Abstract
Background
Acting as a cellular cleaner by packaging and transporting defective proteins and organelles to lysosomes for breakdown, autophagic process is involved in the regulation of cell remodeling after cell damage or cell death in both vertebrate and invertebrate. In human, limitations on the regenerative capacity of specific tissues and organs make it difficult to recover from diseases. Comprehensive understanding on its mechanism within invertebrate have strong potential provide helpful information for challenging these diseases.
Method
In this study, recent findings on the autophagy function in three invertebrates including planarian, hydra and leech with remarkable regenerative ability were summarized. Furthermore, molecular phylogenetic analyses of DjATGs and HvATGs were performed on these three invertebrates compared to that of Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila melanogaster, Mus musculus and Homo sapiens.
Results
In comparison with Scerevisiae, C elegans, D melanogaster, M musculus and human, our analysis exhibits the following characteristics of autophagy and its function in regeneration within invertebrate. Phylogenetical analysis of ATGs revealed that most autophagy-related genes (ATGs) were highly similar to their homologs in other species, which indicates that autophagy is a highly conservative biological function in both vertebrate and invertebrate. Structurally, almost all the core amino acids necessary for the function of ATG8 in mammal were observed in invertebrate HvATG8s and DjATG8s. For instance, ubiquitin-like domain as a signature structure in each ATG8, was observed in all ATG8s in three invertebrates. Basically, autophagy plays a key role in the regulation of regeneration in planarian. DjATG8-2 and DjATG8-3 associated with mTOR signaling pathway are sophisticated in the invertebrate tissue/organ regeneration. Furthermore, autophagy is involved in the pathway of neutralization of toxic molecules input from blood digestion in the leech.
Conclusions
The recent investigations on autophagy in invertebrate including planarian, hydra and leech suggest that autophagy is evolutionally conserved from yeast to mammals. The fundamental role of its biological function in the invertebrate contributing to the regeneration and maintenance of cellular homeostasis in these three organisms could make tremendous information to confront life threatening diseases in human including cancers and cardiac disorders.
Similar content being viewed by others
Background
Autophagy is an evolutionarily conserved process, which plays a crucial role in maintaining cellular homeostasis by removing defective proteins, organelles and invading pathogens [1, 2]. Based on different mechanisms by which intracellular cargos are delivered to lysosomes, three forms of autophagy have been identified—chaperone-mediated autophagy (CMA), microautophagy and macroautophagy (the usual autophagy) [3, 4]. Multiple lines of evidence suggest that autophagic degradation is triggered by various stress responses, such as hypoxia [5], inflammation [6], and nutrient deficiency [7]. Due to its crucial role in maintaining cellular homeostasis, dysfunction of autophagyis thought to be associated with numerous diseases, including cancer, age-related disorders, infection, regeneration, et al. For example, in cancer, autophagy plays a dual role in different environments and tumor stages [8, 9]. In the early stage of tumorigenesis, autophagy acts as aninhibitor through its cellular qualitycontrol function, while in the late stage of tumorigenesis, autophagy provides a protective mechanism for maintaining cancer cell survival and homeostasis. According to Nilsson, deficient autophagy can disrupt the secretion of Aβ peptides, while the accumulated intracellular Aβ peptides can lead to Alzheimer’s disease (AD)-related pathology [10]. Moreover, autophagy-related genes (ATGs),such as ATG7, CDK5 and Beclin 1, may mediate the cross-talk between molecular mechanisms of autophagy and AD [11].
Regeneration is needed in maintaining homeostasis and adapting to the external environment due to apoptosis. Growing evidence has demonstrated that in mammals, autophagy is responsible for the repair of damaged tissues and the replacement of impaired organs or body parts after injury. For example, in muscle regeneration, autophagy may regulate proteostasis and survival mechanisms in regenerating fiber. Dysfunction of autophagy will lead to a decline in the function and number of muscle satellite cells, while restoration of autophagy can effectively prevent senescence and restore regenerative functions of geriatric satellite cells [12]. Additionally, autophagy plays a vital role in maintaining quiescence and stemness of cells by clearing active and healthy mitochondria in hematopoietic stem cells (HSCs) [13].
Regenerative ability may vary from species, organs, tissues, and even development stages [14]. In human, limitations on the regenerative capacity of specific tissues and organs make it difficult to recover from diseases. Compared with mammals, most invertebrates, such as planarian, hydra and leech, have remarkable abilities to regenerate any missing part after amputation [15,16,17]. A large population of adult stem cells may explain the astonishing regenerative abilities of planarians and hydras, while leeches, which have only a few stem cells, achieve their regeneration by dedifferentiation of tissue cells and migration and proliferation of stem cells [17]. Consistent with observations in vertebrates, autophagy appears to be a response to starvation as well as to injury in planarians and hydras [18, 19]. In starving animals, dramatic increase in the number of autophagic vacuoles was detected. An appropriate regulation of autophagy guarantees regeneration efficient in these invertebrates [19, 20]. In regenerating hydra, excessive autophagy induced by Kazal1 silencing leads to death [21]. Treatment with rapamycin, a depressor of autophagy, delays the early phases of head regeneration in both fed and starved hydra. Besides, the autophagy inhibitors Wortmannin and Bafilomycin can also slightly delay head regeneration [19]. Gtdap-1, the planarian ortholog of human death-associated protein-1 (DAP-1), is involved in remodeling by a process of autophagy during planarian regeneration and starvation [18].
Investigating the cellular function of autophagy in regeneration process will allow us to know more about the situation in proliferation-related diseases and will contribute to the development of therapeutic strategies for human disorders. In comparison with vertebrates, invertebrates including planarian, hydra and leech present special characteristics that make them be valuable models to study the relationship between autophagy and regeneration: (1) in contrast to mammals where autophagy only occurs at specific times or in very specific organs, they offer unique models where autophagy occurs continuously due to their un-paralleled regenerative capability and continual process of change. (2) using them to study autophagy means addressing roles of autophagy in regeneration at a whole-organism level, but not at an organ level or asystem level [19, 22, 23]. Therefore, to further assess the role of autophagy in regeneration, ATGs and functional roles of autophagy in planarian, hydra and leech are mainly described in this article.
ATG family and mTORC1-related remodeling within invertebrates
ATG proteins involved in autophagy in general
Autophagy-related genes (ATGs) are essential for the formation of autophagosomes. Since the discovery of autophagy-related (ATG) genes initially in yeast, identification of ATG genes was undertaken in higher eukaryotes [24, 25]. Mammals contain almost all of them as well as a series of factors specific to higher eukaryotes.
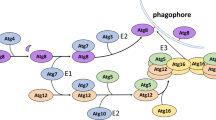
Among these ATGs, one subset which is referred to as the “core” molecular machinery, plays a crucial role at different stages of autophagic process, i.e. initiation, elongation, maturation and fusion with lysosomes [3]. In mammals, these core ATG genes can be divided into several functional groups: (1) ULK1-ATG13-FIP200-ATG101 complex, (2) class III phosphatidylinositol 3-kinase (PtdIns3K) complex I, (3) two ubiquitin-like conjugation systems (ATG8/LC3 conjugation system and ATG12 conjugation system) and (4) ATG9 and its cycling system (ATG2, ATG9, ATG18) [26].
In mammals, initiation of autophagy occurs through ULK complex consisting of ULK1/2, ATG13, FIP200 and ATG101. ULK1/2, a homolog of yeast ATG1, contains an N-terminal kinase domain, a LIR motif and two C-terminal MIT domains [27]. Autophosphorylation of ULK1at Thr180 is crucial for activation [28]. MIT domain of ULK1 binds to MIM domain of ATG13, and ATG13 recruits ULK1 to FIP200 (a focal adhesion kinase family-interacting protein of 200 kDa). FIP200, a hybrid molecule of ATG17 and ATG11 [27], contains an N-terminal ATG17-like domain, a LIR motif, a coiled-coil region and a C-terminal Claw domain. Both ATG13 and FIP200 can stabilizeULK1/2 and increase its kinase activity [29, 30]. Besides MIM domain, ATG13 in mammals also contains an N-terminal HORMA and a LIR motif. The LIR domains of ULK1 and ATG13 in humans can mediate their interaction with ATG8s [31]. The ATG13 containing HORMA domain forms a heterodimer with ATG101 containing HORMA domain [32]. Therefore, the association of ATG101 with ATG13 is the key to autophagy induction [33]. Notably, ATG101 is an entirely novel ATG protein in mammals [34], contributing to maintaining the stability and basal phosphorylation of ATG13 and ULK1 [35, 36]. The WF-finger motif of ATG101 can recruit downstream proteins to the autophagosome formation site in mammals [37], and the C-terminal region is responsible for the binding of phosphatidylinositol 3-kinase (PtdIns3K) complex [32].
