Abstract
Background
Angiogenesis and activation of the epidermal growth factor (EGFR) pathway play an essential role in tumor proliferation and metastasis. Targeting angiogenesis or EGFR alone does not yield adequate tumor control in most solid tumors. Overcoming intrinsic and/or acquired resistance may need a doublet or triplet therapy strategy. Herein, we report the safety and feasibility of dual EGFR blockade with EGFR monoclonal antibody and EGFR tyrosine kinase inhibitor combined with anti-VEGF antibody in advanced solid tumors.
Methods
We conducted a phase I study combining erlotinib, cetuximab, and bevacizumab. Patients with advanced or metastatic solid tumors (excluding colorectal and non-small cell lung cancers) were analyzed for safety, toxicity profile, and response. Anti-tumor activity was evaluated per response evaluation criteria in solid tumors (RECIST 1.0).
Results
Thirty-six patients received treatment on a range of dose-levels. The most frequent tumor types enrolled were cervical (n = 10), head and neck squamous cell (n = 10), and follicular thyroid (n = 4) cancers. The most common treatment-related grade ≥ 2 adverse events were rash (56%), hypomagnesemia (17%), pruritus (11%), diarrhea (8%), and tumor-related bleeding (8%). Seventeen of 19 patients (89%) treated at the maximum tolerated dose did not present treatment-related dose-limiting toxicity. Fifteen (63%) of the 24 evaluable patients achieved a disease control (stable disease ≥ 4 months (n = 14) and partial response (n = 1). The median number of prior lines of therapies was 3 (range 1–10).
Conclusions
The triplet combination of erlotinib, cetuximab, and bevacizumab was well tolerated, conferring clinical benefit in heavily pretreated patients. Future studies are warranted with second or third-generation EGFR tyrosine kinase triplet combinations in the EGFR pathway aberrant patients.
Trial Registration: ClinicalTrials.gov Identifier: NCT00543504. Sponsor(s): National Cancer Institute (NCI), MD Anderson Cancer Center
Similar content being viewed by others
Background
Genome driven precision oncology has primarily been focused on monotherapy for single-gene alterations [1]. While this has led to many successful targeted therapies [2,3,4], resistance to targeted therapies develop. One strategy to manage innate and acquired resistance is combination therapies with other targeted agents. Resistance to BRAFV600E in BRAF monotherapy was overcome by combining BRAF and MEK inhibition in melanoma [5,6,7]. Similarly, combined inhibition was successful in patients with non-small cell lung cancer (NSCLC) and anaplastic thyroid cancer, that led to US Federal Drug Administration (FDA) approval in these diseases. Contemporaneously, EGFR was identified as an innate resistance mechanism in BRAF V600E positive colorectal cancer (CRC). A triplet combination of epidermal growth factor receptor (EGFR) monoclonal antibody and BRAF + MEK inhibitors showed clinical benefit [8]. In addition, recent precision oncology studies like WINTHER and I-PREDICT used customized combination strategies to address multiple pathways [9, 10]. The first iteration of the NCI-MATCH, National Cancer Institute-Molecular Analysis for Therapy Choice, or EAY131, a phase II precision medicine trial, sought to determine whether matching certain drugs in adults whose tumors have specific gene abnormalities will effectively treat their cancers, regardless of tumor types. The second-generation NCI-match planned is the combo-match for doublet therapies that tests combination therapy targeting.
Activation of the EGFR pathway plays a vital role in tumor proliferation of several solid tumors [11]. Cetuximab, a monoclonal antibody against EGFR, is commonly used in CRC [12, 13] and head and neck squamous cell cancers (HNSCC) [14, 15]. Erlotinib, a first-generation EGFR tyrosine kinase inhibitor is approved for the treatment of NSCLC [16, 17]. Preclinical studies showed that combination of monoclonal antibodies and tyrosine kinase inhibitors synergistically inhibit the growth of NSCLC and CRC cell lines [18,19,20].
Angiogenesis, mediated by the vascular endothelial growth factor receptor (VEGFR) and its ligands (VEGF), is critical for tumor growth and metastasis [21]. Bevacizumab is a recombinant anti-VEGF monoclonal antibody and is approved alone or in combination with chemotherapy for treatment of CRC, NSCLC, glioblastoma, cervical, ovarian, and renal cell cancers [22,23,24,25,26].
