Abstract
Background
Angiogenesis is generally involved during the cancer development and hematogenous metastasis. Vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) are considered to have an important role in tumor-associated angiogenesis. However, the effects of simultaneously targeting on VEGF and EGFR on the growth and angiogenesis of colorectal cancer (CRC), and its underlying mechanisms remain unknown.
Methods
Immunohistochemical staining was used to detect the VEGF and EGFR expression in different CRC tissue specimens, and the correlation between VEGF/EGFR expression with the clinicopathologic features was analyzed. Cell counting kit‑8 (CCK-8) and transwell assays were used to assess the cellular proliferation and invasion of CRC cells after treated with anti-VEGF antibody and/or anti-EGFR antibody in vitro, respectively. Moreover, in vivo tumor formation was performed on nude mice model, and the tumor microvessel density (MVD) was determined by anti-CD34 staining in different groups. Finally, we evaluated the impact of anti-VEGF antibody and/or anti-EGFR antibody on the activation of downstream signaling effectors using western blot.
Results
VEGF and EGFR were upregulated in CRC tissues, and their expression levels were correlated with hepatic metastasis. Blockage on VEGF or EGFR alone could inhibit the cellular proliferation and metastasis while their combination could reach a good synergism in vitro. In addition, in vivo xenograft mice model demonstrated that the tumor formation and angiogenesis were strongly suppressed by combination treatment of anti-VEGF and anti-EGFR antibodies. Besides, the combination treatment significantly reduced the activation of AKT and ERK1/2, but barely affected the activation of c-Myc, NF-κB/p65 and IκBα in CRC cells tumors. Interestingly, anti-VEGF antibody or anti-EGFR antibody alone could attenuate the phosphorylation of STAT3 as compared with negative control group, whereas the combined application not further suppressed but at least partially restored the activation of STAT3 in vivo.
Conclusions
Simultaneous targeting on VEGF and EGFR does show significant inhibition on CRC tumor growth and angiogenesis in mice model, and these effects are mainly attributed to suppression of the AKT and ERK signaling pathways.
Similar content being viewed by others
Background
Colorectal cancer (CRC) is one of the most common malignant tumors in the Western World, China, and other countries [1–3]. The prognosis of CRC at an early stage is favorable, as a result of improved detection of early cancer and wider implementation of radical surgery, but the prognosis of unresectable, advanced CRC is not yet satisfactory. When tumor lesions are not fully resectable or become metastatic, patients will have very limited options for target agents and conventional chemotherapy. As a result, the overall outcome of patients is barely satisfied mainly due to distant metastases formation, especially hepatic and other hematogenous metastases [4–7], since angiogenesis and hematogenous metastasis are intrinsically connected. Thus, countermeasures against tumor angiogenesis seem to be a promising strategy for improving the prognoses of these cancer patients.
Angiogenesis, the process leading to the formation of new blood vessels, plays an important role in tumor development and distant metastasis [8], and its induction is mediated by numerous angiogenic factors [9]. Among these factors, vascular endothelial growth factor (VEGF) and its receptors are the most potent molecules activating endothelial cells metastasis and increasing vascular permeability [10–12]. Inhibition of VEGF activity has been reported to suppress the proliferation of cancer cells and improve the prognosis for unresectable CRC patients [13].
In addition, epidermal growth factor receptor (EGFR), which plays an important role in tumorigenesis, is overexpressed in many types of cancers, especially in CRC [14, 15]. According to the European and US guidelines, EGFR targeting-therapy has been recommended for the treatment of metastatic colorectal cancer (mCRC) [12]. However, not all patients have a good response to anti-EGFR treatment, and there is important clinical value for identifying predictors of treatment benefit or lack thereof [16]. Resistance to anti-EGFR therapies is mediated, at least partly, through activating VEGF-mediated intracellular cascade [17, 18]. Therefore, a strategy that simultaneously targets on VEGF and EGFR agents appears to be promising in preclinical and clinical studies for the treatment of CRC.
However, very few studies have been conducted to determine the therapeutic effects of targeting both VEGF and EGFR for anti-CRC treatment. In this study, we mainly evaluated the effects of targeting both VEGF and EGFR on CRC growth and angiogenesis as well as its relative molecular mechanism using in vitro CRC cell lines and in vivo mouse model systems.
Methods
Patients and specimens
In this study, a total of 60 CRC tumor tissues and 30 corresponding normal tissues were prepared for immunohistochemistry assay. Normal tissues were cut at least 5 cm away from tumor margin. All the specimens were collected from patients with CRC who were treated at the Affiliated Hospital of Zunyi Medical University between May 2015 and December 2015. The study was conducted in accordance with the 1975 declaration of Helsinki and with approval from the Ethics Committee of the Affiliated Hospital of Zunyi Medical University. Written informed consent was obtained from all participants. None of the cases received neoadjuvant therapy before surgery. After surgical resection, the resected specimens were re-evaluated before the current study by two pathologists.