Class III PtdIns3K complex I, consisting of VPS34, VPS15, Beclin1 and ATG14(L)/Barkor, is a functional effector of ULK complex and contributes to promoting autophagy elongation [29]. VPS34, composed of an N-terminal lipid-binding C2 domain, a helical domain and a C-terminal kinase domain, is responsible for phosphorylating phosphatidylinositol and thus producing P13P [38]. VPS15 contains an N-terminal kinase domain, a HEAT domain and a C-terminal WD40 repeat domain. Beclin-1, a homology of ATG6, contains a coiled-coil domain and a BABA domain [39]. ATG14L is composed of a coil-coil domain and a BATs domain [27]. When ULK1 phosphorylates BECN1 on Ser14, the ATG14L-containing VPS34 complex is then activated. The cysteine-rich domain near the N-terminal of ATG14L plays a vital role in its starvation-induced translocation to the phagophore initiation sites [40]. BATs domain is required for ER localization of PI3KC3-C1, whereas the C-terminal region of VPS34 determines the orientation on the membrane [41].
In mammals, ATG8 protein is comprised of seven homologs: LC3A, LC3B, LC3C, LC3B2, GABARAP, GABARAP‐L1 and GABARAP‐L2/GATE‐16 [42]. All ATG8/LC3 proteins contain conserved C-terminal ubiquitin-like structures despite the lack of similarity in amino acid sequence [43]. The ubiquitin-like structure, comprising four β-strands and two α-helices, is responsible for the protein–protein interaction (PPI) [44]. The two amino-terminal α helices, which differ among ATG8 proteins, have their specific roles during autophagy. Emerging evidence suggests that LC3 mediates the elongation step, while GABARAP and GABARAPL2 are involved in the sealing and fusion of autophagosome [45]. Among four homologs (ATG4A, B, C, D) of the protease ATG4 in mammals, ATG4B, which is composed of a conserved papain-like domain and a unique short-finger domain according to the structural studies [27], plays a crucial role in processing all ATG8 family proteins [46]. In the process of autophagy, ATG8 is cleaved by ATG4at C-terminus to generate the cytosolic ATG8-1 with a glycine residue. Then, the glycine residue is covalently conjugated in a reaction catalyzed by ATG7/ATG3.
ATG7 is an E1-like enzyme that includes two domains, the N-terminal domain (ATG7-NTD) which can specifically recruit two distinct autophagic E2-like proteins, ATG3 and ATG10 [47], and the C-terminal domain (ATG7-CTD)which is involved in binding and activating ATG8 and ATG12 [27]. The ATG12 can be conjugated to ATG5 in a reaction catalyzed by ATG7 and ATG10. The ATG12-ATG5 conjugate can be directly recruited to phagophore by ATG16L in the interaction between noncovalently and ATG5 via a coiled-coiled domain [48]. The ATG12-ATG5-ATG16L complex can interact with ATG3 and facilitate the transfer of ATG8-like proteins from ATG3 to phosphatidyl ethanolamine (PE).
ATG9 is a six-transmembrane protein, the only known transmembrane protein in ATG core proteins, with both the N and C terminal in the cytosol. The function of ATG9 remains a mystery. In mammalian cells, ATG9 (called mATG9) resides in a unique endosomal-like compartment and on endosomes [49]. The mATG9 is required for the formation of phagophores and its trafficking to phagophore is regulated by TBC1D14 and TRAPPIII independent of early autophagy proteins, such as ULK1 [50]. And the fusion of ATG9 vesicles may provide the membrane structures for the growing phagophore [51].
ATG family within invertebrates
Attention has been shifted from higher eukaryotes (e.g. yeast) to invertebrates in identifying the cellular basis of autophagy and the homologs of ATGs [52,53,54]. During evolution, ATGs have been duplicated and lost, thus resulting in the extinction and expansion of some subfamilies of autophagy-related genes. For instance, multiple ATG8 genes can be found in mammals, whereas there is only a single ATG8 gene in fungal species (e.g. yeast) [42]. Increasing number of yeast ATG orthologs were identified in Hydra vulgaris (H. vulgaris) and Dugesia japonica (D. japonica).
DjATGs include thirteen single genes and three ATG8 family-encoding genes (DjATG8-1, DjATG8-2, and DjATG8-3). Analysis of detailed biochemical index of these DjATG proteins showed their lengths ranged from 106 (DjATG12) to 1790 amino acids (DjATG2). The predicted molecular weights ranged from 11.9 kDa (DjATG12) to 205.9 kDa (DjATG2), pI ranged from 4.75 (DjATG3) to 9.16 (DjATG8-2), and gravity ranged from −0.644 (DjATG8-1) to 0.044 (DjATG9), suggesting that there were significant variations and potential functional differentiation. Based on sequence alignment, DjATGs could be divided into two groups: group with high identity and group with low identity. The former group includes DjATG3, DjATG4, DjATG5, DjATG7, DjATG8 and DjATG12 (> 35%), while the rest falls into the latter group (Table1).
ATG protein sequences ofHomo sapiens (H. sapiens), Mus musculus (M. musculus), Drosophila melanogaster (D. melanogaster), Caenorhabditis elegans (C. elegans) and Saccharomyces cerevisiae (S. cerevisiae) were collected and aligned with those of D. japonica. Phylogenetically, some gene families were highly similar to their homologs in other species (Fig. 1). For instance, ATG5, ATG8 and ATG12 of six species were clustered together, suggesting that they were evolutionally conserved and might have originated from a common ancestor. However, the separation of ATG1, ATG2, ATG9, ATG10 and ATG13 by other ATGs indicated a relatively high variation in protein sequences.
Molecular phylogenetic analysis of ATGs by Maximum Likelihood. The evolutionary tree is presented to compare each subgroup with family members present in other species. Bootstrap analysis was performed with 1000 replicates. Evolutionary analyses were conducted in MEGA-X. The proteins were analyzed as intact sequences. The analysis involved genes from D. japonica (Dj), S. cerevisiae (Sc), C. elegans (Ce), D. melanogaster (Dm), M. musculus (Mm), and H. sapiens (Hs). The names in red color are the D. japonica ATGs
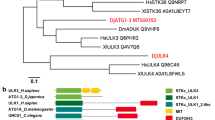
Compared to the single ATG8 gene present in yeast, there are three ATG8 orthologues present in D. japonica. Sequence alignment of ATG8s displayed 20 amino acids with conserved sequences in all proteins (black), indicating a highly conserved primary amino acid sequence (Fig. 2). ATG8-interacting motif (AIM) interacts with two adjacent hydrophobic pockets (HP1 and HP2) of ATG8, with HP1 composed of Glu17, Ile21, Pro30, Ile32, Lys48 and Leu50, and HP2 composed of Tyr49, Val51, Pro52, Leu55, Phe60 and Val63 [79]. Under the interaction of ATG8 and ATG3, Val31, Lys46, Lys48, Tyr49, Leu50, Val51, Val63 and Ile64 play crucial roles. Besides, new evidence has indicated that Arg65, Phe104 and Tyr106 in yeast ATG8 contribute to the conjugation of ATG8 to PE and the C-terminal glycine [120]. Results suggested that almost all the core amino acids, except Ile32, Tyr49, Leu55, Phe60 and Val63, are necessary for the function of ATG8 were observed in all proteins. Notably, in D. japonica, a mutant of Val31 was observed. Besides, the 6th and 22nd amino acids in DjATG8-2 in D. japonica are glutamine and lysine respectively; but in other proteins, they are lysine and arginine.
Genomic DNA of Hydra Vulgaris encodes six ATGs from HvATG4 and HvATG8 gene families, and others encoded by a single gene. HvATGs were composed of 118 (HvGABARAP and HvGABARAPL2) to 1296 amino acids (HvATG2), with corresponding molecular weights from 13.7 kDa (HvGABARAPL2) to 145.5 kDa (HvATG2), pI from 4.79 (HvATG3) to 9.45 (HvLC3C), and gravity from -0.672 (HvBCEN1) to -0.082 (HvATG9). Based on sequence alignment, most HvATGs, including HvATG4, HvATG5, HvBECN1, HvATG9, HvATG10, HvATG12 and HvATGATG16L1, were highly similar to those of mammals, while HvATG3, HvATG7, HvATG8s and HvATG101 share high identity with other species (> 35%) (Table 1).
