Abstract
Co-culture of Bacillus coagulans and Candida utilis was firstly investigated in the efficient treatment of Lactobacillus fermentation wastewater (LFW) containing total organic carbon (TOC) of 22.0 g/L and total nitrogen (TN) of 2.4 g/L. The utilization of lactic acid by C. utilis was responsible for the relief of feedback inhibition to promote the growth of B. coagulans. The removal ratio of TOC by B. coagulans and C. utilis was only 9.1% and 22.7%, respectively, which was improved to 49.0% by co-culture. The removal ratio of TN by B. coagulans and C. utilis was merely 6.3% and 12.5%, respectively, which was also promoted to 44.6% by co-culture. Both the high growth of B. coagulans and the efficient removal of TOC and TN from LFW was achieved with the co-culture, which is not previously reported and very important in the production of probiotics with the resource utilization of LFW.
Similar content being viewed by others
Introduction
Lactic acid bacteria (LAB) are widely distributed in nature and are of great importance and value in agriculture, animal husbandry, food, medicine and manufacturing industries (Leroy and De Vuyst 2014). LAB in the animal body carry out a variety of physiological functions such as regulating normal gastrointestinal tract flora, maintaining micro-ecological balance, and inhibiting the growth of intestinal pathogenic bacteria, which improves the body immunity (Jahn et al. 1996). Mattia Pia Arena provided a fullest possible overview of the antiviral and antifungal activities ascribed to probiotic LAB (Arena et al. 2018). Zeng et al. (2017) and Mao et al. (2017) used LAB as a safe and convenient oral delivery of bioactive peptide and protein drugs for the treatment of diabetes. Furthermore, LAB is commonly used in the manufacture of yoghurt, cheese, sauerkraut (Zhao et al. 2016) and other fermented food (Di Cagno et al. 2016). With the increasing need for LAB, both their culturing scale and the production of Lactobacillus fermentation wastewater (LFW) are rapidly growing. LFW is generally rich with organic compounds such as sugars and protein, as well as lactic acid produced by LAB, which causes pollution and eutrophication in natural water bodies when it is directly released into the environment. Unfortunately, the treatment technology of LFW, especially recycling it as a culture media for other probiotic strains, has not been reported in the literature up to now.
As a typical bacterial probiotic strain, Bacillus coagulans can produce lactic acid and has an ability in the sporulation to overcome harsh stresses, which can inhibit the growth of intestinal pathogens and regulate the animal intestinal micro-ecological balance (Riazi et al. 2009, 2012), and promote animal digestion, and improve animal immunity (Grata and Nabrdalk 2012; Kodali and Sen 2008). Choi et al. (2016) reported that the mixed-culture is a viable approach for the economically feasible production of lactic acid due to the potential use of organic waste for B. coagulans as feedstock.
Candida utilis is another typical eukaryotic probiotic strain that can produce edible protein from various wastes, including bamboo wastewater (Li et al. 2009), rice bran (Rajoka et al. 2004), molasses (Lee and Kim 2001) and potato starch wastewater (Gélinas and Barrett 2007). C. utilis can utilize five-carbon, six-carbon sugars and extracellular organic acids, lactic acid (Oliva and Hang 1979), fumarate, and l-malic acid (Saayman et al. 2000), as carbon sources. In addition, as C. utilis cells are rich in vitamin B and proteins, they can act as a nutrient supplement for livestock, to improve the digestibility of the feed, maintain the microbial balance of the gastrointestinal tract, and enhance the immunity of the animals. Although the individual pure culture of B. coagulans or C. utilis has been well investigated (Yadav and Tarafdar 2012), but no information on the co-culture of them has been reported.
Here the co-culture of B. coagulans and C. utilis using LFW as a medium was investigated, which indicated that the utilization of lactic acid by C. utilis was responsible for the relief of feedback inhibition to promote the growth of B. coagulans. The removal ratios of both total organic carbon (TOC) and total nitrogen (TN) from LFW were all much improved in the presence of B. coagulans and C. utilis simultaneously. The aim in this study is to produce probiotics by the efficient resource utilization of nutrients in LFW, which is not mentioned in the literature and play a vital role in environmental science and biotechnology.