Furthermore, clinical and pre-clinical studies show that the combination of anti-VEGF and anti-EGFR therapy yields improved response rate and survival [27, 28]. The synergistic activity of the combination might be explained by the fact that acquired resistance to EGFR inhibitors is partially due to activation of the VEGF signaling pathway [29, 30]. Herein, we report the feasibility and safety results of a single-center triplet combination of anti-VEGF (bevacizumab) and dual EGFR inhibition (erlotinib, cetuximab) in patients with advanced or metastatic solid tumors.
Methods
This is an investigator-initiated, single-center phase I clinical trial that employed a 3 + 3 dose-escalation design. The primary endpoints were to determine the maximum tolerated dose (MTD) and dose-limiting toxicities (DLT) of bevacizumab in combination with erlotinib and cetuximab. We also evaluated the anti-tumor efficacy of this treatment per response evaluation criteria in solid tumors (RECIST 1.0) [31].
The study was conducted at The University of Texas M. D. Anderson Cancer Center (MDACC) per Institutional Review Board guidelines. The results of the phase I study for tumor-specific cohorts were previously reported for CRC and NSCLC [32, 33]. The study accrual period was from October 2007 to August 2013. The patients reported herein included all patients with heavily pre-treated advanced solid tumors as part of a dose-escalation study conducted in patients with advanced cancer. The dose-escalation portion of the study determined the recommended phase II dose (RP2D) to be bevacizumab 10 mg/kg IV every 2 weeks; cetuximab loading 400 mg/m2, maintenance 250 mg/m2 IV weekly; and erlotinib 150 mg PO daily. The cycle was 28 days. Patients were treated at variable dose levels, depending on the time of study entry (Table 1).
Patients had metastatic or advanced solid tumor not amendable to standard therapy, an Eastern Cooperative Oncology Group (ECOG) performance status 0–2, and adequate hematologic, hepatic, and renal function. Exclusion criteria included hemoptysis, unexplained bleeding, significant cardiovascular disease, intercurrent uncontrolled illness, significant gastrointestinal bleeding within 28 days, hemorrhagic brain metastases, prior abdominal surgery within 30 days, pregnancy, and a history of hypersensitivity to bevacizumab, cetuximab, and/or erlotinib. Treatment with prior cytotoxic therapies must have ended at least 3 weeks before enrollment, and biologic treatment must have completed at least 2 weeks or five drug half-lives before enrollment (whichever is shorter).
Statistical analysis
No formal hypotheses were tested, and analyses were descriptive and exploratory. Non-parametric correlations were determined with Spearman’s rank correlation coefficient.
Results
A total of 36 patients with advanced or metastatic solid tumors received treatment on a range of dose-levels. The most frequent tumor types enrolled were; cervical (n = 10), HNSCC (n = 10), and follicular thyroid (n = 4) cancers. The MTD and the RP2D was determined to be the FDA-approved doses for all three drugs (erlotinib 150 mg orally daily, cetuximab 400 mg/m2 loading dose, then 250 mg/m2 intravenous (IV) weekly and bevacizumab 10 mg/kg IV every 2 weeks). This combination was safe and well tolerated.
Out of the 19 patients treated at the RP2D, 7 patients (37%) required a dose reduction because of grade 2–3 skin rash (n = 6) and grade 3 elevated liver enzymes (n = 1). The most frequent treatment-related grade ≥ 2 adverse events likely related to the EGFR inhibition by cetuximab and erlotinib were: rash (56%), hypomagnesemia (17%), pruritus (11%), diarrhea (8%) and likely related to antiangiogenic effect of bevacizumab were: hypertension, bleeding, and fistula (Table 2).
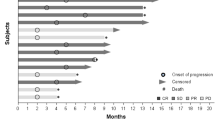
Of the 24 evaluable patients, 14 patients (58%) presented a disease control (defined as stable disease or partial response per RECIST 1.0 of more than 16 weeks), including patients who previously received bevacizumab, erlotinib and/or cetuximab (Fig. 1).
Although only one patient achieved a partial response, 14 patients had a clinical benefit and some durable disease control from the treatment. This might be related to the different pattern of the response of targeted therapies and antiangiogenics, and the radiologic criteria used (RECIST 1.0) has many limits in assessing the response to these treatments [34].
