Abstract
Background
Tannery wastewater contains the most hazardous pollutants. Therefore, identifying potentially efficient, low-cost and locally available filter media as an adsorbent for the treatment of tannery wastewater is critical. The aim of this study is to identify and assess the ability of identified adsorbents and compare their efficiency. The volcanic rocks of pumice and scoria were collected from the rift valley area of Oromia region, Ethiopia and their chemical characteristics were determined using X-ray fluorescence analysis. Batch mode experimental study design was carried out. The rocks were crushed and effective size was determined by using a standard sieve. The composite tannery wastewater was collected from Dire tannery, Addis Ababa, Ethiopia and treated with pumice and scoria. Two adsorption kinetics and isotherm models were conducted to predict the removal mechanism and capacity of the adsorbents on the reduction of NO3–N, PO4–P and [Cr]T from tannery wastewater. Analysis of wastewater samples was done before and after different retention time. R statistical software and Originlab pro 2017 was run for data analysis and graphing.
Results
The untreated tannery wastewater revealed that the mean concentration of BOD5, COD, TSS, orthophosphate, ammonium, nitrite, nitrate, sulfide, sulfate and chromium were beyond the permissible limits. Nitrate removal efficiency of scoria and pumice were 99 and 95% respectively at retention time of 72 h. Phosphate removal was better by scoria on the first 24 and 48 h. The efficiency of pumice to remove sulfate was 83–84%, whereas scoria shows 75–77%. In the first 24 and 48 h retention time, pumice and scoria achieved 76 and 71% in chromium reduction respectively.
Conclusion
This study revealed that both scoria and pumice have a potential capacity to treat tannery wastewater. Conversely comparing the average efficiency to reduce hazardous pollutants scoria showed better results than pumice.
Similar content being viewed by others
Background
Industrial wastes are usually generated from different industrial processes, as a result the amount and toxicity of waste released from industrial activities varies with the type of industrial processes. Among all the industrial wastewater tannery wastewaters are the most source of hazardous pollutants (Shen 1999). Tannery wastewater contains the most hazardous pollutants of industry. Major problems caused by tannery wastewater containing heavy metals, nutrients, toxic chemicals, chloride, lime with high dissolved and suspended salts, and other pollutants. In developing countries, many industrial units are operating in a small and medium scale. These industrial units can generate a considerable pollution load by discharging untreated or partially treated effluents directly into the nearby environment (Asfaw 2014).
In Ethiopia currently, there are more than 30 tannery industries in operation. Among them the majority found in the Oromia region, especially Modjo town and around six established in the capital city Addis Ababa. These tanneries have 153,650 sheep and goat skin soaking capacity and 9725 cowhides soaking capacity per day together they also employ 4577 persons (UNIDO 2012).
The total wastewater discharge estimation from tanneries is about 400 million m3/year in Ethiopia. About 90% of world leather production use chrome-tanning processes rather than vegetable tanning. In Chrome tanning process tanneries utilize chromium in the form of basic chromium-sulphate for hide stabilization against microbial degradation and provision of flexibility of the leather. In chrome tanning process about 60–80% of chromium reacts with the hides and about 20–40% of the chromium amount remaining in the solid and liquid wastes (Rezic and Zeiner 2008).
Tanneries generate wastewater in the range of 30–35 L/kg of skin or hide processed with variable pH and high concentrations of suspended solids, BOD and COD. Major problems are due to wastewater containing heavy metals, toxic chemicals, chloride, lime with high dissolved and suspended salts and other pollutants (Durai et al. 2011). Hexavalent chromium from tannery wastewater is one of the major concerns of environmental pollution. This is due to discharge of tannery wastewater in large quantities without or with partial treatment (Lofrano et al. 2008).
Developing countries face numerous challenges related to preserving the environment from industrial wastewater pollution. Like many other developing countries, Ethiopia also grieves from environmental pollution problems of wastewater particularly industrial wastewater. This issue seems to be a subject which has not yet received adequate attention during the development of industries. Therefore, there is a need to develop efficient and low-cost wastewater treatment technologies for the removal of heavy metals and other pollutants. Among these technologies, adsorption and filtration is a user-friendly technique for this purpose.
In filtration technique, wastewater containing suspended matter is added to the top of the filter medium as the wastewater filters through the porous medium, the suspended matter in the wastewater is removed by a different of mechanisms. These mechanisms are straining, sedimentation, impaction, interception, adhesion, adsorption, flocculation and biological degradation especially for organic removals on the top of the filter medium. A natural characteristics of filter medium are important in the pollutant removal performance. Some of them are effective size, size distribution, slope, density and porosity (Boller and Kavanaugh 1995).
Adsorption on the other hand is the process of accumulation of a substance on the surface of another substance. When a solid surface is exposed to a water or wastewater, molecules from the solution phase accumulate or concentrate at the solid surface. It is recognized as one of the most effective wastewater purification technique used in several industries. The basic principle of adsorption is mass transfer and adsorption of a molecule from a liquid into a solid surface. It will happen if the pollutant has low solubility in the wastewater, greater affinity for the substrate than wastewater and the combination of the two (Rao et al. 2007).