Cell lines and culture conditions
The human CRC cell lines HT29, SW480, SW620 and LoVo were obtained from Cell bank of Chinese Academy of Sciences (Shanghai, China). All the cancer cells were cultured in McCoy 5A, RPMI-1640 or Leibovitz’s L-15 medium supplemented with 10 % fetal bovine serum (FBS) (HyClone, Logan, UT, USA), 100 IU/mL penicillin and 100 μg/ml streptomycin. All the cells were cultured in a humidified atmosphere of 5 % CO2 at 37 °C.
Quantitative Real‑time PCR
Total RNA was extracted from cells with Trizol (Invitrogen, USA) and reverse transcribed using RT reagent Kit (TakaRa, Japan) according to the manufacturer’s instructions. Quantitative reverse transcription-PCR (qRT-PCR) analysis was performed as previous described [19]. The sequences of primers in this section are the followings: (1) VEGF: 5′-CTTGCCTTGCTGCTCTACCT-3′ (forward) and 5′-CTGCATGGTGATGTTGGACT-3′ (reverse); (2) EGFR: 5′-GAGAGGAGA ACTGCCAGAA-3′ (forward) and 5′-GTAGCATTTATGGAGAGTG-3′ (reverse); (3) GAPDH: 5′-GAAGGTGAAGGTCGGAGTC-3′ (forward) and 5′-GAAGATGGTGATGGGATTTC-3′ (reverse). GAPDH was used as an internal control.
Western blot analysis
Western blot analysis was performed as previous described [19]. The following commercial antibodies were used in this study: VEGF, EGFR, phospho-c-Myc, total c-Myc, phospho-NF-κB/p65, total NF-κB/p65, phospho-IκBα and total IκBα (Abcam, UK), phospho-AKT, total AKT, phospho-STAT3, total STAT3, phospho-ERK1/2 and total ERK1/2 (Invitrogen, USA), GAPDH and β-actin (Immunology Consultants Laboratory, USA).
Cell counting kit‑8 assay
The Cell Counting Kit-8 (CCK-8) assay kit (Dojindo, Kumamoto, Japan) was used to determine the impact of anti-human VEGF mAb and/or anti-human EGFR mAb on cell proliferation. The concentrations of anti-VEGF or anti-EGFR used in these assays are as following: 0, 0.25, 0.5, 1 and 2 μg/ml. Cells were plated in 96-well plates at a density of 1 × 104 cells per well for 48 h. 10 μl CCK-8 solution was added to the cells for 2.5 h at 37 °C, and the viability of the cells was measured at 450 nm using an ELISA reader (BioTek, Winooski, VT, USA) according to the manufacturer’s instructions. For each experimental condition, 3 wells were used, and the experiments were repeated 3 times.
Invasion assay
Invasion assays were performed as reported [20]. Transwell invasion assays were performed in Corning Matrigel invasion chamber containing an 8 μm pore-size polycarbonate membrane with a uniform layer of BD Matrigel basement membrane matrix (BD Biosciences, USA). Three independent experiments were performed with triplicate wells.
in vivo tumor xenograft model
Female BALB/C nude mice (5–6 weeks old) were used for xenograft studies. 2 × 106 of control and experimental cells suspended in phosphate-buffered saline (PBS) were injected subcutaneously into the right armpit of mice (six mice each group). Four groups of mice were tested. Group A was injected with CRC cells (SW620/LoVo) and non-specific mouse IgG. Group B was injected with CRC cells and the anti-mouse VEGF mAb (10 μg), which could react with human and mouse source VEGF protein. Group C was injected with CRC cells and the anti-mouse EGFR mAb (10 μg), which could react with human and mouse source EGFR protein. Group D was injected with CRC cells and the anti-mouse VEGF mAb (10 μg) and anti-mouse EGFR mAb (10 μg). Tumor volume was determined by external measurement according to the formula (d2 × D)/2 [21]. Mice were sacrificed after 35 days, and tumors were harvested, weighted and examined histologically.
Immunohistochemical studies
Immunohistochemical assay for paraffin-embedded tissues were performed as reported [19, 20]. The evaluation principle was quantified based on the immunoreactive score (IRS), which was calculated as a product of staining intensity (SI) and percentage of positive cells (PP). SI is determined as follows: no staining (score 0), light yellow (score 1), buffy (score 2) and brown (score 3). PP is determined as follows: less than 5 % (score 0), 6 %–25 % (score 1), 26 %–50 % (score 2), 51 %–75 % (score 3) and >76 % (score 4). By multiplying SI and PP, the final weighed expression score was ranged from 0 to 12. Five random fields in each section were selected for the evaluation. The sections scoring at least 3 points in our study were indicating positive protein expression.