Molecular phylogenetic analysis of ATG proteins revealed that most HvATGs, except HvATG13 and HvATG14, were highly similar to their homologs in other species, indicating that ATGs in H. vulgaris, H. sapiens, M. musculus, D. melanogaster, C. elegans and S. cerevisiae had a common ancestor (Fig. 3). The sequence alignment of HvATG8s with other species indicated that HvATG8s had highly conserved primary amino acid sequences. Sequence alignment of ATG8s displayed 19 amino acids with conserved sequences in all proteins (black), including the core amino acids described above. Interestingly, the 40th amino acid in HvATG8is valine, while in other ATG8s, it is iso-leucine. The ubiquitin-like domain, a signature structure in each ATG8, was composed of 103–115 amino acids, as shown in Table 2 (Fig. 4).
Phylogenetic analysis of ATG family proteins by Maximum Likelihood. The evolutionary tree is presented to compare each subgroup with family members present in other species. Bootstrap analysis was performed with 1000 replicates. Evolutionary analyses were conducted in MEGA-X. The proteins were analyzed as intact sequences. Phylogenetic relationships of ATGs from H. vulgaris (Hv), S. cerevisiae (Sc), C. elegans (Ce), D. melanogaster (Dm), M. musculus (Mm), and H. sapiens (Hs). The names in red color are the H. vulgaris ATGs
DjATG8 family contributes to tissue remodeling after amputation
A number of evidences have suggested the impact of autophagy during regeneration. For instance, induced autophagy in mice can increase microtubule stability through the degradation of SCG10, an MT-destabilization protein, thus promoting axon regeneration after injury [121]. A recent study showed that in a hypomorphic ATG16L1 mouse with autophagy attenuated but still present, the recovery of skeletal muscle following cardiotoxin mediated damage was slower [122]. Autophagy also plays an important role in maintaining the proliferation of intestinal stem cells of fruit fly during aging and regeneration [123].
Using Planarians as an in vivo autophagy model, many studies carry on their experiments on the animal for remarkable plasticity and regenerating process. A study on D. japonica showed that DjATG8-2 (a homolog of Schistosoma haematobium GABARAPL2) and DjATG8-3 (a homolog of yeast ATG8) are involved in the tissue remodeling of planarians during regeneration [20]. Both DjATG8 proteins contain conserved ATG8 domains and three conserved amino acid residues (Arg65, Phe104 and Tyr106), which are essential for the conjugation of ATG8 to PE and C-terminal glycine; DjATG8-3 has similar structures in yeast ATG8 protein, with AIM peptide sites buried in two distinct pockets (W and L). The formation of autophagosomes is inhibited when expression levels of DjATG8-2and DjATG8-3 are down-regulated by RNAi. Then, both DjATG8-2and DjATG8-3 are expressed in blastema by WISH. During regeneration, up-regulation of expression levels of DjATG8-2 and DjATG8-3 is observed. However, the regeneration will be slowed down due to RNA interference of DjATG8-2 or DjATG8-3,and the loss of DjATG8-3will induce death after amputation and karyolysis in nucleus of planarian. In conclusion, the study of Kang et al. indicated that DjATG8-2 and DjATG8-3 play an essential role in the tissue remodeling of planarians during regeneration.
mTOR signaling pathway associated autophagy in remodeling and regeneration
Mechanistic target-of-rapamycin (mTOR), a serine/threonine kinase, involves two functional complexes: mTORC1 and mTORC2. mTORC1, as a central regulator in cell metabolism and proliferation, is composed of mTOR catalytic subunit, Raptor, mLST8 and two inhibitory subunits (PRAS40 and DEPTOR) [27]. FKBP12-rapamycin complex binds to FKBP12-rapamycin-binding (FRB) domain, inhibiting the kinase activity of mTOR [124]. Tuberous sclerosis (TSC) tumor suppressor complex (TSC1/TSC2) indirectly inhibits mTORC1 activity by negatively regulating the activity of Rheb via the GTPase-activating protein (GAP) activity of TSC2 [125]. Activation of growth factor/PI3K/AKT signaling pathway, ERK1/2, and p90 ribosomal S6 kinase (RSK1) can inactivate TSC1/TSC2 complex, leading to the activation of mTOR [126,127,128]. In contrast, AMPK phosphorylates TSC2, resulting in the inhibition of mTORC1 activity [129].
In growing cells, autophagy is negatively regulated by high mTORC1 activity rather thanmTORC2. For instance, mTORC1 inhibits autophagy through direct phosphorylation ULK1 at the Ser758 site to prevent the interaction between ULK1 and AMPK, which is crucial for ULK1 activation [130]. mTORC1 can also prevent the formation of autophagosome through phosphorylation of ATG14L in VPS34 complex [131]. The prevention of nuclear translocation of transcription factor E3 (TFE3) and microphthalmia-associated transcription factor (MITF) by mTOR1 can provide an autophagy inhibition mechanism at the transcriptional level [132, 133]. Besides, accumulating evidence suggests that autophagy can also be regulated by acetylation. Wan et al. found that the phosphorylation of histone acetyl-transferase (HAT) p300 by mTOR leads to suppression of starvation-induced autophagy [134].
More studies have shown that mTOR is one of the critical regulatory signaling pathways of tissue regeneration in vertebrates and invertebrates. In mammalian cells, mTOR plays a different or even opposing role in diverse neuronal injury models. It’s reported that the mTOR signaling pathway differently regulates central and peripheral axon regeneration in mice [135]. Inhibition of mTOR by rapamycin dramatically can diminish the axon regeneration from embryonic cortical neurons. In contrast, mTOR is not required for adult DRG axonal regenerative ability. However, injury-induced neuronal mTOR activity boosts Stat3 signaling in PNS neurons, contributing to axon regeneration [136]. Moreover, the treatment of injured sciatic nerve of a rat with rapamycin, in which autophagy is induced by inhibiting the activation of mTOR, promotes the nerve regeneration and rebuilds the motor function [137]. Additionally, the overexpression of mutant HDAC5AA in rats can result in an increase in HDAC5 cytoplasmic localization and activate the mTOR pathway, thus enhancing the regeneration ability of RGCs after optic nerve injury [138]. mTOR is also an important regulator for muscle regeneration. Peroxisome proliferator activated receptors γ (PPARγ) can be stimulated with nutmeg, which may be involved in myogenesis process of cardiac muscle. In aging rats, treatment with nutmeg may induce AKT-mTOR-autophagy pathway, thus increasing the muscle mass [139].
In D. melanogaster, TOR is required for the proliferation, growth and survival of germline stem cells (GSCs). When exposed to ionizing radiation, foxo paused the cell cycle of the damaged stem cells. TOR was able to overcome the action of foxo, and the stem cells resumed dividing and regenerating the damaged tissue [140]. What’s more, TOR activation in D. melanogaster intestinal stem cells (ISCs) is required for the rapid activation of ISC proliferation in response to a challenge [141].
Rapamycin that acts as a negative regulator of mTOR, efficiently induces autophagy in both intact and regenerating hydra. The transiently excessive autophagy might delay the early phase of head regeneration. During head regeneration, mTOR expression remains constant in the early phase of regeneration, progressively decreases in the early-late phase of regeneration and is finally dramatically up-regulated in the late phase of regeneration. It suggests that autophagy might participate in head regeneration at the early and early-late stages when mTOR is low, but inhibited at the late stage of regeneration [19]. A special hydra species named H. oligactis (Ho) undergoes aging when the temperature drops to 10 °C. Induction of an efficient autophagy is able to rescue epithelial cell cycling. However, in aging animals, rapamycin treatment restores epithelial proliferation but does not rescue the autophagy flux, suggesting that the positive effects are regulated by a distinct mechanism [142].
The role of mTOR signaling pathway in regeneration has also been identified in planarians. In Schmidtea mediterranea (S. mediterranea), inhibition of mTOR with RNA interference disrupts the behavior of neoblasts at the systemic level and severely restricts cell proliferation [143]. Emerging evidence has shown that mTOR signaling acts antagonistically with Smed-smg-1 (a homolog of PIKK). Smed-smg-1 (RNAi) results in a hyper-responsiveness to injury. Regenerative blastemas remain undifferentiated leading to lethal ectopic outgrowth. Loss of mTORC1 (Smed-tor RNAi or Smed-raptor RNAi) is capable of reversing the effects of Smed-smg-1 (RNAi) by decreasing proliferation [144]. Rapamycin treatment can also prevent the tissue homeostasis and regeneration defects observed in Smed-PTENRNAi worms [145]. Besides, mTOR down-regulation leads to elongation of telomeres in planarian stem cells [146].
mTOR is reported to be involved in the regulation of regeneration in D. japonica, which is consistent with its role in S. mediterranea [147]. During regeneration, the expression level of DjTOR in posterior blastemas (PBs) surrounding the wound is up-regulated. Notably, the inhibition of DjTORwill lead to asymmetric blastemas and remarkable reduction growth, while rapamycin can successfully inhibit DjTORand induce autophagyin D. japonica. Therefore, worms treated with rapamycin displayed asymmetric blastemas and neuronal defects. In conclusion, DjTOR is involved in the regulation of regeneration in D. japonica.