Depending on the type of force of attractions between the pollutant and adsorbent, the adsorption process can be divided into two types. Physical adsorption or chemical adsorption. If the force of attraction between pollutant and adsorbent is weak that is a Vanderwaal force of attraction, the process is called physical adsorption or commonly known as physisorption. Physical adsorption takes place with the formation of multilayer of pollutant on the adsorbent. But if the force of attraction between pollutant and adsorbent is chemical forces of attraction or chemical bond, the process is called chemical adsorption or chemisorption. Chemisorption takes place with the formation of a single layer of pollutant on the adsorbent. In general, there are factors influencing adsorption such as surface area, nature of the pollutant, hydrogen ion concentration (pH) of the wastewater, temperature, mixed solutes and nature of adsorbent (Dabrowski 2001).
Adsorption has been identified as one of the most promising mechanism for removal of dissolved heavy metal fractions and nutrients from wastewater. Although commercial adsorbents are available for use in adsorption, they are very expensive, resulting in various new low-cost adsorbents being studied by researchers. (Babel and Kurniawan 2003), reviewed the technical feasibility of various low-cost adsorbents for heavy metals removal from wastewater and concluded that the use of low-cost adsorbents may contribute to the sustainability of the surrounding environment and offer promising benefits for commercial purpose in the future.
Therefore, identifying potentially efficient, low-cost and locally available filter media as an adsorbent is critical for proper practice of environmental management by tanning industries. On the other hand ordinary sand for filter media is costly because of construction-expansion in the country, not available readily and not efficient in the removal of hazardous pollutants by adsorption hence there is a need to substitute pumice and scoria instead of sand filtration.
Pumice is a light, porous volcanic rock that forms during explosive eruptions (Fig. 1). It resembles a sponge as it consists of a network of gas bubbles frozen amidst fragile volcanic glass and minerals. All types of magma (basalt, andesite, dacite, and rhyolite) will form pumice, however it is most commonly formed from rhyolite. During an explosive eruption, volcanic gases dissolved in the liquid portion of magma also expand rapidly to create a foam or froth; in the case of pumice, the liquid part of the froth quickly solidifies to glass around the glass bubbles. Pumice is considered a glass because it has no crystal structure. Like many of the materials considered in this report, it is an aluminosilicate (Akbal et al. 2000).
Studies have shown using substrate rich in iron (Fe), aluminum (Al) or calcium (Ca) concentrations enhances phosphate removal in experimental subsurface wetlands beyond that which can be achieved by using native soils (Arias et al. 2001). It was believed that sedimentation of particulate phosphorus and sorption of soluble phosphorus (onto the pumice) were responsible, and that the high concentrations of iron (18.2%), aluminum (13.7%), calcium (12.7%) and magnesium (7.3%) in the pumice were the source of this high sorption ability. The low specific gravity and high porosity of pumice make it important for a number of applications in water and wastewater treatment processes. Pumice was used as a filter medium and as a support material for microbial growth in water and wastewater treatment (Farizoglu et al. 2003).
The other volcanic ash is scoria generally denser than the pumice. Scoria is somewhat porous material with high surface area and strength with density larger than one. Scoria is an excellent medium which holds water in its pores and allow air circulation to the root zone of the plant. Both pumice and scoria are widely available in the Rift valley area of Ethiopia.
Scoria is bomb-sized, generally vesicular pyroclastic rock with basaltic composition, which is reddish brown to black in color and is of low density (Fig. 2). It has been used in several industrial applications, such as the manufacturing of a lightweight concrete mixture, a heating-insulating material, low-cost fillers in paints, and Sorbents (Moufti et al. 2000; Alemayehu and Lennartz 2009). Scoria is abundant in many places worldwide including Central America, Southeast Asia (Vietnam, etc.), East Africa (Ethiopia, Kenya, etc.), and Europe (Greece, Italy, Spain, Turkey, etc.) (Kwon et al. 2005; Alemayehu and Lennartz 2009).
Sorption of contaminants onto scoria mainly takes place at the outside surface at the initial stage. Changes of ionic composition during sorption experiments suggest that cation exchange is likely the dominant mechanism of heavy metals sorption onto scoria, while considerable As(III) removal by scoria is explained by specific sorption of the neutral As(III) species and electrical adsorption of negatively charged As(V) species via As oxidation onto hematite (Kwon et al. 2010). The experimental investigation conducted demonstrates that the scoria is able to concurrently reduce concentrations of heavy metals and arsenic in aqueous solutions. Kwon et al. (2010), recommend that scoria can be used as an economic and efficient Sorbent to treat contaminated water with heavy metals.
Taking into account the growth of industrialization in Ethiopia and the expected demand for industrial wastewater management, low-cost, appropriate and eco-friendly approaches will play a critical role in the development of future wastewater treatment technology in the country. In this practical approach, this work deal with the principles of adsorption and filtration for the removal of hazardous pollutants from tannery wastewater by identifying these two volcanic ashes (scoria and pumice) as a filter media instead of conventional sand.
Methods
Study area and period
This study has been conducted in Addis Ababa University by transporting sample wastewater from Dire tanning industry located at Kolfe Keranio sub city Addis Ababa Ethiopia from May to August 2016.
Study design
Batch mode comparative experimental study design has been carried out to assess the ability and determine the efficiency of scoria and pumice filter media on removal of hazardous pollutants by the treatment of industrial wastewater, the case of tannery wastewater filtration.