Quantification of microvessel density
Tumor MVD was determined as described [22]. The slides were examined under × 100 magnication to identify the highest vascular density area within the tumor, and one field magnified 200-fold in each of five vascularized areas was counted. The average of the five areas was recorded as the MVD level of this case. Any brown-staining endothelial cell or endothelial cell cluster that was clearly separate from adjacent microvessels, tumor cells, and other connective tissue elements was considered as a single, countable microvessel.
IL6 ELISA
Supernatants collected from CRC cell xenografts were assayed by the IL6 ELISA Kit (Invitrogen) according to the manufacturer’s instructions. Experiments were performed in duplicates.
Statistical analysis
All values were represented as the mean ± SEM from at least three independent experiments. Clinical correlative studies were performed by Pearson’s χ2-test using SPSS19.0 software system. Student’s t-test for two groups or one-way analysis of variance (ANOVA) for three or more groups were performed to evaluate the statistical significance by using GraphPad Prism 5 software. P values less than 0.05 were considered statistically significant.
Results
Clinical significance of VEGF/EGFR expression in CRC tissues
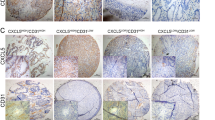
It has been widely recognized that VEGF and EGFR are overexpressed in CRC tissues. In this study, we also detected the expression of VEGF and EGFR in different colorectal tissues. The VEGF and EGFR expression levels were evidently higher in liver-metastatic CRC samples than that in non-metastatic CRC samples or noncancerous samples (Fig. 1a and b). The VEGF and EGFR expression levels in non-metastatic CRC tissues were also higher than that in normal tissues (Fig. 1a and b). In addition, the results of immunohistochemical staining showed that positive signals of VEGF and EGFR were mainly occurred in the cell membrane and cytoplasm (Fig. 1c).
VEGF and EGFR expression are significantly upregulated in liver-metastatic CRC tissues. a Results of VEGF staining were evaluated by the staining scores. b Results of EGFR staining were evaluated by the staining scores. c Immunohistochemistry analysis of VEGF and EGFR expression in different colorectal tissues. *P < 0.05
To further identify the clinical importance of VEGF/EGFR in CRC, we analyzed the correlationship between the VEGF/EGFR protein level with clinicopathological characteristics, including age, gender, tumor size, histology, tumor location, differentiation status, hepatic metastasis and TNM stage. Strikingly, VEGF expression was evidently correlated with tumor size, hepatic metastasis and TNM stage (Table 1). However, no relationship was found between the VEGF expression and other clinicopathological characteristics including age, gender, histology, tumor location and differentiation status (Table 1). In addition, EGFR expression was evidently correlated with tumor size, differentiation status, hepatic metastasis and TNM stage (Table 1). However, no relationship was found between the EGFR expression and other clinicopathological characteristics including age, gender, histology, and tumor location (Table 1). Taken together, these data strongly indicated that VEGF and EGFR were positively correlated with the metastasis of CRC.
VEGF/EGFR expression in CRC cell lines
Furthermore, we detected the VEGF/EGFR expression in CRC cell lines and found that VEGF/EGFR expression in the highly invasive CRC cell lines (SW620 and LoVo) were evidently up-regulated than those in the minimally metastatic CRC cell lines (SW480 and HT29) (Fig. 2).
Effects of combination anti-VEGF mAb and anti-EGFR mAb on CRC cells growth and invasion in vitro
In order to confirm the role of anti-VEGF mAb (monoclonal antibody) or anti-EGFR mAb on CRC cells growth in vitro, SW620 and LoVo cells were treated with different concentrations of anti-VEGF mAb or anti-EGFR mAb. Cell counting kit‑8 (CCK-8) assay kit was used to detect proliferation activity of these cells. The results showed that anti-VEGF mAb or anti-EGFR mAb could independently prohibit the cell proliferation in a concentration dependent manner (Additional file 1: Figure S1). Considered facilitately observed the experimental results, we chose moderate anti-VEGF mAb or anti-EGFR mAb concentration (0.5 μg/ml) to explore the proliferation and invasion of CRC cells in vitro. As shown in Fig. 3a, the proliferation of SW620/LoVo cells was obviously inhibited in the presence of both anti-VEGF mAb and anti-EGFR mAb, compared with the presence of anti-VEGF mAb or anti-EGFR mAb alone. Transwell assay identified that the invasion of SW620/LoVo cells was suppressed in the presence of anti-VEGF mAb or anti-EGFR mAb alone, compared with negative control group (Fig. 3b and c). When both anti-VEGF mAb and anti-EGFR mAb were present, the mobility of these cells was further reduced (Fig. 3b and c). These results revealed that both anti-VEGF mAb and anti-EGFR mAb could suppress growth and metastasis of CRC cells in culture.