Materials and methods
Microbial strains and culture conditions
Bacillus coagulans (ATCC 7050) and C. utilis (ATCC 3052) were bought from Institute of Microbiology, Chinese Academy of Sciences. LFW (Hebei Yiran Biotechnology Co., Ltd.) of Bifidobacterium bifidum was centrifuged (10,000×g for 10 min), filtered (0.22 μ) and sterilized at 121 °C for 20 min, and the initial pH was adjusted to 7.2 by using 40 g/L NaOH solution. B. coagulans grown on beef peptone medium (Chandra et al. 2007) and C. utilis grown on Yeast standard medium (Niu et al. 2011) were used as seeds, inoculated into the pretreated LFW and cultured at 35 °C with the shaking rate of 200 r/min for 4 days. The experiments were carried out in the 100 mL Erlenmeyer flasks with 20 mL culture volume. l.0 mL culture solution of B. coagulans (cell density 4.67 × 107/mL) or C. utilis (cell density 1.53 × 107/mL) was used as inoculum. The samples of three bottles were taken to determine pH, lactic acid, TOC, TN and cell density everyday, respectively.
Identification and count of cell density with flow cytometry
The total biomass of both B. coagulans and C. utilis was measured by a spectrophotometer (722 s, China) at a wavelength of 680 nm (OD680 nm). A flow cytometry (Partec CY-S-3001, German) was further used to identify and count the different cell densities of B. coagulans and C. utilis, respectively. The properties measured include a particle’s relative size, relative granularity or internal complexity, and the number of the particle. For flow cytometer measuring, the gain numeric of handle system parameter was set as follows: FSC 155, SSC 290, FL1 248, FL2 361.5. Suspended cells from 0.2 to 150 μ in size was kept by sieving. 10 μL of sample was diluted with 990 μL phosphate buffer solution (PBS; pH 7.0) to 100 fold, and 800 μL diluted sample was prepared in 2 mL transparent test tubes for a flow cytometer. All samples were measured with the excitation and emission wavelengths of 355 nm at room temperature. Each sample was pumped at 2 m/s for about 1.5 min.
Determination of pH, TOC, TN and total phosphorus (TP)
A pH meter (PHS-25, Leici, China) was directly used to measure pH of the culture sample. TOC and TN in culture solution that was centrifuged (10,000×g for 5 min) and filtered (0.22 μm) with the dilution of ultrapure water were determined on a TOC-V CPH (CPH-CPN, Shimadzu, Japan). TP in LFW was also measured by phosphomolybdenum blue spectrophotometry (Li and Liu 2006).
Determination of lactic acid
An amount of 2 mL of culture broth was centrifuged at 12,000×g for 10 min and the supernatant of 200 μL was taken and diluted 20 times with ultrapure water to measure the concentration of lactic acid on a HPLC system (Shimadzu LC-10A TVP, Shimadzu Co., Ltd., Japan) at the wavelength of 210 nm (De Baere et al. 2013). The mobile phase was 99% (v/v) 0.02 mol/L potassium dihydrogen phosphate solution (adjusted pH to 2 with phosphoric acid) and 1% (v/v) acetonitrile using the column of Agilent ZORBAX SB-Aq (150 mm × 4.6 mm, 5 μm) at 35 °C. The flow rate was 1 mL/min and the injection volume was 20 μL.
Results
Main physicochemical indexes in LFW
As shown in Table 1, TOC, TN and TP of LFW in the absence of LAB are 22.1 g/L, 2.4 g/L and 28.5 mg/L, respectively. Furthermore, lower pH 3.8 of LFW may be caused by the production of organic acids such as lactic acid by LAB.
Identification and determination of cell densities of B. coagulans and C. utilis with flow cytometer
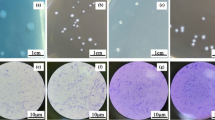
Figure 1 shows the cell scatter diagram of flow cytometer with FSC as the abscissa and SSC as the ordinate, which indicated that the scatter distribution areas of B. coagulans (R1) and C. utilis (R2) were concentrated on the lower left (Fig. 1a) and upper right (Fig. 1b) of the coordinate system, respectively. The cell scatter diagram in the presence of both B. coagulans (R1) and C. utilis (R2) (Fig. 1c) showed that the cells of B. coagulans and C. utilis could be clearly separated and identified with the different cell size, and their cell numbers could also accurately count with flow cytometer, respectively.
