surgical intervention can remove such tumors, the potential for an etiologic field effect with recurrent disease is real. A downstream effector of E7 oncoprotein, enhancer of zeste homolog 2 (EZH2), is known to promote proliferation and to pose a block in differentiation and in turn, could lead to HPV-induced malignant transformation. However, the EZH2 pathway is amenable to low toxicity therapies designed to promote differentiation to a more benign state and prevent recurrent disease by inhibiting the incorporation of HPV into the genome. This is the first study using clinical specimens to demonstrate EZH2 protein expression in oropharyngeal carcinoma (OPC).
Methods
The study included eight patients with oropharyngeal carcinoma, confirmed p16INK4a- positive by immunohistochemistry (IHC). The tissue expression of E6/E7 messenger RNA (mRNA) was measured by RNAscope® in-situ hybridization technology. Expression of EZH2, Ki-67, and mitotic indices were assessed by morphoproteomic analysis. Biomedical analytics expanded the results with data from Ingenuity Pathway Analysis (IPA) and KEGG databases to construct a molecular network pathway for further insights.
Results
Expression of E6 and E7 oncogenes in p16INK4a- positive oropharyngeal carcinoma was confirmed. EZH2 and its correlates, including elevated proliferation index (Ki-67) and mitotic progression were also present. Biomedical analytics validated the relationship between HPV- E6 and E7 and the expression of the EZH2 pathway.
Conclusion
There is morphoproteomic and mRNA evidence of the association of p16INK4a-HPV infection with the E6 and E7 oncogenes and the expression of EZH2, Ki-67 and mitotic progression in oropharyngeal carcinoma. The molecular network biology was confirmed by biomedical analytics as consistent with published literature. This is significant because the biology lends itself to targeted therapeutic options using metformin, curcumin, celecoxib and sulforaphane as therapeutic strategies to prevent progression or recurrence of disease.
Similar content being viewed by others
Background
HPV-associated oropharyngeal carcinoma (OPC) has been reported to account for up to 60% of this subtype of head and neck cancer cases [1]. The E7 oncoprotein of HPV has been linked with the upregulation of p16INK4a protein, which serves as a surrogate marker of HPV-associated oropharyngeal carcinoma [1]. Although the prognosis of HPV-associated oropharyngeal carcinoma has been associated with a 58% reduction in mortality risk vis-à-vis the HPV-negative cases [1, 2], there is still the risk of recurrent disease and an opportunity for therapeutic intervention. Relatedly, high EZH2 expression in patients with head and neck squamous cell carcinoma was associated with advanced T stage and portended a poor survival outcome [3]. A possible connection is that E7 oncoprotein in cervical squamous cell carcinoma has been associated with the activation of EZH2 expression by HPV16 E7 at the transcriptional level [4]. However, no reports of the association of E7 in HPV-associated OPC and EZH2 pathway expression in clinical specimens from patients with OPC are currently cited in the National Library of Medicine’s MEDLINE Database (https://www.ncbi.nlm.nih.gov/pubmedhealth/).
The purpose of this report is to address gaps in the knowledge of EZH2 expression in OPC patient specimens by: 1. providing morphoproteomic and mRNA evidence of the association of p16INK4a-HPVinfection with the E6 and E7 oncogenes in oropharyngeal carcinoma; 2. documenting and correlating both the expression of E6 mRNA in such cases with cell cycle progression and the expression of E7 mRNA with p16INK4a, EZH2 and Ki-67 and mitotic progression; 3. confirming the biology of HPV-associated oropharyngeal carcinoma with biomedical analytics; and 4. investigating targeted therapeutic options based on biomedical analytics and preclinical data.
Methods
Patient population
Eight adult patients (7 males and 1 female) ranging in age from 51 to 72 years were included in this study. The anatomical locations of their biopsy-proven squamous cell carcinomas included palatine tonsil in five and tongue base in three.