Combined application of anti-VEGF and anti-EGFR antibodies suppresses the proliferation and invasion of CRC cells in vitro. a The proliferation rate of SW620 and LoVo cells were analyzed by CCK-8 assay in different groups. b Invasion assay of SW620 and LoVo cells in different groups. c Invasion of SW620 and LoVo cells were quantitatively analyzed in different groups. Columns are the average of three independent experiments ± SEM. *P < 0.05; **P < 0.01
Effect of anti-VEGF mAb and anti-EGFR mAb on CRC cells tumorigenicity in vivo
Cultured SW620 cells were subcutaneously injected in mice, and tumor formation was observed 35 days after injection (Fig. 4a). In addition, tumor weight was measured in these groups. As a result (Fig. 4b), the average tumor weight of SW620 cells in the presence of both anti-VEGF mAb and anti-EGFR mAb was 0.198 ± 0.022 g, which was significantly lower (P < 0.05) than that of mice inoculated with anti-VEGF mAb (0.412 ± 0.036 g), anti-EGFR mAb (0.440 ± 0.038 g), and negative control group (0.952 ± 0.056 g). When LoVo cells were injected with non-specific mouse IgG, the average tumor weight was 1.134 ± 0.083 g. It was 0.462 ± 0.062 g in the presence of anti-VEGF mAb, 0.506 ± 0.059 g in the presence of anti-EGFR mAb, and 0.244 ± 0.025 g in the presence of both anti-VEGF and anti-EGFR antibodies (Fig. 4b). These results suggested that both anti-VEGF mAb and anti-EGFR mAb could inhibit CRC cells tumorigenicity in vivo.
Suppression of CRC cells tumorigenicity by anti-VEGF and anti-EGFR antibodies in vivo. a Representative photographs of tumor formation in mice in response to SW620 cells. b Five weeks later, the tumors were resected and weighted. The weight analyzed with Student’s t-test. Data represent means ± SEM. *P < 0.05
Suppression of tumor angiogenesis by CRC cells after application of anti-VEGF mAb and anti-EGFR mAb
Accordingly, the amount of microvessel density (MVD) determined using anti-CD34 mAb immunostaining in the same mouse tumors (Fig. 5a and b). The number of positive cells in SW620 tumors with non-specific mouse IgG was 49.00 ± 3.22 per field-of-view, 27.00 ± 3.46, 30.33 ± 3.18 per field-of-view in the presence of anti-VEGF mAb or anti-EGFR mAb, and 12.67 ± 2.96 in the presence of both antibodies. In the LoVo tumors, there were 53.00 ± 3.46 positive cells per field-of-view in negative control group. 25.00 ± 2.89, 30.33 ± 3.93 cells were observed in the presence of anti-VEGF mAb or anti-EGFR mAb, and 14.00 ± 3.79 cells were observed in the presence of both antibodies (Fig. 5b). These results indicated that both anti-VEGF and anti-EGFR antibodies could reduce tumor angiogenesis.
Suppression of CRC cells tumor angiogenesis by anti-VEGF and anti-EGFR antibodies. a Representative photographs of anti-CD34 staining in SW620 cells tumors. b The numbers of positively CD34 stained cells in subcutaneous SW620 and LoVo cells tumors. The data are representative of at least three different experiments ± SEM. *P < 0.05
The activity of VEGF and EGFR-dependent signaling in CRC cells tumors after application of anti-VEGF and anti-EGFR antibodies
As both VEGF and EGFR can activate phosphatidylinositol 3-kinase (PI3K), mitogen activated protein kinase (MAPK) and janus kinase (JAK) signaling pathways [23–26], we examined the downstream effectors, AKT, extracellular signal-regulated kinase (ERK) and signal transducer and activator of transcription 3 (STAT3), respectively. As expected, VEGF or EGFR inhibition by mAb downregulated the phosphorylation of AKT, ERK1/2 and STAT3 as compared with negative control group (Fig. 6a, b and Additional file 2: Figure S2A, B). In addition, the phosphorylation of AKT and ERK1/2 was further reduced in the presence of both anti-VEGF mAb and anti-EGFR mAb as compared with anti-VEGF mAb or anti-EGFR mAb alone (Fig. 6a, b and Additional file 2: Figure S2A, B). Unfortunately, when combined treatment with anti-EGFR and anti-VEGF antibodies, the phosphorylation of STAT3 was not further suppressed, but at least, partially restored (Fig. 6a, b and Additional file 2: Figure S2A, B). It has been widely recognized that STAT3 signaling pathway links inflammation to cell transformation, and STAT3 activation is dependent on IL6 levels [27]. To explore whether IL6 expression associates with the activation of STAT3 signaling, we detected the expression of IL6 in the same mouse tumors. The levels of IL6 were slightly decreased in the presence of anti-VEGF mAb or anti-EGFR mAb alone, compared with negative control group. However, when both anti-VEGF mAb and anti-EGFR mAb were present, IL6 levels were significantly up-regulated in CRC cell tumors (Additional file 3: Figure S3). These results suggested that anti-VEGF and anti-EGFR antibodies could attenuate PI3K and ERK signaling, but not IL6/STAT3 signaling in CRC cell tumors.