The growth of B. coagulans and C. utilis using LFW as culture medium
Figure 2 showed the growth of total biomass (OD680nm) of B. coagulans and C. utilis using LFW as a culture medium, which indicated that B. coagulans could slightly grow but C. utilis grew relatively well in pure single culture. The maximum biomass was observed after day 3 in the presence of both B. coagulans and C. utilis, showing that, compared with those of single pure culture, the growth was highly enhanced in co-culture. Further investigation on the growth of two microbial strains in LFW tested by flow cytometer (Fig. 3) showed that the cell densities of B. coagulans and C. utilis were all increased from 6.56 × 105 to 19.14 × 105/mL and from 6.56 × 105 to 7.26 × 105/mL in the pure culture during the period of 4 days, respectively. However, in co-culture, both the growth of C. utilis (cell density from 6.56 × 105 to 8.24 × 105/mL) and especially B. coagulans (cell density from 6.56 × 105 to 215.49 × 105/mL) were much improved at the same time.
Removal of lactic acid
Figure 4 showed the removal of lactic acid by B. coagulans and C. utilis, indicating that the concentrations of lactic acid was kept almost constant in control and slightly increased in the presence of B. coagulans, respectively, however initial lactic acid of 7.7 g/L was completed removed at 72 h by C. utilis both in single and co-culture, which showed that lactic acid could be produced by B. coagulans but removed by C. utilis, respectively.
Removals of TOC and TN by B. coagulans and C. utilis
As shown in Figs. 5 and 6, both TOC and TN declined slightly in single culture and apparently in co-culture. The removal ratios of TOC by B. coagulans and by C. utilis were 9.1% and 22.7%, respectively, which was improved to 49.0% in co-culture during the period of 96 h. The removal ratios of TN by B. coagulans and by C. utilis were 6.3% and 12.5%, which was also enhanced to 44.6% in co-culture at 96 h. The most efficiencies for the removals of both TOC and TN from LFW were achieved in the presence of B. coagulans and C. utilis simultaneously. Initial pH of 7.0 fluctuated from 6.0 to 9.0 during the period of 96 h (Fig. 7), which have not apparently adversely effects on the growth of B. coagulans and C. utilis.
Discussion
With the increasing need for LAB, both their culturing scale and the production of LFW are rapidly growing, which is generally rich with organic compounds such as sugars and protein, as well as lactic acid produced by LAB (Table 1 and Fig. 4). The release of LFW into natural water body may cause cyanobacterial bloom that can produce toxins such as microcystins and nodularin (Wang et al. 2005), which threatens the safety of drinking water and human health. Because many nutrients such as sugar, protein and organic acids are remained in LFW (Table 1 and Fig. 4), which may waste the resource if it is simply treated by traditional processing. Unfortunately, the treatment technology of LFW, especially recycling it as a culture media for other probiotic strains, has not been reported in the literature up to now. So we firstly investigated the co-culture of two probiotic strains using LFW as a medium.
The size of the cells and their granularity, registered by a flow cytometer, may be used for separation of the mixture (Cruz and Bellakov 1996). Corzo et al. (1999) detected Synechococcus and Prochlorococcus-like populations by flow cytometry in a eutrophic reservoir. Here in the co-culture of B. coagulans and C. utilis in LFW, the cell densities of them could be measured with a flow cytometer (Fig. 1), respectively, which is very important in the identification and determination of the different microbial biomass in co-culture.
Generally, OD680nm represents the total microbial biomass (Fig. 2), which cannot identify the cell densities of the different microbial strains. Here we successfully identified and measured the cell densities of both B. coagulans and C. utilis by flow cytometer (Fig. 3), respectively. Both B. coagulans and C. utilis are microbial probiotic strains and only pure culture rather than co-culture of them were reported up to now (Niu et al. 2011; Li and Liu 2006). When B, coagulans and C utilis were singly cultured in LFW, C utilis rather than B. coagulans grew well (Figs. 2, 3). However, in the presence of C. utilis, the growth of both especially B. coagulans was much improved (Figs. 2, 3). Firstly, the production of lactic acid may cause the effect of feedback inhibition on the growth of B. coagulans, but the metabolism of lactic acid by C. utilis can relieve the feedback inhibition to promote the growth of B. coagulans (Figs. 3, 4), which is a very important mechanism in co-culture. Oliva and Hang (1979) also found that C. utilis can grow and remove lactic acid from a continuous flow system of pickled wastewater. On another hand C. utilis might provide a number of nutritional factors such as amino acids and vitamins to enhance the growth of B. coagulans. Furthermore, the production of lactic acid from sugar remained in LFW by B. coagulans might also provide carbon source to enhance the growth of C. utilis, so the growths of both were improved each other in co-culture (Figs. 3, 4).