Exploratory analysis of mutations in EGFR, BRAF, KRAS, NRAS, MET, PIK3CA, and TP53 genes was done in a Clinical Laboratory Improvements Amendments (CLIA)-approved laboratory at MD Anderson Cancer Center on archived tissue.
Only two patients were identified to have EGFR mutations. One patient with epithelioid sarcoma had a pathogenic activating mutation in exon 18 (EGFR p.G719D) and achieved a stable disease per RECIST 1.0 for more than 6 months, with 18% decreased of the target lesions as compared with baseline. Another patient with salivary gland carcinoma with an EGFR p.D770N mutation (exon 20) showed no response to treatment and presented new metastases at the first restaging.
Discussion
Dual EGFR blockade with EGFR monoclonal antibody and oral EGFR tyrosine kinase inhibitor was shown be additive or synergistic with predictable safety profile [35,36,37]. Cetuximab and erlotinib contributed to significant decrease in cellular proliferation without achieving substantial cell death, and enhanced shifting of cancer stem cells from mesenchymal states to epithelial phenotype, thereby reducing local invasion and metastasis in HNSCC cell lines [37]. Wheler and colleagues demonstrated that cetuximab and erlotinib combination was well tolerated and five out of 20 patients (25%) had achieved partial response and stable disease ≥ 6 months in patients with NSCLC [35].
VEGF and EGFR signaling pathways are intercorrelated; via up-regulating VEGF by EGFR expression and VEGF up-regulation independently contributing to EGFR resistance [29, 38,39,40,41]. Preclinical evidence suggested that inhibiting both pathways suppress AKT and ERK signaling and have notably shrunken the tumor growth in CRC cells lines [39]. In preclinical models and early phase trials, combination of VEGF and EGFR inhibition has shown activity in advanced solid tumors, including CRC, NSCLC, breast cancer, renal cell carcinoma and HNSCC [28, 42,43,44].
Recently, the results of a randomized, double-blind phase III study of erlotinib with ramucirumab (anti-VEGF therapy) or placebo in previously untreated EGFR-mutant metastatic non-small-cell lung cancer (RELAY) were reported, and the doublet therapy showed clinical benefit and results were positive [45]. Improving upon a doublet may warrant a triplet, and our trial shows safety, and feasibility of a triplet combination.
Falchook et al., had previously demonstrated the result of phase 1, dose-escalation study combining dual EGFR inhibition with anti-VEGF treatment in heavily pretreated patients with CRC [33]. Thirty-four percent had achieved either stable disease or partial response and most patients tolerated the regimen without dose-limiting toxicities. Hence, we are reporting the regimen in non-CRC and non-NSCLC cohorts (Fig. 1).
EGFR exon 20 insertions confer intrinsic resistance or lack of response to first-generation EGFR inhibitors such as erlotinib, compared to patients harboring other EGFR mutations [46, 47]. Also in preclinical models, exon 20 deletions have also been shown to confer resistance to cetuximab, while retaining sensitivity to other drugs such as poziotinib [48] and pan-ERBB inhibitors, such as neratinib and dacomitinib [49]. Robichaux and colleagues showed that first 11 patients with NSCLC carrying EGFR exon 20 mutations had achieved an objective response rate of 64% in a phase II trial [50]. Osimertinib and other third-generation EGFR inhibitors are still under investigation in patients with NSCLC harboring these mutations.
Although PIK3CA mutations in exon 20 (H1047R) have been identified as potential predictive biomarkers for non-response to cetuximab in KRAS-wild-type tumors, PIK3CA mutations in exon 9 have not been associated with resistance to EGFR inhibitors [51]. Interestingly all three patients who were found to have mutations in exon 9 (E542K and E545K) had a stable disease for more than 16 weeks.
There are several limitations of this study, including a small number of patients who had molecular testing, precluding from robust analysis. Since this was employed in advanced solid malignancies, EGFR mutation was not a criterion to enroll in the trial. However, our results show that combination of dual EGFR inhibition by erlotinib and cetuximab with bevacizumab is well-tolerated with the most common adverse event being manageable rash, in heavily pretreated patients with multiple solid tumors with a median of 3 prior systemic treatments. In addition, this trial was carried out in an era when comprehensive genomic panel was not routine in all patients. Moreover, results show the necessity of developing predictive biomarkers of treatment and integrating correlative studies in the clinical trials. Furthermore, not only the gene mutated is important, but also the annotation of each mutation within a gene. Functional annotation has become crucial in genomic medicine, and several algorithms have been developed.