Data collection protocols
Data collection and molecular analyses were performed in accordance with the guidelines of the University of Texas McGovern Medical School Committee for the Protection of Human Subjects Institutional Review Board (IRB).
Molecular analyses
Molecular analyses included in-situ hybridization for the expression of HPV-HR18 E6/E7 mRNA using the RNAscope® technology from Advanced Cell Diagnostics (https://acdbio.com/). Morphoproteomic analysis and biomedical analytics were also performed as part of the molecular analysis in our CLIA and CAP certified Consultative Proteomics Laboratory in order to define the biology of the patients’ tumors, to provide correlative expressions, and to ascertain targeted therapeutic options designed to reduce the progression or recurrence of the HPV-associated oropharyngeal carcinomas.
In-situ hybridization
RNAscope® 2.5HD Red Assay was performed to evaluate expression in all 8 tissue specimens. The test assayed 18 high-risk HPV serotypes: HPV-HR18 HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82, E6/E7 mRNA. Hs-PPIB was used as a positive control marker for sample quality control (QC) and to evaluate RNA quality in all the tissue samples. Bacterial gene dapB was used as a negative control. Standard pretreatment assay conditions were determined to be optimal for the samples in the study set. All the samples in the study passed QC with strong PPIB expression and no/negligible dapB background. A semi-quantitative scoring system of 0-4 was utilized.
Morphoproteomics
Morphoproteomic analysis applies bright field microscopy and immunohistochemistry directed against various protein analytes to define the biology of a neoplastic process. The analysis uncovers etiopathogenetic occurrences that might be responsible for the process development and the propensity for it to recur [5, 6]. Immunohistochemical probes were applied against the following protein analytes in unstained sections of the patients’ oropharyngeal carcinomas: Ki-67 (G1, S, G2 and M phases of the cell cycle; DakoCytomation, Carpinteria, California, lot #20001030); and enhancer of zeste homolog 2 (EZH2; Cell Signaling Technology, Inc., lot #7). The level of expression of the analytes was graded on a 0 to 3+ scale based on signal intensity indicated by a 3,3′- tetrahydrochloride (DAB) chromogenic (brown) signal, the nuclear estimation of Ki-67 and EZH2 percentages, and mitotic index based on mitotic figures/10 high power fields. The details of the morphoproteomic staining procedure have been previously described [5, 6].
Biomedical analytics
To gain insights into HPV-associated oropharyngeal carcinoma, a standard IPA oropharyngeal pathway network (“ORO”) was constructed from key molecules associated with oropharyngeal carcinoma in the Ingenuity Knowledge Base (www.ingenuity.com). Since IPA does not include viral species, E6/E7 and their interactions associated with HPV (hsa05203) (“HPV” network) were extracted from the KEGG pathway database (http://www.genome.jp/kegg/pathway.html) and manually added to the ORO network. A “patient” pathway network was also constructed from the median patient scores and linked to ORO-HPV. From these graphs and additional data mining of the National Library of Medicine’s MEDLINE database, a single ORO-HPV network model was constructed using IPA Pathway Designer to represent the key modulation and adaptive responses in the signal transduction processes. Therapies were then linked to the ORO-HPV network model to assess potential benefits.
Results
The IHC workup had established the expression of p16INK4a protein in all cases (Fig. 1, Table 1).
Patient 8 biopsy specimen with non-keratinizing oropharyngeal carcinoma compared with concurrent non-neoplastic mucosa. H&E and p16INK4a stained sections of non-keratininzing squamous cell carcinoma versus non-neoplastic squamous epithelium (Frames a and c and b and d, respectively; note strong DAB brown chromogenic signal for p16INK4a in oropharyngeal carcinoma [Frame c] versus absence of expression in non-neoplastic squamous mucosa [Frame d]; original magnification ×200 Frames a-d)
In-situ hybridization
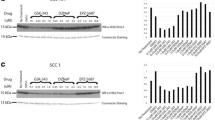
HPV-HR18 E6/E7 was detected at high levels across most samples (score 4) with the exception of patient specimens 5 and 6 that scored at 2 and 3, respectively (see Table 2 and Fig. 2).