Materials, experimental, design and set-up establishments
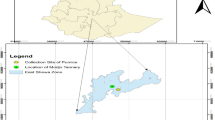
The volcanic rocks were collected from volcanic cones in the rift valley area of Oromia region, East Shewa Zone, Ethiopia, (pumice collected from the area lies in between: 8°28′36″N and 39°14′29″E; scoria collected from the area lies in between: 8°35′47″N and 39°08′45″E) approximately 50–100 km East of Addis Ababa (Fig. 3).
The rocks are local volcanic rocks with various chemical and mineralogical structure and transported to Addis Ababa University. The chemical characteristics of both filter media were determined by XRF analysis (Table 1).
These filter media were crushed and graded. The effective size was determined by using a standard sieve. Based on the analysis the effective size (ES) (d10) of each media was 1.5–4.5 and the uniformity coefficient (UC) (d60/d10) is 3.5–4. After grading the filter materials were washed with tap water and dry in sunlight for 1 week.
Two filtration tanks were made of metal sheet, each with the following dimension, 60 cm height and 28 cm diameter and also were fitted with a half-inch an outlet tap (faucet) 5 cm above from the bottom of each tank. The filtration tanks were installed at College of natural and computational science, Addis Ababa University. After installation the filter media were filled in the filtration tank 10 cm depth with 10–25 mm grain size, drainage layer at the bottom, 30 cm depth filter layer with a grain size of 1.5–4.5 mm in the middle and the distribution layer (flat coarse gravel) was added 5 cm depth at the top of the filter media to protect erosion of filter’s top layers, then it is ready for sample tannery wastewater filtration (Fig. 4).
Wastewater sample collection and filtration
The composite sample tannery wastewater was collected from Dire tannery and transported to Addis Ababa University in 40 L plastic ‘Jerican’for each filter tank. The on-site measurement of the physicochemical parameters was undertaken. The collected raw tannery wastewater was added to the two filtration tank at the time and a sample also transported to Addis Ababa EPA water and wastewater analysis laboratory and Ethiopian Construction Design and Supervision Works Corporation Research Laboratory (ECDSWCRL) for the raw tannery wastewater characterization.
Filtrated sample collection and laboratory analysis
The physicochemical analysis of wastewater samples has been done before and after the treatment with the two filter media, using standard methods (APHA 2005). Optimum operating treatment time was determined for maximum removal of these impurities by running the experiment for 24, 48 and 72 h, respectively. Filtrated samples were taken by 2 L plastic bottle after each fixed retention time that is over 24, 48, and 72 h and transported to Addis Ababa EPA water and wastewater analysis laboratory and ECDSWCRL after taking each sample.
The analytical parameters were pH, DO, BOD5, COD, TSS, ammonium N, nitrite N, nitrate N, phosphate, sulfide, sulfate and chromium. On-site measurement of the wastewater like temperature, pH and DO were carried out at the site in the tannery environmental quality control laboratory using a portable pH meter (Wagtech International N374, M128/03IM, USA) and DO meter (Hach P/N HQ30d, Loveland. CO, USA).
COD, ammonium–nitrogen, nitrite–nitrogen, nitrate–nitrogen, phosphate, sulfide and sulfate were measured by using a spectrophotometer (Hach model DR/3900 portable spectrophotometer, Germany) according to Hach instructions. BOD5 and total Cr were analyzed using BOD sensor and inductive stirring system AQUA LYTIC model type ET618-4 and flame atomic absorption spectrophotometer (AAS), (model AAS NOUA-400, Germany) respectively. Total suspended solids (TSS) were determined according to the standard methods for the examination of water and wastewater gravimetric method (APHA 2005).
The amount of chromium from the wastewater adsorbed onto the filter medium at any time, q t , was calculated as Eq. (1).
At equilibrium, q t = q e and C t = C e ; therefore the amount of adsorbed chromium, q e , was calculated by Eq. (2).
where C0, C t and C e are the initial concentration, concentration at any time and equilibrium concentrations of chromium in the wastewater (g/L), respectively, V is the volume of the wastewater (L), and W is the mass of the filer medium (kg) (Elmorsi 2011).
The removal efficiency of the filter medium for the selected parameters including chromium were calculated as Eq. (3).
where C0 is the parameter concentration in the untreated wastewater and C t is the parameter concentration in the treated wastewater at the final hydraulic retention time t.
Statistical data analysis
Mean and standard deviations were calculated to estimate the concentration of each parameter of the samples. The hypothesis has been tested by student t test using R statistical software: R version 3.2.2 (2015-08-14), Platform: x86_64-w64-mingw32/x64 (64-bit) to determine whether an observed difference between the means of the groups is statistically significant or not, based on the treatment efficiency of the filter materials. OriginPro 2017 64-bit was used also for graphing and plotting for adsorption kinetics and isotherms model analysis.
Data quality management
To assure quality of the data by minimizing the errors the following measures had been undertaken: Apparatuses were calibrated; expiry date of reagents had been checked before starting the real analysis and standard control also prepared. Each test had been triplicated.
Result and discussion
Physicochemical characteristics of Dire tannery wastewater
The raw wastewater was taken from Dire tannery around Kolfe Keranio Sub city Asko area, Addis Ababa, Ethiopia and transported to the Addis Ababa EPA laboratory and ECDSWCRL for physicochemical analysis. Based on this investigation the mean concentrations of selected physicochemical parameters were presented in Table 2.