Combined application of anti-VEGF and anti-EGFR antibodies attenuates the activation of AKT and ERK, but not STAT3, c-Myc and NF-κB in vivo. a Western blot analysis was performed to determine the activation of AKT, ERK1/2 and STAT3 in different SW620 cells tumors. b Western blot assay of different LoVo cells tumors (one clone, a). c Western blot analysis was performed to determine the activation of c-Myc, NF-κB/p65 and IκBα in different SW620 cells tumors. d Western blot assay of different LoVo cells tumors (one clone, c)
Notably, other signaling effectors, such as c-Myc oncogene [28] and nuclear factor kappa B (NF-κB) [29] are frequently reported to involve in the development of many types of tumors. In addition, IκBα functions as an inhibitor of NF-κB, which interacts with p65 to form an inactive NF-κB/IκBα complex, and then inhibits the activation of NF-κB signaling pathway [30, 31]. In order to determine whether anti-VEGF and anti-EGFR antibodies could suppress the activation of these signaling effectors, we examined the expression of c-Myc, NF-κB/p65 and IκBα in CRC cell tumors by western blot. We found that the activation of phospho-c-Myc, phospho-NF-κB/p65 and phospho-IκBα was not marked increased or decreased in the presence of anti-VEGF antibody or anti-EGFR antibody compared with the control group (Fig. 6c, d and Additional file 2: Figure S2C, D). Moreover, when both anti-VEGF and anti-EGFR antibodies were present, there were also no significant differences in the activation of these factors (Fig. 6c, d and Additional file 2: Figure S2C, D). Taken together, these findings indicated that anti-VEGF and anti-EGFR antibodies couldn’t sufficient to inhibiting the activation of c-Myc and NF-κB.
Discussion
It has been demonstrated that overexpression of VEGF/EGFR is correlated with the progression and metastasis of CRC [32–34]. Despite that previous studies found the suppressing role of anti-VEGF/EGFR antibody on CRC development [35, 36], the potential effect of combination anti-VEGF and anti-EGFR antibodies on CRC growth and angiogenesis remains little known. In this study, we have shown that both increased VEGF and EGFR were associated with hepatic metastases in CRC. Additionally, we found that anti-VEGF and EGFR antibodies could not only reduce CRC cells proliferation and invasion in vitro, but also inhibit the tumor growth and angiogenesis in vivo mainly through prohibiting the activation of AKT and ERK signaling pathways. However, monoclonal antibodies targeting VEGF and EGFR may be un-sufficient to controlling the activity of other signaling pathways such as IL6/STAT3 signaling, which may exemplify the underlying mechanism of anti-tumor resistance.
Animal studies have manifested that inhibition of VEGF suppresses both tumor angiogenesis and tumor growth in vivo [37, 38]. Preclinical and clinical studies also suggest that inhibition of VEGF pathway causes direct and rapid changes to the tumor vasculature, and improves the overall survival rate of mCRC patients [39, 40]. In addition, there is accumulating evidence suggesting that before the selection of anti-VEGF agents, anti-EGFR agents deliver their maximum efficacy in mCRC patients when given early in the treatment strategy [41]. Of note, many studies have indicated that EGFR has a potent effect on tumor-associated angiogenesis and combined treatment with anti-EGFR and anti-VEGF antibodies have at least additive antitumor activity [42, 43]. Importantly, clinical trials have also produced promising data: combining the anti-VEGF monoclonal antibody bevacizumab with the anti-EGFR antibody cetuximab or the EGFR tyrosine kinase inhibitor erlotinib increases benefit compared with either of these anti-EGFR agents alone or combined with chemotherapy [44]. In this study, we found that the proliferation and invasion of CRC cells were obviously inhibited in the presence of both anti-VEGF and anti-EGFR antibodies in culture. Furthermore, when anti-EGFR combined with anti-VEGF treatment, the tumor growth and angiogenesis were significantly suppressed compared with other groups. These findings provided new evidence supporting the collaboration of anti-VEGF and anti-EGFR antibodies in inhibiting tumor growth and angiogenesis.