Patient 8 biopsy specimen with non-keratinizing oropharyngeal carcinoma compared with adjacent non-neoplastic mucosa. Red RNAscope® 2.5 HD in-situ hybridization (ISH) assay for HPV-HR18 E6/E7 mRNA performed on the non-keratinizing squamous cell carcinoma revealed strong red chromogenic cytoplasmic expression (4+ semi-quantitative score, see Table 2) in the tumor (lower right and middle, Frame a) and no expression in the adjacent non-neoplastic (upper left, Frame a). Contrast with the dapβ negative control in Frame b. (original magnification ×200 for Frames a and b)
Morphoproteomics
Nuclear expressions of EZH2 (enhancer of zeste homolog 2) and Ki-67 (G1, S, G2 and M phases of the cell cycle) and mitotic indices (mitotic figures/10 high power fields) by visual estimation on each case revealed a median of 90% and 70% for EZH2 and Ki-67, respectively and a range of 16 to 105 for the mitotic indices (Table 1 and Fig. 3, frames a and b, c and d, and e and f, respectively for EZH2, Ki-67 expression, and mitotic progression).
Patient 8 biopsy specimen with non-keratinizing oropharyngeal carcinoma compared with concurrent non-neoplastic mucosa. Enhancer of zeste homolog 2 (EZH2) and Ki-67 (G1, S, G2 and M phases of the cell cycle) show strong (3+ on a scale of 0-3+) nuclear expression in a majority of the non-keratinizing squamous cell carcinoma (NKSCC) versus similar expression primarily limited to the basal and suprabasal cells of the non-neoplastic squamous mucosa (Frames a and c versus b and d, respectively). Mitotic progression in the corresponding H&E coincides with the EZH2 and Ki-67 expression with multiple mitotic figures evident in the NKSCC (Frame e) with no mitotic figures in the adjacent non-neoplastic squamous mucosa (Frame f). (DAB brown chromogenic signal for frames a-d; original magnifications ×200 for frames a-d and ×400 for Frames e and f)
Biomedical analytics
In order to provide a visual comparison, biomedical analytics generated a normalized score by patient of the comparative analytes for Ki67, mitotic index, p16INK4a, EZH2 and E6/E7 mRNA. This is illustrated in the bar chart (Fig. 4).
ORO-HPV network model
There were extensive crossover interactions between the HPV pathway and the ORO pathway. Three molecules of interest - EZH2, MKI67 (Ki67) and CDKN2A (p16 INK4a) were present – and affected – in the combined pathway network (not shown.) This combined ORO-HPV network model contained 1086 molecular interactions, or links, in the 3 linked pathways of ORO, HPV, and median patient. Of the 1086 links, 513 bridged between the ORO network and the HPV network. For the patient, the level of CDKN2A affected more molecules associated with HPV than with ORO. In return, more molecules from HPV than ORO affected the patient network, with TP53 and RB1 being the major influencers. 174 molecules from ORO affected HPV and patients; whereas 224 molecules from HPV affected ORO and patient.
Therapeutic interactions
The complex ORO-HPV network model was edited to focus on the key molecules: E6/E7 mRNA, EZH2, Ki-67 (MK167) plus related interactions from the National Library of Medicine’s MEDLINE Database. The potential efficacy of sulforaphane and the metformin and curcumin therapies – the latter in part through the upregulation of microRNAs – were graphically demonstrated (Fig. 5). EZH2 was identified as a possible therapeutic target. It can be seen from the networks that EZH2 (Fig. 5, upper left) is a key network point that is activated by E6/E7. Mir-26A can be seen in the upper right hand corner of Fig. 5; it needs to be upregulated to decrease EZH2.