This study revealed that the mean concentration of BOD5, COD and TSS were 1081 ± 160, 12,913 ± 6875 and 2426 ± 515.20 mg/L respectively (Table 2). This result is basically similar to different studies in Ethiopia with slight differences for different parameters, for example a study done at Modjo tannery indicated that the mean concentration of COD was laid between 7950 and 15,240 mg/L with the mean of 11,123 ± 563.90 mg/L (Leta et al. 2003). Another study also undertaken with same tannery wastewater showed that the mean concentration of BOD5 was 1054 ± 448 mg/L (Tadesse and Seyoum 2015). But the concentration of total suspended solid was found to range from 1849 to 2840 mg/L (Table 2) this result is a bit greater than some studies, for instance a study done in India indicated 1244 mg/L (Mandal et al. 2010).
Nutrients like orthophosphate, ammonium, nitrite and nitrate concentration of Dire tannery were characterized in this study, the result revealed that 168 ± 74, 314 ± 60, 1.7 ± 0.30, 124 ± 13 mg/L respectively. This result is comparable to a study done by Sivakumar et al. (2015) which indicates the concentration of nitrate in untreated tannery effluent as 116 mg/L. The result of ammonium is in the range of the results done at Bahir Dar tannery wastewater characterization (96–420 mg/L) (Wosnie and Wondie 2014). According to Sugasini and Rajagopal (2015), the nitrite concentration of untreated tannery wastewater was 1.3 mg/L almost parallel to this study finding which accounts 1.7 ± 0.30 mg/L (Table 2). Whereas the concentration of orthophosphate in this study was 168 ± 74 mg/L, this result shows that the concentration of phosphate in Dire tannery wastewater is higher than other study results done previously to characterize another tannery wastewaters. The variation may be due to the utilization of phosphorus containing chemicals for different purposes and tanning activities in Dire tannery.
The total suspended solids in Dire tannery found to be 2426 mg/L this result is more or less similar with the results of tannery wastewater analyzed by Banuraman and Meikandaan (2013). The concentration level of both sulfide and sulfate were 417 ± 131 and 1307 ± 224 mg/L respectively. In this case the amount of sulfide found in this study wastewater was more or less equivalent to study done by Islam et al. (2014) that is 380 ± 50 mg/L. Sugasini and Rajagopal (2015), also characterize the tannery wastewater based on their result the concentration of sulfate was 1517 mg/L which is almost parallel to this investigation. In terms of chromium concentration, Dire tannery comprised 35.7 ± 90 mg/L is similar to other different results presented from various tannery wastewaters in Ethiopia for example a study done by Leta et al. (2003) indicates 32.2 ± 5.70 mg/L. On the other hand two more study results showed, the chromium concentration lied to be in the ranges of this investigation 28–45 mg/L as in Table 2 (Tadesse and Seyoum 2015; Asaye 2009).
Even though Wastewater of each tannery process consists of varying pH and temperature values, this study results (9.1 ± 3.10 and 20.6 ± 2.34 °C) respectively were analogous to different studies. Likewise a large variation exists in values of physicochemical parameters in general like BOD5, COD, TSS, phosphate, sulfide, sulfate, etc. in every tannery wastewater characteristics, this may be because of different tanning process, methods, technology and raw material utilization by various tanning industries.
Adsorption kinetics for the pollutant removal process of tannery wastewater treatment using pumice and scoria filter medium
From the experimental data three selected pollutant parameters were tested to fit into different kinetic models for the adsorption rate, the removal mechanism and process and predict information about the interaction between these two naval adsorbents (filter medium) and those selected three pollutants (NO3–N, PO4–P and [Cr]T) in the tannery wastewater (Martins et al. 2013). In this study, two models were used, these are the pseudo-first-order (Lagergren 1898) and the pseudo-second-order kinetics model (Ho and McKay 1999).
Pseudo-first-order equation
Pseudo-first-order equation was given first by Lagergren (1898) to determine the rate constant of adsorption process as Eq. (4).
where q e and q t are the amounts of the pollutants adsorbed (g/kg) at equilibrium and at time t (h), respectively and k1 is the rate constant of adsorption (h−1). Values of k1 and q e were calculated from the slope and the intercept of the plots of log (q e − q t ) versus t for NO3–N, PO4–P and [Cr]T adsorption from the tannery wastewater onto pumice and scoria shown in Figs. 5a and 6a respectively.
The results in Tables 3 and 4 show that the values of correlation coefficients (R2) were high for all parameters adsorption on to both adsorbents except chromium adsorption onto scoria (Table 4) and the experimental q e value agree with the calculated value with very slight difference for all parameters except PO4–P and [Cr]T adsorption onto scoria (Tables 3, 4). Therefore, this study revealed that the adsorption of the three pollutants onto both adsorbents fit with the pseudo-first-order kinetic model except [Cr]T does not fit only onto scoria substrate.
Pseudo-second-order equation
Pseudo-second-order equation formulated from the adsorption equilibrium (Ho and McKay 1999). The equation can be expressed in Eq. (5):
where q e and q t are the sorption capacity (g/kg) of pollutants at equilibrium and at time t respectively and k2 is the rate constant for pseudo-second order sorption (kg g−1 h−1). For the boundary conditions t = 0 to t = t and q t = 0 to q t = q t , the above equation has been integrated and linearized to make Eq. (6).
where k2 (kg g−1 h−1) is the adsorption rate constant of pseudo-second-order equation. The value of q e and k2 can be obtained from the slope and the intercept of the plot of (t/q t ) versus t respectively for those selected pollutants adsorption from the tannery wastewater onto pumice Fig. 5b and scoria Fig. 6b.