No information is provided on the treatment of LFW containing much amount of TOC and TN (Table 1), here we firstly investigated the resource utilization of LFW as a medium to culture other probiotic strains. The results indicated that the removals of both TOC and TN from LFW in co-culture of B. coagulans and C. utilis were much better than those of single culture (Figs. 5, 6). The previous studies also indicated that simultaneous removal of nutrients (ammonium and phosphate) and COD was better by the co-culture of Chlorella vulgaris and Pseudomonas putida than those of single culture, indicating that nutrients uptake capability of C. vulgaris was enhanced in the presence of P. putida.
In summary, the flow cytometer was successfully used to identify and count the different cell densities of both B. coagulans and C. utilis (Fig. 1) using LFW containing much amount of TOC and TN (Table 1) as medium. Compared with B. coagulans, C. utilis relatively grew well in LFW in a single culture (Figs. 2, 3). The growths of two microbial strains especially B. coagulans were much improved in co-culture and the removal of lactic acid by C. utilis was responsible for the relief of feedback inhibition to increase the growth of B. coagulans (Figs. 2, 3 and 4). The promotion of microbial growth in co-culture was responsible for the efficient removals of TOC and TN (Figs. 5, 6), respectively, which came true the production of probiotics by the resource utilization of LFW as a medium.
Abbreviations
- LAB:
-
lactic acid bacteria
- LFW:
-
lactobacillus fermentation wastewater
- TOC:
-
total organic carbon
- TN:
-
total nitrogen
- TP:
-
total phosphorus
References
Arena MP, Capozzi V, Russo P, Drider D, Spano G, Fiocco D (2018) Immunobiosis and probiosis: antimicrobial activity of lactic acid bacteria with a focus on their antiviral and antifungal properties. Appl Microbiol Biotechnol 102(23):9949–9958
Chandra R, Raj A, Purohit HJ, Kapley A (2007) Characterization and optimization of three potential aerobic bacterial strains for kraft lignin degradation from pulp paper waste. Chemosphere 67(4):839–846
Choi G, Kim J, Lee C (2016) Effect of low pH start-up on continuous mixed-culture lactic acid fermentation of dairy effluent. Appl Microbiol Biotechnol 100(23):10179–10191
Corzo A, Jiménez-Gómez F, Gordillo FJL, García-Ruízetc R, Niell FX (1999) Synechococcus and Prochlorococcus-like populations detected by flow cytometry in a eutrophic reservoir in summer. J Plankton Res 21:1575–1581
Cruz GPA, Bellakov G (1996) Fuzzy gating and its application in flow cytometry. Proc Conf 29:154–162
De Baere S, Eeckhaut V, Steppe M, De Maesschalck C, De Backer P, Van Immerseel F, Croubels S (2013) Development of a HPLCeUV method for the quantitative determination of four short-chain fatty acids and lactic acid produced by intestinal bacteria during in vitro fermentation. J Pharm Biomed Anal 80:107–115
Di Cagno R, Filannino P, Gobbetti M (2016) Novel fermented fruit and vegetable-based products. In: Ojha K, Tiwari B (eds) Novel food fermentation technologies. Food engineering series. Springer, Cham, pp 279–291
Gélinas P, Barrett J (2007) Protein enrichment of potato processing waste through yeast fermentation. Bioresour Technol 98(5):1138–1143
Grata K, Nabrdalk M (2012) Anti-fungal activity of Bacillus spp. against Fusarium spp. Proc Ecopole 6(1):1
Jahn HU, Ullrich R, Schneider T, Liehr RM, Schieferdecker HL, Holst H, Zeitz M (1996) Immunology and tropical effects of Saccharomyces boulardii on the small intestine in healthy human volunteers. Digestion 57:95–104
Kodali VP, Sen R (2008) Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotech J 3(2):245–251
Lee BK, Kim JK (2001) Production of Candida utilis biomass on molasses in different culture types. Aquac Eng 25(2):111–124
Leroy F, De Vuyst L (2014) Fermented food in the context of a healthy diet: how to produce novel functional foods. Curr Opin Clin Nutr Metab Care. 17:574–581