Discussion
This report provides morphoproteomic and mRNA evidence of the association of p16INK4a-HPVinfection with the E6 and E7 oncogenes in oropharyngeal carcinoma. The E6 oncoprotein has been shown to bind with and promote the degradation of wild type p53 by activating the ubiquitin ligase E6AP [1, 7], and thereby, potentially to facilitate cell cycle progression [1, 8, 9]. Similarly, the E7 oncoprotein via the upregulation of EZH2 should promote cell cycle progression [1, 4]. Morphoproteomics and biomedical analytics provide correlates in our cases in the form of Ki-67 (G1, S, G2 and M phases) with mitotic progression. The seemingly paradoxical but ineffective increase in p16INK4a, a cyclin-dependent kinase inhibitor [10], coincides with E7 expression in our cases of oropharyngeal carcinoma [1]. In addition to its association with cell cycle progression in our study, enhancer of Zeste homolog 2 (EZH2) – a histone methyltransferase – is potentially tumorigenic by virtue of the fact that it methylates and inactivates tumor suppressor genes and contributes to a state of proliferation and dedifferentiation in tumors and in facilitating their migratory potential [11,12,13,14,15,16]. This could account, in part, for the non-keratinizing/poorly keratinizing component of HPV-associated squamous cell carcinomas. The recent manuscript by Idris et al. [17] showed that inhibition of EZH2 has anti-tumorigenic effects on oropharyngeal squamous cell carcinoma (OPSCC) cells in culture that is more pronounced in HPV-positive cell lines. This preclinical evidence plus the results of our study support the applicability of our approach for human patients.
Biomedical analytics confirmed the correlations and the interactive biology of E6/E7 mRNA-associated oropharyngeal carcinoma with the morphoproteomic findings in our study and illustrated the potential targeted therapies of metformin, curcumin, celecoxib and sulforaphane.
Rationale for the application of these agents against HPV-associated oropharyngeal carcinoma are provided as follows: pharmacogenomic upregulation of microRNAs, miR-26a and miR-101, by metformin and a curcumin analog in preclinical studies correspondingly decreased the expression of EZH2 [18, 19] and metformin delays cell cycle progression [20]; curcumin has anti-tumoral activity against HPV-associated cells by selectively inhibiting the expression of viral oncogenes E6 and E7 [21, 22]; celecoxib in HPV-18-infected cervical cancer cells has been shown to restore p53 by suppressing viral oncoprotein E6 expression by down-modulating COX-2 and retrieving p53 from COX-2 association [23]; and sulforaphane suppresses EZH2 expression in skin cancer cells via a proteasome-dependent mechanism [24]. Lindsay et al. recently underscored the opportunities for novel therapeutic targets, such as EZH2, in OPC [25]. Although our pilot study was limited by a small patient population, the results are encouraging. We are in the process of developing a specific clinical protocol for patients with a confirmatory biopsy of HPV -oropharyngeal carcinoma (p16INK4a+/EZH2+) with high Ki-67 expression and mitotic progression. The protocol would incorporate the listed therapeutic agents in a combinatorial fashion and be applied following a confirmatory biopsy of HPV-oropharyngeal carcinoma (p16INK4a+/EZH2+ with high Ki-67 expression and mitotic progression) prior to the implementation of standard tumor board-recommended therapy for the individual patient.
Conclusions
Our study showed that p16INK4a-HPV infection is associated with the E6 and E7 oncogenes and with the expression of EZH2, Ki-67, and mitotic progression in oropharyngeal carcinoma. This biology lends itself to targeted therapeutic options using metformin, curcumin, celecoxib and sulforaphane as therapeutic strategies to prevent progression or recurrence of disease.