The results in Figs. 5b and 6b show linear plots with very high values of R2 on the adsorption of PO4–P and [Cr]T onto scoria. But the value R2 for NO3–N adsorption onto pumice was low. In addition the result showed that, good agreement between experimental and calculated values of q e in the adsorption kinetics of PO4–P and [Cr]T onto pumice adsorbent (Tables 3, 4). Therefore, the adsorption of PO4–P and [Cr]T onto the scoria represents a good fit with pseudo-second-order kinetics. In this case the adsorption of PO4–P and [Cr]T onto the scoria process suspected to be chemisorption Alemayehu et al. (2011).
In terms of R2, Table 3 clearly shows, all the three pollutant removal process in the pumice containing filter tank strongly agree with pseudo-first-order kinetic model than second-order. A result of chromium adsorption kinetics and equilibrium study done by Pandey et al. (2010) showed the same trend with this kinetic study findings.
The NO3–N is strongly agree with Pseudo first-order kinetic model by considering both correlation coefficients (R2) and experimental and calculated q e value, but PO4–P agree with second-order kinetics in terms of both kinetic parameters using scoria adsorbent. Similarly [Cr]T removal process onto scoria substrate also agrees with the second-order model based on the value of R2, which is comparable to a study done in South Korea and Nigeria on the adsorption of Cr(VI) ion onto different natural adsorbents (Ali et al. 2016; Owalude and Tella 2016). However, in this kinetic model for [Cr]T onto the same adsorbent (scoria), the experimental q e is not in-line with the calculated one (Table 4).
Adsorption equilibrium isotherm modeling of chromium removal from tannery wastewater
In this study, two different adsorption isotherm models, namely Langmuir and Freundlich were used to evaluate the affinity of the two studied adsorbents (filter medium) for the removal of [Cr]T from the real tannery wastewater.
Langmuir isotherm
The Langmuir isotherm model assumes that a monolayer adsorption at specific homogenous sites such as [Cr]T in real tannery wastewater is adsorbed on the adsorbent surface in this specific case pumice and scoria. Equation (7) is the Langmuir expression.
where q m (the maximum capacity of adsorption, g/kg) and K L (a constant related to the affinity of the binding sites, L/kg) are the Langmuir isotherm constants. Both q m and K L can be determined from the slope and intercept of the plot C e /q e versus C e which is the linear form of Langmuir equation that gives a straight line showed in Figs. 7a and 8a.
The Langmuir isotherm constants of [Cr]T onto pumice and scoria substrates were displayed in Tables 5 and 6 respectively. The high value of correlation coefficients (R2) = 0.9464 in pumice substrate indicated minimal deviation from the fitted equation showing that the adsorption of [Cr]T onto pumice follows Langmuir equation and also it shows that pumice has a maximum adsorption capacity of [Cr]T than scoria (Tables 5, 6). Even though the previous researchers used laboratory scale test with synthetic solution unlike this study, Alemayehu et al. (2011) obtained similar result with this finding.
Freundlich isotherm
The Freundlich exponential equation presumes that the adsorption process takes place on a heterogeneous surface. The linear form of Freundlich equation is given in Eq. (8).
where K F (L/kg) is an indicator of the multilayer adsorption capacity and 1/n is the adsorption intensity and indicates both the relative distribution of energy and the heterogeneity of the adsorbent sites. Figures 7b and 8b representing the linear plot of log q e versus log C e at constant temperature. The value of K F and 1/n (Tables 5, 6) were determined from the intercept and the slope of the plot of log q e versus log C e respectively.
Linear regression is the widely used approach in evaluating the fit between experimental data and the various isotherm models, this can be determined from the value of (R2) (Ncibi 2008). In this study, R2 (0.9174) of Freundlich isotherm on the adsorption of [Cr]T onto pumice is greater than the value of R2 (0.7857) onto scoria substrate. On the other hand the value of R2 is greater in the Langmuir isotherm model than Freundlich in both substrates. Therefore adsorption of [Cr]T onto both substrates fit with the Langmuir and Freundlich isotherm model with R2˃ 0.75.
Comparison of the efficiency of pumice and scoria substrate for tannery wastewater treatment
Wastewater from the leather industry is known to be heavily contaminated with inorganic and organic pollutants. Treatment processes for such type of high strength wastewaters is challenging. In this case adsorption and filtration method are more effective than chemical and biological processes, but at the same time more expensive. So alternative adsorbent must be investigated. In this study two volcanic rocks pumice and scoria were identified and tested for their ability in removal of hazardous pollutants and compared the efficiency for the treatment of tannery wastewater by adsorption and filtration techniques.
Based on this finding the pollutant removal efficiency of pumice is better than scoria to remove BOD5 at each retention time, but in terms of COD removal reveres result was obtained. In general the two substrates were poor to remove both BOD5 and COD this is obvious that the organic matters cannot be removed by adsorption some may be removed biologically at the top of the filter medium. Better removal efficiency has been seen by Scoria to remove TSS, the minimum removal efficiency (65%) was achieved by pumice at RT = 24 h and the maximum removal efficiency (84%) was obtained by scoria at RT = 72 h. This better removal efficiency was seen because most of the TSS may be removed by the straining mechanism of filtration in both filter materials (Table 7).