The mitogen-activated extracellular signal-regulated kinase (MEK)/ERK and PI3K/AKT signaling pathways are often concurrently activated in CRC, which are associated with the progression, metastasis and drug resistance of CRC [45–47]. VEGF and EGFR are known to function as two upstream effectors of the PI3K and MAPK pathways [23, 24]. In this study, we demonstrated that anti-VEGF antibody cooperated with anti-EGFR antibody in suppressing the phosphorylation of AKT and ERK1/2 in nude mouse model. In addition, STAT3 is persistently activated in many human cancers during cancer development and progression [48]. Interestingly, we found that anti-VEGF antibody or anti-EGFR antibody alone could attenuate the activation of STAT3, but simultaneous targeting of both VEGF and EGFR partially restored the phosphorylation of STAT3 in vivo. Although VEGF and EGFR can activate JAK/STAT3 signaling pathway, a variety of extracellular stimuli especially IL6 expression, is necessary for the activation of STAT3 signaling [25–27]. Hence, we detected the expression of IL6 in CRC cell tumors, and found that the trend of IL6 levels were consistent with that of STAT3 activation in the same tumors. One plausible explanation is that when anti-VEGF mAb or anti-EGFR mAb alone inhibits tumor growth, it also slightly decreases the expression of IL6 because of destruction of some tumor cells, while anti-VEGF and anti-EGFR antibodies simultaneously suppress tumorigenesis, the surviving tumor cells increase IL6 levels to escape the killing effect. Of note, it has been widely recognized that inhibition of JAK/STAT3 signaling is participated in chemotherapeutic sensitivity of CRC patients [49, 50]. These data suggested that combined application of anti-VEGF and anti-EGFR antibodies could sufficient suppress the activation of AKT and ERK signaling, but not IL6/STAT3 signaling pathway, and may indicate the underlying mechanism of chemotherapeutic resistance.
Of further interest, we examined the impact of both anti-VEGF and anti-EGFR antibodies on the activation of c-Myc and NF-κB in CRC cell tumors. Unfortunately, we found no significant differences between the phosphorylation of c-Myc, NF-κB/p65, IκBα, and the application of anti-VEGF antibody and/or anti-EGFR antibody in vivo. Despite that previous studies suggested the enigmatic regulation mechanisms of c-Myc and NF-κB activation, the role of activated c-Myc and NF-κB in tumor drug resistance is very affirmative [51, 52]. Of note, persistent activation of STAT3 signaling promotes uncontrolled growth and survival through dysregulation of gene expression including c-Myc, and thereby contributes to oncogenesis [53]. In addition, constitutive and persistent NF-κB activation in cancer cells is partly dependent on STAT3 status [54]. These findings and our results implied a plausible hypothesis that the invalid effect of both anti-VEGF and anti-EGFR antibodies on the activity of c-Myc and NF-κB is partly attributed to the STAT3 status.
Progression-free and overall survival in patients with mCRC was improved greatly by the addition of anti-VEGF and/or anti-EGFR to standard chemotherapy, in either first- or second-line treatment. [12, 55]. However, several clinical results have suggested that VEGF and EGFR combinatory therapy do not improve the overall prognosis in CRC [56]. In the present work, we convincingly showed that simultaneous targeting of both VEGF and EGFR could further suppress the proliferation and invasion of CRC cells in vitro, and further inhibit CRC cell tumor growth and angiogenesis through downregulation of AKT and ERK signaling in vivo. Although our data is not in line with clinical results, the STAT3 status, at least, may partly explain the inefficiencies of VEGF/EGFR co-inhibition in clinical trial.
Conclusions
In conclusion, this study demonstrates that combined application of anti-VEGF and anti-EGFR antibodies could inhibit CRC growth and angiogenesis mainly by suppressing AKT and ERK signaling pathways in mice model. However, other molecular targets including STAT3, c-Myc and NF-κB may contribute to enhance the risk of drug-resistance to chemotherapy targeting VEGF and EGFR.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32.
Field K, Lipton L. Metastatic colorectal cancer-past, progress and future. World J Gastroenterol. 2007;13:3806–15.
Nordlinger B, Van Cutsem E, Gruenberger T, Glimelius B, Poston G, Rougier P, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985–92.
Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93:1115–22.
Smith MD, McCall JL. Systematic review of tumour number and outcome after radical treatment of colorectal liver metastases. Br J Surg. 2009;96:1101–13.
Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–24.
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31.
Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76.
Cheng YD, Yang H, Chen GQ, Zhang ZC. Molecularly targeted drugs for metastatic colorectal cancer. Drug Des Devel Ther. 2013;7:1315–22.
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Falchook GS, Kurzrock R. VEGF and dual-EGFR inhibition in colorectal cancer. Cell Cycle. 2015;14:1129–30.
Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4:361–70.
Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA. Systematic review: Anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med. 2011;154:37–49.
Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–79.
Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008;5:521–30.
Ding C, Luo J, Yu W, Gao S, Yang L, Chen C, et al. Gab2 is a novel prognostic factor for colorectal cancer patients. Int J Clin Exp Pathol. 2015;8:2779–86.
Ding C, Luo J, Li L, Li S, Yang L, Pan H, et al. Gab2 facilitates epithelial-to-mesenchymal transition via the MEK/ERK/MMP signaling in colorectal cancer. J Exp Clin Cancer Res. 2016;35:5.
Naito S, von Eschenbach AC, Giavazzi R, Fidler IJ. Growth and metastasis of tumor cells isolated from a human renal cell carcinoma implanted into different organs of nude mice. Cancer Res. 1986;46:4109–15.
Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8.