Bloodstream infection and autophagy via leech
Leeches are well-known for their blood-feeding habits and their extensive use in many human diseases. In relief of venous congestion and plastic and reconstructive surgery [148, 149], the efficient lysis and catabolism of blood can provide an abundance of nutrients for leeches. However, the degradation of hemoglobin, the most abundant protein in vertebrate blood, results in the generation of amino acids and heme, which may be toxic or even lethal [150, 151]. For example, under laboratory breeding conditions, signs of death of cells or even organisms given blood meals were observed [152, 153]. In order to maintain homeostasis, several mechanisms have been developed to neutralize toxic molecules in blood-feeding animals [154, 155]. It is reported that in Ae. Aegyptigiven blood meals, expression level of autophagy-related genes significantly increases [156]. Autophagy has also been shown to be a survival factor and involved in protecting epithelial cells from the toxic molecules caused by blood degradation in leeches [153].
In the previous studies, numerous vesicles with an electron-dense content in cytoplasm of midgut cells in Piscicola geometra were observed. They were originally described to be involved in the enzyme accumulation [157]. However, further study showed that the electron-dense content is formed by residual bodies of autolysosomes [153]. It was observed that autophagy occurred in all regions of digestive system (esophagus, crop, posterior crop caecum, and intestine) in adult non-feeding and feeding specimens. During autophagy, the autophagosomes engulfing the damaged organelles fused with lysosomes to form autolysosomes. Then cell membrane was disrupted by the accumulation of autophagosomes, autolysosomes or residual bodies, releasing autophagosomes, autolysosomes or residual bodies into midgut lumen. In digestive cells, autophagy occurred only in about 10–30% of cells before blood feeding, and was significantly up-regulated during and after bloodfeeding, compared with juvenile and non-feeding specimens, in which the process was absent. This suggests that autophagy is involved in the neutralization of toxic molecules caused by blood digestion in midgut epithelium of adult leeches.
Conclusion
The identification ofautophagic process and a number of orthologs of ATGs in planarian, hydra and leech suggest that autophagy is evolutionarily conserved from yeast to mammals. Phylogenetical analysis of ATG proteins suggests that ATG proteins involved in ATG8 and ATG12 ubiquitin-like conjugation systems share high identity with their homologs, indicating that they might originate from a common ancestor. Distant homologs of ATG proteins were also found in both planarian and hydra, suggesting that they might have different functions. Notably, compared to D. melanogaster, C. elegans and S. cerevisiae, HvATGs show a higher identity with H. sapiens and M. musculus, suggesting that hydra can be used as a powerful model for uncovering the role of autophagy in human diseases.
Understanding the mechanisms of regenerative process has a clinical interest due to its effectiveness in many treatments for tissue repair and age-related diseases. Autophagy is strongly activated not only in starving planarians and hydras but also during regeneration. In leeches, autophagy is involved in the neutralization of toxic molecules caused by blood digestion. The results discussed above suggest that autophagy also plays a role in these three organisms when it can contribute to the regeneration and maintenance of cellular homeostasis. However, the control mechanisms of autophagy remain unclear, and the analysis of the relationship between autophagy and regeneration will provide a more comprehensive view of therapeutic strategies for human diseases.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AA:
-
Amino acids
- AIM:
-
Autophagy interacting motifs
- AMPK:
-
AMP-activated protein kinase
- ATG:
-
Autophagy-related gene
- CDK 5:
-
Cyclin Dependent Kinase 5
- CTD:
-
C-terminal domain
- CTX:
-
Cardiotoxin
- DRG:
-
Dorsal root ganglion
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular regulated protein kinases
- FIP200:
-
Focal adhesion kinase (FAK) family interacting protein of 200 kDa
- FRB:
-
FKBP12-rapamycin binding
- GABARAP:
-
γ‐Aminobutyric acid receptor‐associated protein
- GATE-16:
-
Golgi-associated ATPase enhancer of 16 kDa
- HAT:
-
Histone acetyltransferase
- LC3:
-
Microtubule‐associated protein light chain three
- LIR:
-
LC3-interacting region
- MITF:
-
Microphthalmia-associated transcription factor
- Mm:
-
Mus musculus
- mTORC:
-
Mechanistic target of rapamycin complex
- Mw:
-
Molecular weight
- NTD:
-
N-terminal domain
- PE:
-
Phosphatidylethanolamine
- PNS:
-
Peripheral nervous system
- PPARγ:
-
Peroxisome proliferator activated receptors γ
- PtdIns3K:
-
Phosphatidylinositol 3-kinase
- RSK1:
-
Ribosomal S6 kinase
- TFE3:
-
Transcription factor E3
- mTOR:
-
Mammalian target of rapamycin
- TSC:
-
Tuberous sclerosis
- ULK:
-
Unc-51-like kinase
- VPS:
-
Vacuolar protein sorting
- WD:
-
Tryptophan-aspartate
References
Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460–73. https://doi.org/10.1089/ars.2013.5371.
Morishita H, Mizushima N. Diverse cellular roles of autophagy. Annu Rev Cell Dev Biol. 2019;35:453–75. https://doi.org/10.1146/annurev-cellbio-100818-125300.
Xie Z, Klionsky DJ. Autophagosome formation core machinery and adaptations. Nat Cell Biol. 2007;9(10):1102–9. https://doi.org/10.1038/ncb1007-1102.
Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221(1):3–12. https://doi.org/10.1002/path.2697.
Daskalaki I, Gkikas I, Tavernarakis N. Hypoxia and selective autophagy in cancer development and therapy. Front Cell Dev Biol. 2018;6:104. https://doi.org/10.3389/fcell.2018.00104.
Monkkonen T, Debnath J. Inflammatory signaling cascades and autophagy in cancer. Autophagy. 2018;14(2):190–8. https://doi.org/10.1080/15548627.2017.1345412.
Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res. 2014;24(1):42–57. https://doi.org/10.1038/cr.2013.166.
Li X, He S, Ma B. Autophagy and autophagy-related proteins in cancer. Mol Cancer. 2020;19(1):12. https://doi.org/10.1186/s12943-020-1138-4.
Cao Q, Bai P. Role of autophagy in renal cancer. J Cancer. 2019;10(11):2501–9. https://doi.org/10.7150/jca.29285.
Nilsson P, Loganathan K, Sekiguchi M, et al. αβ secretion and plaque formation depend on autophagy. Cell Rep. 2013;5(1):61–9. https://doi.org/10.1016/j.celrep.2013.08.042.
Uddin MS, Stachowiak A, Mamun AA, Tzvetkov NT, Takeda S, Atanasov AG, Bergantin LB, Abdel-Daim MM, Stankiewicz AM. Autophagy and Alzheimer’s disease: from molecular mechanisms to therapeutic implications. Front Aging Neurosci. 2018;10:04. https://doi.org/10.3389/fnagi.2018.00004.
García-Prat L, Martínez-Vicente M, Perdiguero E, Ortet L, Rodríguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Muñoz-Cánoves P. Autophagy maintains stemness by preventing senescence. Nature. 2016;529(7584):37–42. https://doi.org/10.1038/nature16187.
Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, Passegué E. Autophagy maintains the metabolism and function of young and old stem cells. Nature. 2017;543(7644):205–10. https://doi.org/10.1038/nature21388.
Yuan J, Wang Z, Zou D, Peng Q, Peng R, Zou F. Expression profiling of planarians shed light on a dual role of programmed cell death during the regeneration. J Cell Biochem. 2018;119(7):5875–84. https://doi.org/10.1002/jcb.26779.
Zeng A, Li H, Guo L, Gao X, McKinney S, Wang Y, Yu Z, Park J, Semerad C, Ross E, Cheng LC, Davies E, Lei K, Wang W, Perera A, Hall K, Peak A, Box A, Sánchez AA. Prospectively isolated tetraspanin(+) neoblasts are adult pluripotent stem cells underlying Planaria regeneration. Cell. 2018;173(7):1593–608. https://doi.org/10.1016/j.cell.2018.05.006.
Galliot B. Hydra, a fruitful model system for 270 years. Int J Dev Biol. 2012;56(6–8):411–23. https://doi.org/10.1387/ijdb.120086bg.
Grimaldi A, Banfi S, Bianchi C, Gabriella G, Tettamanti G, Noonan DM, Valvassori R, de Eguileor M. The leech: a novel invertebrate model for studying muscle regeneration and diseases. Curr Pharm Des. 2010;16(8):968–77. https://doi.org/10.2174/138161210790883417.