Abbreviations
- CAP:
-
College of American Pathologists
- CLIA:
-
Clinical Laboratory Improvement Amendments
- DAB:
-
3,3'-Diaminobenzidine
- EZH2:
-
Enhancer of zeste homolog 2
- H&E:
-
Hematoxylin and eosin stain
- HPV:
-
Human papillomavirus
- IHC:
-
Immunohistochemistry
- IPA:
-
Ingenuity Pathway Analysis
- IRB:
-
Institutional Review Board
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- Ki-67/MKI67:
-
Proliferation marker protein Ki-67
- mRNA:
-
messenger ribonucleic acid
- p16INK4a/CDKN2A:
-
Cyclin-dependent kinase inhibitor 2A
- QC:
-
Quality control
- RB1:
-
Retinoblastoma-associated protein
- RNA:
-
Ribonucleic acid.
References
Spence T, Bruce J, Yip KW, Liu FF. HPV associated head and neck cancer. Cancers (Basel). 2016 Aug 5;8(8):pii:E75. doi:https://doi.org/10.3390/cancers8080075.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DL, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
Chang JW, Gwak SY, Shim GA, Liu L, Lim YC, Kim JM, Jung MG, Koo BS. EZH2 is associated with poor prognosis in head-and-neck squamous cell carcinoma via regulating the epithelial-to-mesenchymal transition and chemosensitivity. Oral Oncol. 2016;52:66–74.
Holland D, Hoppe-Seyler K, Schuller B, Lohrey C, Maroldt J, Durst M, Hoppe-Seyler F. Activation of the enhancer of zeste homologue 2 gene by the human papillomavirus E7 oncoprotein. Cancer Res. 2008;68(23):9964–72.
Brown RE. Morphoproteomics: exposing protein circuitries in tumors to identify potential therapeutic targets in cancer patients. Expert Rev Proteomics. 2005;2(3):337–48.
Brown RE. Morphogenomics and morphoproteomics: a role for anatomic pathology in personalized medicine. Arch Pathol Lab Med. 2009;133(4):568–79.
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–36.
Feng W, Xiao J, Zhang Z, Rosen DG, Brown RE, Liu J, Duan X. Senescence and apoptosis in carcinogenesis of cervical squamous carcinoma. Mod Pathol. 2007 Sep;20(9):961–6.
Feng W, Duan X, Liu J, Xiao J, Brown RE. Morphoproteomic evidence of constitutively activated and overexpressed mTOR pathway in cervical squamous carcinoma and high grade squamous intraepithelial lesions. Int J Clin Exp Pathol. 2009;2(3):249–60.
Komata T, Kanzawa T, Takeuchi H, Germano IM, Schreiber M, Kondo Y, Kondo S. Antitumour effect of cyclin-dependent kinase inhibitors(p16 (INK4A), p18(INK4C), p19(INK4D), p21(WAF1/CIP1) and p27(KIP1)) on malignant glioma cells. Br J Cancer. 2003;88(8):1277–80.
Yamaguchi H, Hung MC. Regulation and role of EZH2 in cancer. Cancer Res Treat. 2014;46:209–22.
Sher F, Boddeke E, Copray S. Ezh2 expression in astrocytes induces their dedifferentiation toward neural stem cells. Cell Reprogram. 2011;13:1–6.
Eskander RN, Ji T, Huynh B, Wardeh R, Randall LM, Hoang B. Inhibition of enhancer of zeste homolog 2 (EZH2) expression is associated with decreased tumor cell proliferation, migration, and invasion in endometrial cancer cell lines. Int J Gynecol Cancer. 2013;23:997–1005.
Behrens C, Solis LM, Lin H, Yuan P, Tang X, Kadara H, Riquelme E, Galindo H, Moran CA, Kalhor N, Swisher SG, Simon GR, Stewart DJ, et al. EZH2 protein expression associates with the early pathogenesis, tumor progression, and prognosis of non-small cell lung carcinoma. Clin Cancer Res. 2013;19:6556–65.
Smits M, Nilsson J, Mir SE, van der Stoop PM, Hulleman E, Niers JM, de Witt Hamer PC, Marquez VE, Cloos J, Krichevsky AM, Noske DP, Tannous BA, Würdinger T. miR-101 is down-regulated in glioblastoma resulting in EZH2-induced proliferation, migration, and angiogenesis. Oncotarget. 2010;1:710–20.