Giordano G, Febbraro A, Tomaselli E, Sarnicola ML, Parcesepe P, Parente D, et al. Cancer-related CD15/FUT4 overexpression decreases benefit to agents targeting EGFR or VEGF acting as a novel RAF-MEK-ERK kinase downstream regulator in metastatic colorectal cancer. J Exp Clin Cancer Res. 2015;34:108.
Thomaidis T, Maderer A, Formentini A, Bauer S, Trautmann M, Schwarz M, et al. Proteins of the VEGFR and EGFR pathway as predictive markers for adjuvant treatment in patients with stage II/III colorectal cancer: results of the FOGT-4 trial. J Exp Clin Cancer Res. 2014;33:83.
Wang T, Liu J, Xiao XQ. Cantharidin inhibits angiogenesis by suppressing VEGF-induced JAK1/STAT3, ERK and AKT signaling pathways. Arch Pharm Res. 2015;38:282–9.
Ding CB, Yu WN, Feng JH, Luo JM. Structure and function of Gab2 and its role in cancer (Review). Mol Med Rep. 2015;12:4007–14.
Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706.
Huang H, Weng H, Zhou H, Qu L. Attacking c-Myc: targeted and combined therapies for cancer. Curr Pharm Des. 2014;20:6543–54.
Cortés Sempere M, Rodríguez Fanjul V, Sánchez Pérez I, Perona R. The role of the NFkappaB signaling pathway in cancer. Clin Transl Oncol. 2008;10:143–7.
Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–96.
Zhang NN, Sun QS, Chen Z, Liu F, Jiang YY. Homeostatic regulatory role of Pokemon in NF-κB signaling: stimulating both p65 and IκBα expression in human hepatocellular carcinoma cells. Mol Cell Biochem. 2013;372:57–64.
Zafirellis K, Agrogiannis G, Zachaki A, Gravani K, Karameris A, Kombouras C. Prognostic significance of VEGF expression evaluated by quantitative immunohistochemical analysis in colorectal cancer. J Surg Res. 2008;147:99–107.
Mokhtari M, Ardestani MM, Movahedipour M. An immunohistochemical study of EGFR expression in colorectal cancer and its correlation with lymph nodes status and tumor grade. J Res Med Sci. 2012;17:741–4.
Simone G, Mangia A, Malfettone A, Rubini V, Siciliano M, Di Benedetto A, et al. Chromogenic in situ hybridization to detect EGFR gene copy number in cell blocks from fine-needle aspirates of non small cell lung carcinomas and lung metastases from colo-rectal cancer. J Exp Clin Cancer Res. 2010;29:125.
Komatsu Y, Ishioka C, Shimada K, Yamada Y, Gamoh M, Sato A, et al. Study protocol of the TRICOLORE trial: a randomized phase III study of oxaliplatin-based chemotherapy versus combination chemotherapy with S-1, irinotecan, and bevacizumab as first-line therapy for metastatic colorectal cancer. BMC Cancer. 2015;15:626.
Liu X, George GC, Tsimberidou AM, Naing A, Wheler JJ, Kopetz S, et al. Retreatment with anti-EGFR based therapies in metastatic colorectal cancer: impact of intervening time interval and prior anti-EGFR response. BMC Cancer. 2015;15:713.
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–4.
Asano M, Yukita A, Suzuki H. Wide spectrum of antitumor activity of a neutralizing monoclonal antibody to human vascular endothelial growth factor. Jpn J Cancer Res. 1999;90:93–100.
Carrato A, Gallego-Plazas J, Guillen-Ponce C. Anti-VEGF therapy: a new approach to colorectal cancer therapy. Expert Rev Anticancer Ther. 2006;6:1385–96.
Papadimitriou K, Rolfo C, Dewaele E, Van De Wiel M, Van den Brande J, Altintas S, et al. Incorporating anti-VEGF pathway therapy as a continuum of care in metastatic colorectal cancer. Curr Treat Options Oncol. 2015;16:18.
Zaniboni A, Formica V. The Best. First. Anti-EGFR before anti-VEGF, in the first-line treatment of RAS wild-type metastatic colorectal cancer: from bench to bedside. Cancer Chemother Pharmacol. 2016.
Shaheen RM, Ahmad SA, Liu W, Reinmuth N, Jung YD, Tseng WW, et al. Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br J Cancer. 2001;85:584–9.
Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:1007–21.
Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res. 2007;5:203–20.
Ye Q, Cai W, Zheng Y, Evers BM, She QB. ERK and AKT signaling cooperate to translationally regulate survivin expression for metastatic progression of colorectal cancer. Oncogene. 2014;33:1828–39.
Troiani T, Napolitano S, Vitagliano D, Morgillo F, Capasso A, Sforza V, et al. Primary and acquired resistance of colorectal cancer cells to anti-EGFR antibodies converge on MEK/ERK pathway activation and can be overcome by combined MEK/EGFR inhibition. Clin Cancer Res. 2014;20:3775–86.