González-Estévez C, Felix DA, Aboobaker AA, Saló E. Gtdap-1 promotes autophagy and is required for planarian remodeling during regeneration and starvation. Proc Natl Acad Sci USA. 2007;104(33):13373–8. https://doi.org/10.1073/pnas.0703588104.
Chera S, Buzgariu W, Ghila L, Galliot B. Autophagy in Hydra: a response to starvation and stress in early animal evolution. Biochim Biophys Acta. 2009;1793(9):1432–43. https://doi.org/10.1016/j.bbamcr.2009.03.010.
Kang J, Dong Z, Wang J, Chen G, Liu D. Autophagy-related Djatg8 is required for remodeling in planarians Dugesia japonica. Biol Open. 2019;8(12):bio045013. https://doi.org/10.1242/bio.045013.
Chera S, de Rosa R, Miljkovic-Licina M, Dobretz K, Ghila L, Kaloulis K, Galliot B. Silencing of the hydra serine protease inhibitor Kazal1 gene mimics the human SPINK1 pancreatic phenotype. J Cell Sci. 2006;119(Pt 5):846–57. https://doi.org/10.1242/jcs.02807.
González-Estévez C. Autophagy in freshwater planarians. Methods Enzymol. 2008;451:439–65. https://doi.org/10.1016/s0076-6879(08)03227-8.
Gonzalez-Estevez C. Autophagy meets planarians. Autophagy. 2009;5(3):290–7. https://doi.org/10.4161/auto.5.3.7665.
Matsuura A, Tsukada M, Wada Y, Ohsumi Y. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene. 1997;192(2):245–50. https://doi.org/10.1016/s0378-1119(97)00084-x.
Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90(4):1383–435. https://doi.org/10.1152/physrev.00030.2009.
Yang Z, Klionsky DJ. Mammalian autophagy core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22(2):124–31. https://doi.org/10.1016/j.ceb.2009.11.014.
Pan L, Liu J, Li Y. Structural basis of autophagy regulatory proteins. Adv Exp Med Biol. 2019;1206:287–32626. https://doi.org/10.1007/978-981-15-0602-4_15.
Lazarus MB, Novotny CJ, Shokat KM. Structure of the human autophagy initiating kinase ULK1 in complex with potent inhibitors. ACS Chem Biol. 2015;10(1):257–61. https://doi.org/10.1021/cb500835z.
Hurley JH, Young LN. Mechanisms of autophagy initiation. Annu Rev Biochem. 2017;86:225–44. https://doi.org/10.1146/annurev-biochem-061516-044820.
Mercer TJ, Gubas A, Tooze SA. A molecular perspective of mammalian autophagosome biogenesis. J Biol Chem. 2018;293(15):5386–95. https://doi.org/10.1074/jbc.R117.810366.
Alemu EA, Lamark T, Torgersen KM, Birgisdottir AB, Larsen KB, Jain A, et al. ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region (LIR) motifs. J Biol Chem. 2012;287(47):39275–90. https://doi.org/10.1074/jbc.M112.378109.
Kim BW, Jin Y, Kim J, Kim JH, Jung J, Kang S, et al. The C-terminal region of ATG101 bridges ULK1 and PtdIns3K complex in autophagy initiation. Autophagy. 2018;14(12):2104–16. https://doi.org/10.1080/15548627.2018.1504716.
Wallot-Hieke N, Verma N, Schlütermann D, Berleth N, Deitersen J, Böhler P, et al. Systematic analysis of ATG13 domain requirements for autophagy induction. Autophagy. 2018;14(5):743–63. https://doi.org/10.1080/15548627.2017.1387342.
Michel M, Schwarten M, Decker C, Nagel-Steger L, Willbold D, Weiergräber OH. The mammalian autophagy initiator complex contains two HORMA domain proteins. Autophagy. 2015;11(12):2300–8. https://doi.org/10.1080/15548627.2015.1076605.
Hosokawa N, Sasaki T, Iemura S, Natsume T, Hara T, Mizushima N. Atg101, a novel mammalian autophagy protein interacting with Atg13. Autophagy. 2009;5(7):973–9. https://doi.org/10.4161/auto.5.7.9296.
Mercer CA, Kaliappan A, Dennis PB. A novel, human Atg13 binding protein, Atg101, interacts with ULK1 and is essential for macroautophagy. Autophagy. 2009;5(5):649–62. https://doi.org/10.4161/auto.5.5.8249.
Suzuki H, Kaizuka T, Mizushima N, Noda NN. Structure of the Atg101–Atg13 complex reveals essential roles of Atg101 in autophagy initiation. Nat Struct Mol Biol. 2015;22(7):572–80. https://doi.org/10.1038/nsmb.3036.
Rostislavleva K, Soler N, Ohashi Y, Zhang L, Pardon E, Burke JE, et al. Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science. 2015;350(6257):aac7365. https://doi.org/10.1126/science.aac7365.
Menon MB, Dhamija S. Beclin 1 Phosphorylation—at the Center of Autophagy Regulation. Front Cell Dev Biol. 2018;6:137. https://doi.org/10.3389/fcell.2018.00137.
Matsunaga K, Saitoh T, Tabata K, Omori H, Satoh T, Kurotori N, et al. Two Beclin 1-binding proteins, Atg14L and Rubicon, reciprocally regulate autophagy at different stages. Nat Cell Biol. 2009;11(4):385–96. https://doi.org/10.1038/ncb1846.
Ma M, Liu JJ, Li Y, Huang Y, Ta N, Chen Y, et al. Cryo-EM structure and biochemical analysis reveal the basis of the functional difference between human PI3KC3-C1 and -C2. Cell Res. 2017;27(8):989–1001. https://doi.org/10.1038/cr.2017.94.
Shpilka T, Weidberg H, Pietrokovski S, Elazar Z. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol. 2011;12(7):226–36. https://doi.org/10.1186/gb-2011-12-7-226.
Lee YK, Lee JA. Role of the mammalian ATG8/LC3 family in autophagy: differential and compensatory roles in the spatiotemporal regulation of autophagy. BMB Rep. 2016;49(8):424–30. https://doi.org/10.5483/bmbrep.2016.49.8.081.
Noda NN, Ohsumi Y, Inagaki F. ATG systems from the protein structural point of view. Chem Rev. 2009;109(4):1587–98. https://doi.org/10.1021/cr800459r.
Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010;29(11):1792–802. https://doi.org/10.1038/emboj.2010.74.
Fernández ÁF, López-Otín C. The functional and pathologic relevance of autophagy proteases. J Clin Invest. 2015;125(1):33–41. https://doi.org/10.1172/JCI73940.
Kaiser SE, Mao K, Taherbhoy AM, Yu S, Olszewski JL, Duda DM, et al. Noncanonical E2 recruitment by the autophagy E1 revealed by Atg7–Atg3 and Atg7–Atg10 structures. Nat Struct Mol Biol. 2012;19(12):1242–9. https://doi.org/10.1038/nsmb.2415.
Otomo C, Metlagel Z, Takaesu G, Otomo T. Structure of the human ATG12~ATG5 conjugate required for LC3 lipidation in autophagy. Nat Struct Mol Biol. 2013;20(1):59–66. https://doi.org/10.1038/nsmb.2431.
Orsi A, Razi M, Dooley HC, Robinson D, Weston AE, Collinson LM, et al. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. Mol Biol Cell. 2012;23(10):1860–73. https://doi.org/10.1091/mbc.E11-09-0746.
Lamb CA, Nühlen S, Judith D, Frith D, Snijders AP, Behrends C, et al. TBC1D14 regulates autophagy via the TRAPP complex and ATG9 traffic. EMBO J. 2016;35(3):281–301. https://doi.org/10.15252/embj.201592695.
Li W, Zhang L. Regulation of ATG and autophagy initiation. Adv Exp Med Biol. 2019;1206:41–65. https://doi.org/10.1007/978-981-15-0602-4_2.
Tettamanti G, Saló E, González-Estévez C, Felix DA, Grimaldi A, de Eguileor M. Autophagy in invertebrates: insights into development, regeneration and body remodeling. Curr Pharm Des. 2008;14(2):116–25. https://doi.org/10.2174/138161208783378716.
Dixit NS, Shravage BV, Ghaskadbi S. Identification and characterization of the autophagy-related genes Atg12 and Atg5 in hydra. Int J Dev Biol. 2017;61(6–7):389–95. https://doi.org/10.1387/ijdb.160461sg.
Ma K, Zhang Y, Song G, Wu M, Chen G. Identification of autophagy-related gene 7 and autophagic cell death in the planarian Dugesia japonica. Front Physiol. 2018;9:1223. https://doi.org/10.3389/fphys.2018.01223.