With the advances of tumor DNA sequencing, there is a growing interest in personalized cancer therapy with genomically matched treatments and it would be suitable to explore the combination of a third-generation tyrosine kinase inhibitor targeting EGFR with cetuximab and bevacizumab in preselected patients with EGFR activating mutations and excluding patients with concomitant alterations that might confer resistance to the combination, such as KRAS mutations.
Conclusions
Dual EGFR inhibition (erlotinib and cetuximab) combined with bevacizumab is a safe and well tolerated combination, demonstrating antitumor activity in patients with solid tumors, beyond CRC and NSCLC. Future studies are warranted with second or third-generation EGFR tyrosine kinase triplet combinations in the EGFR pathway aberrant patients. There is a critical need to develop and validate predictive biomarkers for genomically matched therapies and personalize cancer treatment.
Lessons learned
-
1.
Dual EGFR inhibition (erlotinib and cetuximab) combined with bevacizumab is a safe and well tolerated combination, demonstrating antitumor activity in patients with solid tumors beyond colorectal and non-small cell lung cancers
-
2.
There is a critical need to develop and validate predictive biomarkers for genomically matched therapies and personalize cancer treatment
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- EGFR:
-
Epidermal growth factor
- RECIST:
-
Response evaluation criteria in solid tumors
- FDA:
-
US Federal Drug Administration
- NCI-MATCH:
-
National Cancer Institute-Molecular Analysis for Therapy Choice
- CRC:
-
Colorectal cancers
- NSCLC:
-
Non-small cell lung cancer
- VEGF:
-
Vascular endothelial growth factor
- MTD:
-
Maximum tolerated dose
- DLT:
-
Dose-limiting toxicities
- CLIA:
-
Clinical Laboratory Improvements Amendments
- RP2D:
-
Recommended phase II dose
- ECOG:
-
Eastern cooperative oncology group
References
Subbiah V, Kurzrock R. Challenging standard-of-care paradigms in the precision oncology era. Trends Cancer. 2018;4(2):101–9.
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–9.
Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–36.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25.
Shi H, Moriceau G, Kong X, Lee MK, Lee H, Koya RC, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012;3:724.
Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19(10):1315–27.
Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381(7):626–36.
Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632–43.
Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019;25(5):744–50.
Rodon J, Soria JC, Berger R, Miller WH, Rubin E, Kugel A, et al. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat Med. 2019;25(5):751–8.
Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366(1):2–16.
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–17.
Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357(20):2040–8.
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–78.
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–27.
Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–32.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46.
Weickhardt AJ, Price TJ, Chong G, Gebski V, Pavlakis N, Johns TG, et al. Dual targeting of the epidermal growth factor receptor using the combination of cetuximab and erlotinib: preclinical evaluation and results of the phase II DUX study in chemotherapy-refractory, advanced colorectal cancer. J Clin Oncol. 2012;30(13):1505–12.
Perez-Torres M, Guix M, Gonzalez A, Arteaga CL. Epidermal growth factor receptor (EGFR) antibody down-regulates mutant receptors and inhibits tumors expressing EGFR mutations. J Biol Chem. 2006;281(52):40183–92.
Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119(10):3000–10.
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–8.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J med. 2014;370(8):699–708.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50.
Tewari KS, Sill MW, Long HJ 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–43.
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83.
Shaheen RM, Ahmad SA, Liu W, Reinmuth N, Jung YD, Tseng WW, et al. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br J Cancer. 2001;85(4):584–9.
Saltz LB, Lenz HJ, Kindler HL, Hochster HS, Wadler S, Hoff PM, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol. 2007;25(29):4557–61.
Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140(2):268–79.
Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008;5(9):521–30.
Kim JH. Comparison of the RECIST 1.0 and RECIST 1.1 in patients treated with targeted agents: a pooled analysis and review. Oncotarget. 2016;7(12):13680–7.
Falchook GS, Naing A, Hong DS, Zinner R, Fu S, Piha-Paul SA, et al. Dual EGFR inhibition in combination with anti-VEGF treatment: a phase I clinical trial in non-small cell lung cancer. Oncotarget. 2013;4(1):118–27.