Papadatos-Pastos D, Rabbie R, Ross P, Sarker D. The role of the PI3K pathway in colorectal cancer. Crit Rev Oncol Hematol. 2015;94:18–30.
Wu J, Zhang J, Shen B, Yin K, Xu J, Gao W, et al. Long noncoding RNA lncTCF7, induced by IL-6/STAT3 transactivation, promotes hepatocellular carcinoma aggressiveness through epithelial-mesenchymal transition. J Exp Clin Cancer Res. 2015;34:116.
Qin A, Yu Q, Gao Y, Tan J, Huang H, Qiao Z, et al. Inhibition of STAT3/cyclinD1 pathway promotes chemotherapeutic sensitivity of colorectal caner. Biochem Biophys Res Commun. 2015;457:681–7.
Wang SW, Sun YM. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (Review). Int J Oncol. 2014;44:1032–40.
Tan J, Yu Q. Molecular mechanisms of tumor resistance to PI3K-mTOR-targeted therapy. Chin J Cancer. 2013;32:376–9.
Li J, Xiang S, Zhang Q, Wu J, Tang Q, Zhou J, et al. Combination of curcumin and bicalutamide enhanced the growth inhibition of androgen-independent prostate cancer cells through SAPK/JNK and MEK/ERK1/2-mediated targeting NF-κB/p65 and MUC1-C. J Exp Clin Cancer Res. 2015;34:46.
Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409–22.
Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-κB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2011;30:1615–30.
García-Alfonso P, Grande E, Polo E, Afonso R, Reina JJ, Jorge M, et al. The role of antiangiogenic agents in the treatment of patients with advanced colorectal cancer according to K-RAS status. Angiogenesis. 2014;17:805–21.
Li X, Wang M, Liu GY, Ma JL. Dual VEGF/EGFR inhibition versus single targeted agent treatment in patients with metastatic colorectal cancer: a meta-analysis of randomized trials. Int J Colorectal Dis. 2016;31:1655-6.
Acknowledgements
We thank the Department of Oncological Surgery, the Affiliated Hospital of Zunyi Medical University for providing CRC sample.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 30970809, 81271636), the Natural Science Foundation of Jiangsu Province (No. BK2009274) and the Special Fund of Clinical Medicine, Jiangsu Province, China (No. BL2012063).
Availability of data and materials
We confirm that all the authors of the manuscript have read and agreed to its content, that readily reproducible materials described in the manuscript will be freely available to any scientist wishing to use them, without breaching participant confidentiality.
Authors’ contributions
CBD conceived and designed the experiments and wrote the manuscript. CBD performed the experiments and analyzed the data. LML and TYY helped in sample and clinical data collection. XBF and GQW were responsible for the review and/or revision of the manuscript. GQW supervised the whole experimental work. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable because this manuscript does not contain any individual persons data.
Ethics approval and consent to participate
All patients provided written informed consent before surgery, and our study were approved by the Ethics Committee of the Affiliated Hospital of Zunyi Medical University according to the 1975 Declaration of Helsinki. All animal experiments were performed with the approval of Medical School of Southeast University Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Figure S1.
Effect of different anti-VEGF mAb or anti-EGFR mAb concentration on the proliferation of SW620 and LoVo cells in vitro. A The proliferation rate of SW620 and LoVo cells were analyzed by CCK-8 assay in different anti-VEGF mAb concentration. B The proliferation rate of SW620 and LoVo cells were analyzed by CCK-8 assay in different anti-EGFR mAb concentration. (TIF 1121 kb)
Additional file 2: Figure S2.
Combined application of anti-VEGF and anti-EGFR antibodies inhibits AKT and ERK signaling pathways in mice model. A The expression of p-AKT, p-ERK1/2 and p-STAT3 were quantitatively analyzed in different SW620 cells tumors. B Western blot assay of different LoVo cells tumors (one clone, A). C The expression of p-c-Myc, p-NF-κB/p65 and p-IκBα were quantitatively analyzed in different SW620 cells tumors. D Western blot assay of different LoVo cells tumors (one clone, C). The data are representative of at least three different experiments ± SEM. *P < 0.05 (TIF 1285 kb)
Additional file 3: Figure S3.
The levels of IL6 in CRC cell tumors. A The expression of IL6 was quantitatively analyzed in different SW620 cells tumors. B ELISA assay of different LoVo cells tumors (one clone, A). The data are representative of at least three different experiments ± SEM. NS: No statistical significance; *P < 0.05 (TIF 1783 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ding, C., Li, L., Yang, T. et al. Combined application of anti-VEGF and anti-EGFR attenuates the growth and angiogenesis of colorectal cancer mainly through suppressing AKT and ERK signaling in mice model. BMC Cancer 16, 791 (2016). https://doi.org/10.1186/s12885-016-2834-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2834-8