Chan EY, Longatti A, McKnight NC, Tooze SA. Kinase-inactivated ULK proteins inhibit autophagy via their conserved C-terminal domains using an Atg13-independent mechanism. Mol Cell Biol. 2009;29(1):157–71. https://doi.org/10.1128/MCB.01082-08.
Petherick KJ, Conway OJ, Mpamhanga C, Osborne SA, Kamal A, Saxty B, et al. Pharmacological inhibition of ULK1 kinase blocks mammalian target of rapamycin (mTOR)-dependent autophagy. J Biol Chem. 2015;290(18):11376–83. https://doi.org/10.1074/jbc.C114.627778.
Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, et al. Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol Cell. 2015;59(2):285–97. https://doi.org/10.1016/j.molcel.2015.05.031.
Puente C, Hendrickson RC, Jiang X. Nutrient-regulated phosphorylation of ATG13 inhibits starvation-induced autophagy. J Biol Chem. 2016;291(11):6026–35. https://doi.org/10.1074/jbc.M115.689646.
Joo JH, Dorsey FC, Joshi A, Hennessy-Walters KM, Rose KL, McCastlain K, et al. Hsp90-Cdc37 chaperone complex regulates Ulk1- and Atg13-mediated mitophagy. Mol Cell. 2011;43(4):572–85. https://doi.org/10.1016/j.molcel.2011.06.018.
Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, et al. Induction of autophagy and inhibition of tumorigenesis by Beclin 1. Nature. 1999;402(6762):672–6. https://doi.org/10.1038/45257.
Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2008;105(49):19211–6. https://doi.org/10.1073/pnas.0810452105.
Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15(7):741–50. https://doi.org/10.1038/ncb2757.
Fan W, Nassiri A, Zhong Q. Autophagosome targeting and membrane curvature sensing by Barkor/Atg14(L). Proc Natl Acad Sci U S A. 2011;108(19):7769–74. https://doi.org/10.1073/pnas.1016472108.
Matsunaga K, Morita E, Saitoh T, Akira S, Ktistakis NT, Izumi T, et al. Autophagy requires endoplasmic reticulum targeting of the PI3-kinase complex via Atg14L. J Cell Biol. 2010;190(4):511–21. https://doi.org/10.1083/jcb.200911141.
Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, et al. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152(1–2):290–303. https://doi.org/10.1016/j.cell.2012.12.016.
Tan X, Thapa N, Liao Y, Choi S, Anderson RA. PtdIns(4,5)P2 signaling regulates ATG14 and autophagy. Proc Natl Acad Sci U S A. 2016;113(39):10896–901. https://doi.org/10.1073/pnas.1523145113.
Mizushima N, Sugita H, Yoshimori T, Ohsumi Y. A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J Biol Chem. 1998;273(51):33889–92. https://doi.org/10.1074/jbc.273.51.33889.
Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD, et al. A protein conjugation system essential for autophagy. Nature. 1998;395(6700):395–8. https://doi.org/10.1038/26506.
Vierstra RD. The expanding universe of ubiquitin and ubiquitin-like modifiers. Plant Physiol. 2012;160(1):2–14. https://doi.org/10.1104/pp.112.200667.
Pang Y, Yamamoto H, Sakamoto H, Oku M, Mutungi JK, Sahani MH, et al. Evolution from covalent conjugation to non-covalent interaction in the ubiquitin-like ATG12 system. Nat Struct Mol Biol. 2019;26(4):289–96. https://doi.org/10.1038/s41594-019-0204-3.
Komatsu M, Tanida I, Ueno T, Ohsumi M, Ohsumi Y, Kominami E. The C-terminal region of an Apg7p/Cvt2p is required for homodimerization and is essential for its E1 activity and E1–E2 complex formation. J Biol Chem. 2001;276(13):9846–54. https://doi.org/10.1074/jbc.M007737200.
Nitta A, Hori K, Tanida I, Igarashi A, Deyama Y, Ueno T, et al. Blocking LC3 lipidation and ATG12 conjugation reactions by ATG7 mutant protein containing C572S. Biochem Biophys Res Commun. 2019;508(2):521–6. https://doi.org/10.1016/j.bbrc.2018.11.158.
Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408(6811):488–92. https://doi.org/10.1038/35044114.
Qiu Y, Zheng Y, Taherbhoy AM, Kaiser SE, Schulman BA. Crystallographic characterization of ATG proteins and their interacting partners. Methods Enzymol. 2017;587:227–46. https://doi.org/10.1016/bs.mie.2016.09.058.
Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20(21):5971–81. https://doi.org/10.1093/emboj/20.21.5971.
Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19(5):2092–100. https://doi.org/10.1091/mbc.E07-12-1257.
Romanov J, Walczak M, Ibiricu I, Schüchner S, Ogris E, Kraft C, et al. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J. 2012;31(22):4304–17. https://doi.org/10.1038/emboj.2012.278.
Hanada T, Satomi Y, Takao T, Ohsumi Y. The amino-terminal region of Atg3 is essential for association with phosphatidylethanolamine in Atg8 lipidation. FEBS Lett. 2009;583(7):1078–83. https://doi.org/10.1016/j.febslet.2009.03.009.
Yamaguchi M, Noda NN, Nakatogawa H, Kumeta H, Ohsumi Y, Inagaki F. Autophagy-related protein 8 (Atg8) family interacting motif in Atg3 mediates the Atg3–Atg8 interaction and is crucial for the cytoplasm-to-vacuole targeting pathway. J Biol Chem. 2010;285(38):29599–607. https://doi.org/10.1074/jbc.M110.113670.
Ngu M, Hirata E, Suzuki K. Visualization of Atg3 during autophagosome formation in Saccharomyces cerevisiae. J Biol Chem. 2015;290(13):8146–53. https://doi.org/10.1074/jbc.M114.626952.
Sakoh-Nakatogawa M, Kirisako H, Nakatogawa H, Ohsumi Y. Localization of Atg3 to autophagy-related membranes and its enhancement by the Atg8-family interacting motif to promote expansion of the membranes. FEBS Lett. 2015;589(6):744–9. https://doi.org/10.1016/j.febslet.2015.02.003.
Li YT, Yi C, Chen CC, Lan H, Pan M, Zhang SJ, et al. A semisynthetic Atg3 reveals that acetylation promotes Atg3 membrane binding and Atg8 lipidation. Nat Commun. 2017;8:14846. https://doi.org/10.1038/ncomms14846.
Liu S, Zhang F, Wang Y, Wang H, Chen X, Hu Y, et al. Characterization of the molecular mechanism of the autophagy-related Atg8–Atg3 protein interaction in Toxoplasma gondii. J Biol Chem. 2018;293(37):14545–566. https://doi.org/10.1074/jbc.RA118.002614.
Qiu Y, Zheng Y, Grace CRR, Liu X, Klionsky DJ, Schulman BA. Allosteric regulation through a switch element in the autophagy E2, Atg3. Autophagy. 2020;16(1):183–4. https://doi.org/10.1080/15548627.2019.1688550.
Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T, et al. The reversible modification regulates the membrane-binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J Cell Biol. 2000;151(2):263–76. https://doi.org/10.1083/jcb.151.2.263.
Yu ZQ, Ni T, Hong B, Wang HY, Jiang FJ, Zou S, et al. Dual roles of Atg8-PE deconjugation by Atg4 in autophagy. Autophagy. 2012;8(6):883–92. https://doi.org/10.4161/auto.19652.
Ryabovol VV, Minibayeva FV. Autophagic proteins ATG4 and ATG8 in wheat: Structural characteristics and their role under stress conditions. Dokl Biochem Biophys. 2014;458:179–81. https://doi.org/10.1134/s1607672914050056.
Seo E, Woo J, Park E, Bertolani SJ, Siegel JB, Choi D, et al. Comparative analyses of ubiquitin-like ATG8 and cysteine protease ATG4 autophagy genes in the plant lineage and cross-kingdom processing of ATG8 by ATG4. Autophagy. 2016;12(11):2054–68. https://doi.org/10.1080/15548627.2016.1217373.
Hirata E, Ohya Y, Suzuki K. Atg4 plays an important role in efficient expansion of autophagic isolation membranes by cleaving lipidated Atg8 in Saccharomyces cerevisiae. PLoS ONE. 2017;12(7):e0181047. https://doi.org/10.1371/journal.pone.0181047.
Sánchez-Wandelmer J, Reggiori F. Atg4 in autophagosome biogenesis. Oncotarget. 2017;8(65):108290–1. https://doi.org/10.18632/oncotarget.22714.