Lu J, He ML, Wang L, Chen Y, Liu X, Dong Q, Chen YC, Peng Y, Yao KT, Kung HF, Li XP. MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 2011;71:225.
Idris S, Lindsay C, Kostiuk M, Andrews C, Côté DW, O'Connell DA, Harris J, Seikaly H, Biron VL. Investigation of EZH2 pathways for novel epigenetic treatment strategies in oropharyngeal cancer. J Otolaryngol Head Neck Surg. 2016;45(1):54.
Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S, Sarkar FH. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila). 2012;5:355–64.
Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, Sarkar FH. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72(1):335–45.
Cai X, Hu X, Tan X, Cheng W, Wang Q, Chen X, Guan Y, Chen C, Jing X. Metformin induced AMPK activation, G0/G1 phase cell cycle arrest and the inhibition of growth of esophageal Squamous cell carcinomas in vitro and in vivo. PLoS One. 2015;10(7):e0133349.
Divya CS, Pillai MR. Antitumor action of curcumin in human papillomavirus associated cells involves downregulation of viral oncogenes, prevention of NFkB and AP-1 translocation, and modulation of apoptosis. Mol Carcinog. 2006;45:320–32.
Chakraborty S, Das K, Saha S, Mazumdar M, Manna A, Chakraborty S, Mukherjee S, Khan P, Adhikary A, Mohanty S, Chattopadhyay S, Biswas SC, Sa G, et al. Nuclear matrix protein SMAR1 represses c-Fos-mediated HPV18 E6 transcription through alteration of chromatin histone deacetylation. J Biol Chem. 2014;289(42):29074–85.
Saha B, Adhikary A, Ray P, Saha S, Chakraborty S, Mohanty S, Das K, Mukherjee S, Mazumdar M, Lahiri L, Hossain DM, Sa G, Das T. Restoration of tumor suppressor p53 by differentially regulating pro- and anti-p53 networks in HPV-18-infected cervical cancer cells. Oncogene. 2012;31:173–86.
Balasubramanian S, Chew YC, Eckert RL. Sulforaphane suppresses polycomb group protein level via a proteasome-dependent mechanism in skin cancer cells. Mol Pharmacol. 2011;80:870–8.
Lindsay C, Seikaly H, Biron V. Epigenetics of oropharyngeal squamous cell carcinoma: opportunities for novel chemotherapeutic targets. J Otolaryngol Head Neck Surg. 2017;46(1):9.
Acknowledgements
The authors thank Pamela Johnston, HT (ASCP) for technical assistance, Bheravi Patel for secretarial support and help with the graphics, and scientists Marie Lauigan and Paul Terinate at Advanced Cell Diagnostics, Newark, CA.
Funding
Funding for this study was obtained from the Morphoproteomics Initiative, University of Texas McGovern Medical School at Houston.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
RB, MM, RK and SN were involved in all aspects of experimental design, data collection, data analysis and were the main contributors of manuscript preparation. JB was primarily involved in technical aspects of slide analyses. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Authors’ information
RB is a Professor of Pathology who invented the term “morphoproteomics” in 2003 to help define the biology of disease processes. MM is a Bioinformatics Scientist who invented the term “biomedical analytics” and has developed its applications. RK is Chief of the Division of Head and Neck Surgical Oncology at UTHealth.
Ethics approval and consent to participate
Ethics approval for this study was obtained from the University of Texas McGovern Medical School Committee for the Protection of Human Subjects protocol (HSC-MS-15-0676).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Brown, R.E., Naqvi, S., McGuire, M.F. et al. Morphoproteomics, E6/E7 in-situ hybridization, and biomedical analytics define the etiopathogenesis of HPV-associated oropharyngeal carcinoma and provide targeted therapeutic options. J of Otolaryngol - Head & Neck Surg 46, 52 (2017). https://doi.org/10.1186/s40463-017-0230-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40463-017-0230-2