Falchook GS, Naing A, Wheler JJ, Tsimberidou AM, Zinner R, Hong DS, et al. Dual EGFR inhibition in combination with anti-VEGF treatment in colorectal cancer. Oncoscience. 2014;1(8):540–9.
Nishino M, Jagannathan JP, Krajewski KM, O’Regan K, Hatabu H, Shapiro G, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198(4):737–45.
Wheler JJ, Tsimberidou AM, Falchook GS, Zinner RG, Hong DS, Fok JY, et al. Combining erlotinib and cetuximab is associated with activity in patients with non-small cell lung cancer (including squamous cell carcinomas) and wild-type EGFR or resistant mutations. Mol Cancer Ther. 2013;12(10):2167–75.
Horn L, Gettinger S, Camidge DR, Smit EF, Janjigian YY, Miller VA, et al. Continued use of afatinib with the addition of cetuximab after progression on afatinib in patients with EGFR mutation-positive non-small-cell lung cancer and acquired resistance to gefitinib or erlotinib. Lung Cancer. 2017;113:51–8.
Setubal Destro Rodrigues MF, Gammon L, Rahman MM, Biddle A, Nunes FD, Mackenzie IC. Effects of Cetuximab and Erlotinib on the behaviour of cancer stem cells in head and neck squamous cell carcinoma. Oncotarget. 2018;9(17):13488–500.
Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res. 2007;5(3):203–20.
Ding C, Li L, Yang T, Fan X, Wu G. Combined application of anti-VEGF and anti-EGFR attenuates the growth and angiogenesis of colorectal cancer mainly through suppressing AKT and ERK signaling in mice model. BMC Cancer. 2016;16(1):791.
Larsen AK, Ouaret D, El Ouadrani K, Petitprez A. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther. 2011;131(1):80–90.
Poindessous V, Ouaret D, El Ouadrani K, Battistella A, Megalophonos VF, Kamsu-Kom N, et al. EGFR- and VEGF(R)-targeted small molecules show synergistic activity in colorectal cancer models refractory to combinations of monoclonal antibodies. Clin Cancer Res. 2011;17(20):6522–30.
Hainsworth JD, Sosman JA, Spigel DR, Edwards DL, Baughman C, Greco A. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J clinical Oncol. 2005;23(31):7889–96.
Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23(11):2544–55.
Dickler MN, Rugo HS, Eberle CA, Brogi E, Caravelli JF, Panageas KS, et al. A phase II trial of erlotinib in combination with bevacizumab in patients with metastatic breast cancer. Clin Cancer research : an official journal of the American Association for Cancer Res. 2008;14(23):7878–83.
Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–69.
Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17(11):3812–21.
Yasuda H, Park E, Yun CH, Sng NJ, Lucena-Araujo AR, Yeo WL, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med. 2013;5(216):216ra177.
Elamin Y, Robichaux J, Lam V, Tsao A, Lu C, Blumenschein G, et al. OA 12.01 The preclinical and clinical activity of poziotinib, a potent, selective inhibitor of EGFR Exon 20 mutant NSCLC. J Thorac Oncol. 2017;12(11):S1776.
Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67(24):11924–32.
Robichaux JP, Elamin YY, Tan Z, Carter BW, Zhang S, Liu S, et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018;24(5):638–46.
Eng J, Woo KM, Sima CS, Plodkowski A, Hellmann MD, Chaft JE, et al. Impact of concurrent PIK3CA mutations on response to EGFR tyrosine kinase inhibition in EGFR-mutant lung cancers and on prognosis in oncogene-driven lung adenocarcinomas. J Thorac Oncol. 2015;10(12):1713–9.
Acknowledgements
We thank the patients, and their families who participated in this study.
Funding
National Cancer Institute (NCI), and MD Anderson Cancer Center.
Author information
Authors and Affiliations
Contributions
All the authors have significantly contributed to the preparation of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Applicable (obtained).
Consent for publication
Applicable (obtained).
Competing interests
A full list of the authors’ competing interests can be found in Additional file 1.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Complete Statement of Competing Interest
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Subbiah, V., Dumbrava, E.I., Jiang, Y. et al. Dual EGFR blockade with cetuximab and erlotinib combined with anti-VEGF antibody bevacizumab in advanced solid tumors: a phase 1 dose escalation triplet combination trial. Exp Hematol Oncol 9, 7 (2020). https://doi.org/10.1186/s40164-020-00159-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40164-020-00159-1