Maruyama T, Noda NN. Autophagy-regulating protease Atg4: structure, function, regulation and inhibition. J Antibiot. 2017;71:72. https://doi.org/10.1038/ja.2017.104.
Kauffman KJ, Yu S, Jin J, Mugo B, Nguyen N, O’Brien A, et al. Delipidation of mammalian Atg8-family proteins by each of the four ATG4 proteases. Autophagy. 2018;14(6):992–1010. https://doi.org/10.1080/15548627.2018.1437341.
Noda NN, Satoo K, Fujioka Y, Kumeta H, Ogura K, Nakatogawa H, et al. Structural basis of Atg8 activation by a homodimeric E1, Atg7. Mol Cell. 2011;44(3):462–75. https://doi.org/10.1016/j.molcel.2011.08.035.
Kaiser SE, Qiu Y, Coats JE, Mao K, Klionsky DJ, Schulman BA. Structures of Atg7–Atg3 and Atg7–Atg10 reveal noncanonical mechanisms of E2 recruitment by the autophagy E1. Autophagy. 2013;9(5):778–80. https://doi.org/10.4161/auto.23644.
Yamaguchi M, Satoo K, Suzuki H, Fujioka Y, Ohsumi Y, Inagaki F, et al. Atg7 Activates an autophagy-essential ubiquitin-like protein Atg8 through multi-step recognition. J Mol Biol. 2018;430(3):249–57. https://doi.org/10.1016/j.jmb.2017.12.002.
Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, et al. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999;147(2):435–46. https://doi.org/10.1083/jcb.147.2.435.
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–8. https://doi.org/10.1093/emboj/19.21.5720.
Slobodkin MR, Elazar Z. The Atg8 family: multifunctional ubiquitin-like key regulators of autophagy. Essays Biochem. 2013;55:51–64. https://doi.org/10.1042/bse0550051.
Schaaf MB, Keulers TG, Vooijs MA, Rouschop KM. LC3/GABARAP family proteins: autophagy-(un)related functions. FASEB J. 2016;30(12):3961–78. https://doi.org/10.1096/fj.201600698R.
Lystad AH, Simonsen A. Mechanisms and pathophysiological roles of the ATG8 conjugation machinery. Cells. 2019;8(9):E973. https://doi.org/10.3390/cells8090973.
Johansen T, Lamark T. Selective Autophagy: ATG8 Family Proteins, LIR Motifs and Cargo Receptors. J Mol Biol. 2020;432(1):80–103. https://doi.org/10.1016/j.jmb.2019.07.016.
Wang CW, Kim J, Huang WP, Abeliovich H, Stromhaug PE, Dunn WA, et al. Apg2 is a novel protein required for the cytoplasm to vacuole targeting, autophagy, and pexophagy pathways. J Biol Chem. 2001;276(32):30442–51. https://doi.org/10.1074/jbc.M102342200.
Obara K, Sekito T, Niimi K, Ohsumi Y. The Atg18–Atg2 complex is recruited to autophagic membranes via phosphatidylinositol 3-phosphate and exerts an essential function. J Biol Chem. 2008;283(35):23972–80. https://doi.org/10.1074/jbc.M803180200.
Velikkakath AK, Nishimura T, Oita E, Ishihara N, Mizushima N. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol Biol Cell. 2012;23(5):896–909. https://doi.org/10.1091/mbc.E11-09-0785.
Kotani T, Kirisako H, Koizumi M, Ohsumi Y, Nakatogawa H. The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc Natl Acad Sci USA. 2018;115(41):10363–8. https://doi.org/10.1073/pnas.1806727115.
Gómez-Sánchez R, Rose J, Guimarães R, Mari M, Papinski D, Rieter E, et al. Atg9 establishes Atg2-dependent contact sites between the endoplasmic reticulum and phagophores. J Cell Biol. 2018;217(8):2743–63. https://doi.org/10.1083/jcb.201710116.
Osawa T, Kotani T, Kawaoka T, Hirata E, Suzuki K, Nakatogawa H, et al. Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nat Struct Mol Biol. 2019;26(4):281–8. https://doi.org/10.1038/s41594-019-0203-4.
Valverde DP, Yu S, Boggavarapu V, Kumar N, Lees JA, Walz T, et al. ATG2 transports lipids to promote autophagosome biogenesis. J Cell Biol. 2019;218(6):1787–98. https://doi.org/10.1083/jcb.201811139.
Osawa T, Noda NN. Atg2: A novel phospholipid transfer protein that mediates de novo autophagosome biogenesis. Protein Sci. 2019;28(6):1005–122. https://doi.org/10.1002/pro.3623.
Ktistakis NT. Who plays the ferryman: ATG2 channels lipids into the forming autophagosome. J Cell Biol. 2019;218(6):1767–8. https://doi.org/10.1083/jcb.201904159.
Noda T, Kim J, Huang WP, Baba M, Tokunaga C, Ohsumi Y, et al. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the Cvt and autophagy pathways. J Cell Biol. 2000;148(3):465–80. https://doi.org/10.1083/jcb.148.3.465.
He C, Baba M, Klionsky DJ. Double duty of Atg9 self-association in autophagosome biogenesis. Autophagy. 2009;5(3):385–7. https://doi.org/10.4161/auto.5.3.7699.
Mari M, Griffith J, Rieter E, Krishnappa L, Klionsky DJ, Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 2010;190(6):1005–222. https://doi.org/10.1083/jcb.200912089.
Yamamoto H, Kakuta S, Watanabe TM, Kitamura A, Sekito T, Kondo-Kakuta C, et al. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol. 2012;198(2):219–33. https://doi.org/10.1083/jcb.201202061.
Zavodszky E, Vicinanza M, Rubinsztein DC. Biology and trafficking of ATG9 and ATG16L1, two proteins that regulate autophagosome formation. FEBS Lett. 2013;587(13):1988–96. https://doi.org/10.1016/j.febslet.2013.04.025.
Feng Y, Backues SK, Baba M, Heo JM, Harper JW, Klionsky DJ. Phosphorylation of Atg9 regulates movement to the phagophore assembly site and the rate of autophagosome formation. Autophagy. 2016;12(4):648–58. https://doi.org/10.1080/15548627.2016.1157237.
Feng Y, Klionsky DJ. Autophagic membrane delivery through ATG9. Cell Res. 2017;27(2):161–2. https://doi.org/10.1038/cr.2017.4.
Ungermann C, Reggiori F. Atg9 proteins, not so different after all. Autophagy. 2018;14(8):1456–9. https://doi.org/10.1080/15548627.2018.1477382.
Watanabe Y, Kobayashi T, Yamamoto H, Hoshida H, Akada R, Inagaki F, et al. Structure-based analyses reveal distinct binding sites for Atg2 and phosphoinositides in Atg18. J Biol Chem. 2012;287(38):31681–90. https://doi.org/10.1074/jbc.M112.397570.
Zihui L, Siwei Z, Xiaoteng L, Shulin W. Autophagy-related TtATG8 regulates the lifespan of protozoan tetrahymena. J Fudan Univ. 2015;54(10):781–801.
He M, Ding Y, Chu C, Tang J, Xiao Q, Luo ZG. Autophagy induction stabilizes microtubules and promotes axon regeneration after spinal cord injury. Proc Natl Acad Sci USA. 2016;113(40):11324–9. https://doi.org/10.1073/pnas.1611282113.
Paolini A, Omairi S, Mitchell R, Vaughan D, Matsakas A, Vaiyapuri S, et al. Attenuation of autophagy impacts on muscle fibre development, starvation induced stress and fibre regeneration following acute injury. Sci Rep. 2018;8(1):9062. https://doi.org/10.1038/s41598-018-27429-7.
Nagy P, Sándor GO, Juhász G. Autophagy maintains stem cells and intestinal homeostasis in Drosophila. Sci Rep. 2018;8(1):4644. https://doi.org/10.1038/s41598-018-23065-3.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–76. https://doi.org/10.1016/j.cell.2017.02.004.
Wang Y, Zhang H. Regulation of autophagy by mTOR signaling pathway. Adv Exp Med Biol. 2019;1206:67–83. https://doi.org/10.1007/978-981-15-0602-4_3.
Maiese K. Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural Regen Res. 2016;11(3):372–85. https://doi.org/10.4103/1673-5374.179032.
Cherepkova MY, Sineva GS, Pospelov VA. Leukemia inhibitory factor (LIF) withdrawal activates mTOR signaling pathway in mouse embryonic stem cells through the MEK/ERK/TSC2 pathway. Cell Death Dis. 2016;7:e2050. https://doi.org/10.1038/cddis.2015.387.