In nutrient removal potential, scoria shows better efficiency than pumice for example nitrate removal efficiency of scoria and pumice were 99 and 95% respectively at RT = 72 h. Similarly the phosphate removal also better by scoria on the first 24 and 48 h, but at RT = 72 h pumice removed 66% and scoria removed 63% only. This study result is in line with study done in Jimma University Ethiopia using an aqueous solution running over 24 h contact time with similar filter media (Birhane et al. 2014) with a slight difference. This difference may be due to the phosphate concentration, experimental setup and the type of wastewater used for the test between the two studies.
In another study that was tested using four filter materials. The removal of phosphate ranged from 35 to 41% for calcite, 59 to 100% for zeolite, 49 to 100% for sand, and 73 to 100% for iron filings (Reddy et al. 2013). From the indicated four filter materials the result of this paper can be compared in the range of the result obtained from iron filings. That means pumice is similar adsorption characteristics with iron filings. The removal of nitrate and phosphate may be mainly attributed to adsorption, ion exchange and precipitation. The reverse trend was shown to remove sulfate and chromium that indicate pumice had a better efficiency than scoria in all given retention time (Table 7; Fig. 9).
Since industrial effluents like tannery wastewater containing sulfide and sulfate are toxic to aquatic environment, it is essential to reduce them and bring the discharge levels of these species to below the toxic limit. In this investigation the sulfate removal potential of pumice was greater than scoria the efficiency of pumice to remove sulfate was from 83 to 84% whereas scoria shows from 75 to 77%. In general both filter mediums were effective to remove sulfide and sulfate from tannery wastewater.
The better removal performance of pumice may be achieved based on the good sulfate adsorption nature of the pumice and its chemical composition. On the other hand metal ions from the filter material may react with dissolved sulfide ions to form metal sulfides as colloidal suspension, which were coagulated and precipitated and finally filtered out, this mechanism may be contributed mainly to sulfide removal in the filtration tank. In a study carried out to investigate the effect of chemical modification method on sulfate removal efficiency of adsorbents, the removal of sulfate ion using Fe-modified carbon residue was notably higher compare with unmodified carbon residue and commercially available activated carbon (Runtti et al. 2016).
Batch experimental laboratory scale studies were undertaken in a different world on the potential of pumice to reduce the concentration of heavy metals including chromium from aqueous solution in the laboratory. But in this study the chromium removal efficiency of pumice and scoria was done from real tannery wastewater.
In this study pumice had better in chromium reduction potential than scoria. In the first 24 and 48 h retention time pumice and scoria achieved 76 and 71% in chromium reduction respectively (Table 7). A study done by (Alemayehu et al. 2011), showed similar result that was pumice had a better result to remove chromium than scoria. The maximum adsorption yield, 77% for scoria and 80% for pumice, was obtained at low pH in that study. This chromium removal potential difference between the two substrates may be due to the difference in chemical composition (Table 7; Fig. 9).
Considering all the selected tannery wastewater parameters for this study, the average treatment efficiency of pumice and scoria were 58.8 and 63.4% in RT = 24 h, 61.5 and 67.5% at RT = 48 h respectively, and equivalent efficiency was obtained at the third retention time that is over 72 h both filter materials showed 68.3%. Even though there were differences in different types of parameter reduction, the average tannery wastewater treatment efficiency of scoria was greater than pumice at the first 24 and 48 h, but it is not statistically significant at 95% confidence interval, p = 0.3 and 0.2 respectively. Equivalent percentage removal (68.3%) was shown in both substrates at RT = 72 h, but in the overall result, Scoria had shown greater efficiency than pumice but it is not statistically significant at 95% confidence interval, p = 0.2.
Conclusion
Based on the investigation the following major conclusions have been drawn:
Dire tannery wastewater characteristics were very high strength wastewater with different hazardous pollutants. The applied methodology provides an efficient and feasible approach for removal of pollutants.
Pumice had good potential to reduce pollutants from tannery wastewater, especially nutrients, sulfate and chromium but it is poor in terms of reduction of organic pollutants. Scoria also showed promising result in the reduction of nutrient, sulfate and chromium from tannery wastewater. Except sulfate and chromium the other wastewater parameter reduction was directly proportional to the retention time of the filtration system.
More or less both substrates have a potential to treat high strength industrial wastewater like tannery effluent by using different techniques including filtration and can be an alternative to sand filtration. However, when we compare the average efficiency to reduce those selected wastewater parameters scoria showed better results than pumice, even though pumice has better potential to reduce chromium and sulfate than scoria. Therefore the use of locally available low cost adsorbents may contribute to the low technology solution for sustainable wastewater management. Any interested company can use these substrates as a filter medium in the filtration bed or substitute by sand for available filtration bed for wastewater treatment.