Li S, Liu D (2006) Spectrophotometric determination of inorganic phosphorus in environmental water samples with phospho molybdenum blue. Metall Anal 26(3):82–83
Li X, Jia OY, Xu Y, Chen M, Song XY, Yong Q, Yu SY (2009) Optimization of culture conditions for production of yeast biomass using bamboo wastewater by response surface methodology. Bioresour Technol 100(14):3613–3617
Mao RF, Wu DL, Hu SM, Zhou KP, Wang M, Wang YF (2017) Secretory expression and surface display of a new and biologically active single-chain insulin (SCI-59) analog by lactic acid bacteria. Appl Microbiol Biotechnol 101(8):3259–3271
Niu Y, Shen H, Chen J, Xie P, Yang X, Tao M, Ma ZM, Qi M (2011) Phytoplankton community succession shaping bacterioplankton community composition in Lake Taihu. China. Water Res 45(14):4169–4182
Oliva RU, Hang YD (1979) Continuous removal of lactic acid from wastewater by Candida utilis. Appl Environ Microbiol 38:1027–1028
Rajoka MI, Kiani MA, Khan S, Awan MS, Abu-Saeed Hashmi (2004) Production of single cell protion from rice polishing using Candida utilis. World J Microb Biotech 20(3):297–301
Riazi S, Wirawan RE, Badmaev V, Chikindas ML (2009) Characterization of lactosporin, a novel antimicrobial protein produced by Bacillus coagulans ATCC 7050. J Appl Microbiol 106:1370–1377
Riazi S, Dover SE, Chikindas ML (2012) Mode of action and safety of lactosporin, a novel antimicrobial protein produced by Bacillus coagulans ATCC 7050. J Appl Microbiol 113(3):714–722
Saayman M, Van Vuuren HJJ, Van Zyl WH, Viljoen-Bloom M (2000) Differential uptake of fumarate by Candida utilis and Schizosaccharomyces pombe. Appl Microbiol Biotechnol 54(6):792–798
Wang Q, Wang X, Ma H, Ren N (2005) Bioconversion of kitchen garbage to lactic acid by two wild strains of Lactobacillus species. J Environ Sci Heal A 40(10):1951–1962
Yadav BK, Tarafdar JC (2012) Efficiency of Bacillus coagulans as biofertilizer to mobilize native soil organic and poorly soluble phosphates and increase crop yield. Arch Agron Soil Sci 58(10):1099–1115
Zeng Z, Yu R, Zuo FL, Zhang B, Ma HQ, Chen SW (2017) Recombinant Lactococcus lactis expressing bioactive exendin-4 to promote insulin secretion and beta-cell proliferation in vitro. Appl Microbiol Biotechnol 101(19):7177–7186
Zhao N, Zhang C, Yang Q, Guo Z, Yang B, Lu WW, Li DY, Tian FW, Liu XM, Zhang H, Chen W (2016) Selection of taste markers related to lactic acid bacteria microflora metabolism for Chinese traditional Paocai: a gas chromatography–mass spectrometry-based metabolomics approach. J Agric Food Chem 64(11):2415–2422
Authors’ contributions
JYL and PFS designed and executed the experiments, collected and analyzed all data, wrote the manuscript. HY was responsible for initiation and supervision of the study. HY, QXL and ShA participated in the provided critical review of the manuscript. CHY, XLL, YL and HYZ contributed to experimental theory. QQX provided technical support. All authors read and approved the final manuscript.
Acknowledgements
The author thanks to Hebei Yiran Biotechnology Co., Ltd. for the experimental materials provided to us.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data supporting the conclusions are presented in the main article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable. This paper does not contain any studies with human participants or animal performed by any of the authors.
Funding
This study was financially supported in part by the National Natural Science Foundation of China (No. 21177009) and the special Foundation of Doctoral Program, Ministry of Education, China (No. 20120006110001).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, J., Shi, P., Ahmad, S. et al. Co-culture of Bacillus coagulans and Candida utilis efficiently treats Lactobacillus fermentation wastewater. AMB Expr 9, 15 (2019). https://doi.org/10.1186/s13568-019-0743-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-019-0743-3