Schleich S, Teleman AA. Akt Phosphorylates Both Tsc1 and Tsc2 in Drosophila, but neither phosphorylation is required for normal animal growth. PLoS ONE. 2009;4(7):e6305. https://doi.org/10.1371/journal.pone.0006305.
Chen W, Pan Y, Wang S, Liu Y, Chen G, Zhou L, et al. Cryptotanshinone activates AMPK-TSC2 axis leading to inhibition of mTORC1 signaling in cancer cells. BMC Cancer. 2017;17(1):34. https://doi.org/10.1186/s12885-016-3038-y.
Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–41. https://doi.org/10.1038/ncb2152.
Yuan HX, Russell RC, Guan KL. Regulation of PIK3C3/VPS34 complexes by MTOR in nutrient stress-induced autophagy. Autophagy. 2013;9(12):1983–95. https://doi.org/10.4161/auto.26058.
Martina JA, Diab HI, Lishu L, Jeong-A L, Patange S, Raben N, et al. The nutrient-responsive transcription factor TFE3 promotes autophagy, lysosomal biogenesis, and clearance of cellular debris. Sci Signal. 2014;7(309):ra9. https://doi.org/10.1126/scisignal.2004754.
Ozturk DG, Kocak M, Akcay A, Kinoglu K, Kara E, Buyuk Y, et al. MITF-MIR211 axis is a novel autophagy amplifier system during cellular stress. Autophagy. 2019;15(3):375–90. https://doi.org/10.1080/15548627.2018.1531197.
Wan W, You Z, Xu Y, Zhou L, Guan Z, Peng C, et al. mTORC1 phosphorylates acetyltransferase p300 to regulate autophagy and lipogenesis. Mol Cell. 2017;68(2):323–35. https://doi.org/10.1016/j.molcel.2017.09.020.
Huang Z, Wang W, Ma J, Li B, Chen J, Yang H, et al. mTOR signaling pathway differently regulates central and peripheral axon regeneration. Acta Biochim Biophys Sin. 2017;49(8):689–95. https://doi.org/10.1093/abbs/gmx068.
Chen W, Lu N, Ding Y, Wang Y, Chan LT, Wang X, et al. Rapamycin-Resistant mTOR Activity Is Required for Sensory Axon Regeneration Induced by a Conditioning Lesion. eNeuro. 2017;3(6):ENEURO.0356. https://doi.org/10.1523/ENEURO.0358-16.2016.
Huang HC, Chen L, Zhang HX, Li SF, Liu P, Zhao TY, et al. Autophagy promotes peripheral nerve regeneration and motor recovery following sciatic nerve crush injury in rats. J Mol Neurosci. 2016;58(4):416–23. https://doi.org/10.1007/s12031-015-0672-9.
Pita-Thomas W, Mahar M, Joshi A, Gan D, Cavalli V. HDAC5 promotes optic nerve regeneration by activating the mTOR pathway. Exp Neurol. 2019;317:271–83. https://doi.org/10.1016/j.expneurol.2019.03.011.
Pratiwi YS, Lesmana R, Goenawan H, Sylviana N, Setiawan I, Tarawan VM, et al. Nutmeg extract increases skeletal muscle mass in aging rats partly via IGF1-AKT-mTOR pathway and inhibition of autophagy. Evid Based Complement Alternat Med. 2018;2018:2810840. https://doi.org/10.1155/2018/2810840.
Artoni F, Kreipke RE, Palmeira O, Dixon C, Goldberg Z, Ruohola-Baker H. Loss of foxo rescues stem cell aging in Drosophila germ line. Elife. 2017;6:e27842. https://doi.org/10.7554/eLife.27842.
Haller S, Kapuria S, Riley RR, O’Leary MN, Schreiber KH, Andersen J. mTORC1 activation during repeated regeneration impairs somatic stem cell maintenance. Cell Stem Cell. 2017;21(6):806–18. https://doi.org/10.1016/j.stem.2017.11.008.
Tomczyk S, Schenkelaars Q, Suknovic N, Wenger Y, Ekundayo K, Buzgariu W, et al. Deficient autophagy in epithelial stem cells drives aging in the freshwater cnidarian Hydra. Development. 2020;147(2):236638. https://doi.org/10.1242/dev.177840.
Peiris TH, Weckerle F, Ozamoto E, Ramirez D, Davidian D, García-Ojeda ME, et al. TOR signaling regulates planarian stem cells and controls localized and organismal growth. J Cell Sci. 2012;125(Pt 7):1657–65. https://doi.org/10.1242/jcs.104711.
González-Estévez C, Felix DA, Smith MD, Paps J, Morley SJ, James V, et al. SMG-1 and mTORC1 act antagonistically to regulate response to injury and growth in planarians. PLoS Genet. 2012;8(3):e1002619. https://doi.org/10.1371/journal.pgen.1002619.
Oviedo NJ, Pearson BJ, Levin M, Sánchez AA. Planarian PTEN homologs regulate stem cells and regeneration through TOR signaling. Dis Model Mech. 2008;1(2–3):131–43. https://doi.org/10.1242/dmm.000117.
Iglesias M, Felix DA, Gutierrez-Gutierrez O, De Miguel-Bonet MDM, Sahu S, Fernandez-Varas B, et al. Downregulation of mTOR signaling increases stem cell population telomere length during starvation of immortal planarians. Stem Cell Rep. 2019;13(2):405–18. https://doi.org/10.1016/j.stemcr.2019.06.005.
Kang J, Dong Z, Hao Q, Wang J, Chen G, Liu D. The regulation of rapamycin in planarian Dugesia japonica Ichikawa & Kawakatsu, 1964 regeneration according to TOR signaling pathway. Ecotoxicol Environ Saf. 2019;185:109680. https://doi.org/10.1016/j.ecoenv.2019.109680.
Kvist S, Min GS, Siddall ME. Diversity and selective pressures of anticoagulants in three medicinal leeches (Hirudinida: Hirudinidae, Macrobdellidae). Ecol Evol. 2013;3(4):918–33. https://doi.org/10.1002/ece3.480.
Mumcuoglu KY. Recommendations for the use of leeches in reconstructive plastic surgery. Evid Based Complement Alternat Med. 2014;2014:205929. https://doi.org/10.1155/2014/205929.
Kehrer JP. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149(1):43–50. https://doi.org/10.1016/s0300-483x(00)00231-6.
Toh SQ, Glanfield A, Gobert GN, Jones MK. Heme and blood-feeding parasites: friends or foes? Parasit Vectors. 2010;3(1):108. https://doi.org/10.1186/1756-3305-3-108.
Frolov AK, Litvinenko RA. Basic morphofunctional features of pharmaceutic leech (Hirudo verbana Carena, 1820) tissues in various forms of response after hirudotherapeutic procedures. Annals Parasitol. 2015;61(1):27–35.
Rost-Roszkowska MM, Świątek P, Poprawa I, Rupik W, Swadźba E, Kszuk-Jendrysik M. Ultrastructural analysis of apoptosis and autophagy in the midgut epithelium of Piscicola geometra (Annelida, Hirudinida) after blood feeding. Protoplasma. 2015;252(5):1387–96. https://doi.org/10.1007/s00709-015-0774-9.
Sterkel M, Oliveira JHM, Bottino-Rojas V, Paiva-Silva GO, Oliveira PL. The dose makes the poison: nutritional overload determines the life traits of blood-feeding arthropods. Trends Parasitol. 2017;33(8):633–44. https://doi.org/10.1016/j.pt.2017.04.008.
Graça-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, et al. Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol. 2006;36(4):322–35. https://doi.org/10.1016/j.ibmb.2006.01.009.
Eng MW, van Zuylen MN, Severson DW. Apoptosis-related genes control autophagy and influence DENV-2 infection in the mosquito vector. Aedes aegypti Insect Biochem Mol Biol. 2016;76:70–83. https://doi.org/10.1016/j.ibmb.2016.07.004.
Rost-Roszkowska MM, Swiątek P, Kszuk M, Główczyk K, Bielecki A. Morphology and ultrastructure of the midgut in Piscicola geometra (Annelida, Hirudinea). Protoplasma. 2012;249(4):1037–47. https://doi.org/10.1007/s00709-011-0337-7.
Acknowledgments
Not applicable.
Funding
This work was supported by National Natural Science Foundation of China to ZBS (Grant number: 31970430).
Author information
Authors and Affiliations
Contributions
ZBS conceived the study. QS and HL collected and analyzed all the data. QS prepared the manuscript and all authors edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Song, Q., Liu, H., Zhen, H. et al. Autophagy and its role in regeneration and remodeling within invertebrate. Cell Biosci 10, 111 (2020). https://doi.org/10.1186/s13578-020-00467-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13578-020-00467-3