Abbreviations
- APHA:
-
American Public Health Association
- BOD:
-
biological oxygen demand
- COD:
-
chemical oxygen demand
- DO:
-
dissolved oxygen
- ECDSWCRL:
-
Ethiopian Construction Design and Supervision Works Corporation Research Laboratory
- EPA:
-
environmental protection authority
- ES:
-
effective size
- RT:
-
retention time
- TSS:
-
total suspended solids
- TWW:
-
tannery wastewater
- UC:
-
uniformity coefficient
- UNIDO:
-
United Nations Industrial Development Organization
- XRF:
-
X-ray fluorescence
References
Akbal F, Akdemir N, Onar AN (2000) FT-IR spectroscopic detection of pesticide after sorption onto modified pumice. Talanta 53:131–135
Alemayehu E, Lennartz B (2009) Virgin volcanic rocks: kinetics and equilibrium studies for the adsorption of cadmium from water. J Hazard Mater 169:395–401
Alemayehu E, Thiele-Bruhn S, Lennartz B (2011) Adsorption behavior of Cr(VI) onto macro and micro vesicular volcanic rocks from water. Sep Purif Technol 78:55–61
Ali A, Saeed K, Mabood F (2016) Removal of chromium (VI) from aqueous medium using chemically modified banana peels as efficient low-cost adsorbent. Alex Eng J 55:2933–2942
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public health association, Washington
Arias CA, Del Bubba M, Brix H (2001) Phosphorus removal by sands for use as media in subsurface flow constructed reed beds. Water Res 35(5):1159–1168
Asaye K (2009) Evaluation of selected plant species for the treatment of tannery effluent in a constructed wetland system. Dissertation, Addis Ababa University
Asfaw A (2014) Heavy metals concentration in tannery effluents, associated surface water and soil at Ejersa area of East Shoa, Ethiopia. Herald J Geogr Reg Plan 3(3):124–130
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metal uptake from contaminated water. J Hazard Mater 97:219–243
Banuraman S, Meikandaan TP (2013) Treatability study of tannery effluent by enhanced primary treatment. Int J Mod Eng Res 3(1):119–122
Birhane M, Abebe A, Alemayehu E, Mengistie E (2014) Efficiency of locally available filter media on fluoride and phosphate removal for household water treatment system. Chin J Popul Res Environ 12(2):110–115
Boller MA, Kavanaugh MC (1995) Particle characteristics and head loss increase in granular media filtration. Water Res 29(4):139
Dabrowski A (2001) Adsorption—from theory to practice. Adv Coll Interface Sci 93:135–224
Durai G, Rajasimman M, Rajamohan N (2011) Kinetic studies on biodegradation of tannery wastewater in a sequential batch bioreactor. J Biotechnol Res 3:19–26
Elmorsi TM (2011) Equilibrium isotherms and kinetic studies of removal of methylene blue dye by adsorption onto miswak leaves as a natural adsorbent. J Environ Prot 2:817–827
Farizoglu B, Nuhoglu A, Yildiz E, Keskinler B (2003) The performance of pumice as a filter bed material under rapid filtration conditions. Filtr Sep 40(3):41–47
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Islam B, Musa AE, Ibrahim EH, Salma AA, Babiker M (2014) Evaluation and characterization of tannery wastewater. J Forest Prod Ind 3(3):141–150
Kwon JS, Yun ST, Kim SO, Mayer B, Hutcheon I (2005) Sorption of Zn(II) in aqueous solutions by scoria. Chemosphere 60:1416–1426
Kwon JS, Yun ST, Lee JH, Kim SO, Jo HY (2010) Removal of divalent heavy metals (Cd, Cu, Pb, and Zn) and arsenic (III) from aqueous solutions using scoria: kinetics and equilibria of sorption. J Hazard Mater 174(13):307–313
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 24(4):1–39
Leta S, Assefa F, Dalhammar G (2003) Characterization of tannery wastewater and assessment of downstream pollution profiles along Modjo River in Ethiopia. Ethiop J Biol Sci 2(2):157–168
Lofrano G, Aydn E, Russo F, Guida M, Belgiorno V, Meric S (2008) Characterization fluxes and toxicity of leather tanning bath chemicals in a large tanning district area. Water Air Soil Pollut 8:529–542
Mandal T, Dasgupta D, Mandal S, Datta S (2010) Treatment of leather industry wastewater by aerobic biological and Fenton oxidation process. J Hazard Mater 180:204–211
Martins AE, Pereira MS, Jorgetto AO, Martines MA, Silva RI, Saeki MJ, Castro GR (2013) The reactive surface of castor leaf (Ricinus communis L.) powder as a green adsorbent for the removal of heavy metals from natural river water. Appl Surf Sci 276:24–30
Moufti MR, Sabtan AA, El-Mahdy OR, Shehata WM (2000) Assessment of the industrial utilization of scoria materials in central Harrat Rahat, Saudi Arabia. Eng Geol 57:155–162
Ncibi MC (2008) Applicability of some statistical tools to predict optimum adsorption isotherm after linear and non-linear regression analysis. J Hazard Mater 153:207–212
Owalude SO, Tella AC (2016) Removal of hexavalent chromium from aqueous solutions by adsorption on modified groundnut hull. Beni-suef Univ J Basic Appl Sci 5:377–388
Pandey PK, Sharma SK, Sambi SS (2010) Kinetics and equilibrium study of chromium adsorption on zeoliteNaX. Int J Environ Sci Technol 7(2):395–404
Rao BH, Dalinaidu A, Singh DN (2007) Accelerated diffusion test on the intact rock mass. J Test Eval 35(2):111–117
Reddy KR, Xie T, Dastgheibi S (2013) Nutrients removal from urban storm water by different filter materials. Water Air Soil Pollut 225:1778
Rezic I, Zeiner M (2008) Determination of extractable chromium from leather. Monatshefte fur Chemie-Chemical 140(3):325–328
Runtti H, Tuomikoski S, Kangas T, Kuokkanen T, Rämö J, Lassi U (2016) Sulfate removal from water by carbon residue from biomass gasification: effect of chemical modification methods on sulfate removal efficiency. BioResources 11(2):3136–3152
Shen TT (1999) Industrial pollution prevention, 2nd edn. Springer, Berlin, p 40
Sivakumar P, Kanagappan M, Sam Manohar Das S (2015) Physicochemical characteristics of untreated effluent from tannery Industries in Tamil Nadu: a comparative study. Int J Pharm Bio Sci 6(1):446–451
Sugasini A, Rajagopal K (2015) Characterization of physicochemical parameters and heavy metal analysis of tannery effluent. Int J Curr Microbiol Appl Sci 4(9):349–359
Tadesse AT, Seyoum LA (2015) Evaluation of selected wetland plants for removal of chromium from tannery wastewater in constructed wetland, Ethiopia. Afr J Environ Sci Technol 9(5):420–427
UNIDO (2012) United Nations Industrial Development Organization Vienna, Technical assistance project for the upgrading of the Ethiopian leather and leather products industry, independent evaluation report Ethiopia. UNIDO project number: TE/ETH/08/008. https://open.unido.org/api/documents/4763121/download/Independent%20Evaluation%20Report%20-%20ETHIOPIA%20-%20Technical%20assistance%20project%20for%20the%20upgrading%20of%20the%20Ethiopian%20leather%20and%20leather%20products%20industry. Accessed Sept 2016
Wosnie A, Wondie A (2014) Bahir Dar tannery effluent characterization and its impact on the head of Blue Nile River. Afr J Environ Sci Technol 8(6):312–318
Authors’ contributions
All authors have made an essential intellectual contribution to this study. MBA designed the study, conducted the experiments, Collected, analyzed and interpreted the data and wrote the manuscript. SLA involved on the study design, supervised the experiment, provided comments and suggestion for the whole work. MMK supervised the work, drafting and revising the primary manuscript, edited the manuscript, provided pertinent comments and suggestion on the manuscript. All authors read and approved the final manuscript.
Authors’ information
Mekonnen Birhanie Aregu is a Ph.D. Scholar in environmental pollution and sanitation, Center for Environmental Sciences, College of Natural Science, Addis Ababa University and lecturer at School of Public Health, Dilla University. He has given Public health, Environmental Health Science and Technology courses and also contribute in community services and problem solving applied research activities and published several articles in the internationally peer reviewed journals. He is also a senior consultant in environmental health, waste management and emission control.
Seyoum Leta Asfaw (Ph.D.) is an Associate Professor of environmental pollution and sanitation stream, Center for Environmental Sciences, Addis Ababa University. He has given various courses in the stream. He is also supervising and monitoring several Ph.D.s and M.Sc. students in the areas of environmental biotechnology, wastewater treatment and water quality studies, waste to energy (resource recovery and climate change mitigation and adaptation studies), bioremediation, phytoremediation, microbial ecological studies, environmental sanitation. He has published several peer reviewed papers in different international reputable journals. Currently, he is also executive Director for Horn of Africa Regional Environmental Centre and Network (HOARECN).
He has more than 20 years of experience in environmental science and technology studies and management. He had also been a regional program manager for the Bio-resources innovation network for Eastern Africa development. He has also been the principal investigator for a number of research projects such as “Development of innovative technologies for the sustainable treatment of high strength wastewater in East Africa”, a regional research program involving Ethiopia, Kenya, Tanzania and Uganda funded by Sida. He developed an innovative, integrated pilot technology for the treatment of agro-process wastewater, generating biogas, biofertilizer, and clean water at Modjo Tannery, Addis Ababa, Ethiopia.
Professor Mohammed Mazharuddin Khan (Ph.D.) is a professor of Environmental Science at Center for Environmental Sciences, Addis Ababa University. He is the CHARLES DARWIN GOLD MEDAL was conferred on the 27th day of June, 2009 towards the contribution made in the field of Life Science by International Society for Ecological Communication at Vinoba Bhave university, Hazaribagh, Jharkhand, India. He has given several courses like Botany, Biotechnology, Environmental sciences, Ecology, Microbiology, and Environmental Microbiology at postgraduate and undergraduate level. He is also a founder of different organizations, moreover he is a member of more than eight international professional associations and societies and served in different positions. Professor Khan has published several internationally peer reviewed papers.
Acknowledgements
The principal investigator would like to thank Addis Ababa University for financial support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The dataset and materials used for this manuscript is available and can be shared whenever necessary. Data was generated by the author from the field substrate sample collection, workshop and laboratory analysis.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
The first author is grateful to Addis Ababa University in supporting for expenditures during laboratory analysis.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Aregu, M.B., Asfaw, S.L. & Khan, M.M. Identification of two low-cost and locally available filter media (pumice and scoria) for removal of hazardous pollutants from tannery wastewater. Environ Syst Res 7, 10 (2018). https://doi.org/10.1186/s40068-018-0112-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40068-018-0112-2