Abstract
Severe economic losses due to diseases in marine larviculture may be linked to vibriosis. To better understand the pathogenesis of vibriosis and evaluate new ways to prevent and combat this important disease, there is a great need for reliable and reproducible experimental infection models. The present study aimed at developing a challenge model for vibriosis in Dover sole larvae and testing its applicability to study the effect of the probiotic treatment. For that purpose, larvae were challenged at 10 days post hatching with Vibrio anguillarum WT, V. anguillarum HI610 or V. harveyi WT. Following administration of V. anguillarum WT via immersion at 1 × 107 colony forming units/mL, a larval mortality of 50% was observed at 17 days post-inoculation. In a next step, the probiotic potential of 371 isolates retrieved from Dover sole was assessed by screening for their inhibitory effects against Vibrio spp. and absence of haemolytic activity. One remaining isolate (V. proteolyticus) and V. lentus, known for its protective characteristics in seabass larvae, were further tested in vivo by means of the pinpointed experimental infection model. Neither isolate provided via the water or feed proved to be protective for the Dover sole larvae against challenge with V. anguillarum WT. This developed challenge model constitutes a firm basis to expedite basic and applied research regarding the pathogenesis and treatment of vibriosis as well as for studying the impact of (a)biotic components on larval health.
Similar content being viewed by others
Introduction
Dover sole (Solea solea L.) is greatly appreciated in high quality restaurants and has a high market value, making it a very promising candidate for European aquaculture [1, 2]. In addition, farmers developed a renewed interest in Dover sole aquaculture to diversify their operations due to indications of limited market growth for species such as sea bass (Dicentrarchus labrax L.) and sea bream (Sparus aurata L.) [1, 3]. Furthermore, a reliable sole production would reduce fishing pressure on wild Dover sole populations, whereby the main sole stocks only recently recovered after collapsing 20 years ago and are now at or close to being harvested sustainable [1, 3]. As for other marine teleost species, high larval mortality rates (especially during first feeding) and limited knowledge on the nutritional requirements result in juvenile scarcity for stocking purposes, being the main obstacle for large scale aquaculture [1, 4, 5].
One of the major causes for the low and unpredictable survival in marine larviculture are outbreaks of infectious diseases. Vibriosis is one of the most challenging bacterial diseases to tackle in these early life stages [6,7,8] and multiple publications stress the importance of pathogenic Vibrio species in hatcheries and their potential to cause disease [9,10,11]. The causative agents of vibriosis are bacteria belonging to the genus Vibrio, with Vibrio anguillarum being the most prominent member [7, 12]. Important contributions are made to prevent and control infectious diseases, in the past mainly focussing on the use of antimicrobial agents or chemical additives [13]. However, the emerging antimicrobial resistance, the potential transfer of antimicrobial resistance genes to fish or human pathogens [14] and the possibility that antimicrobials can enter the human food chain [15], stress the need to develop reliable alternatives. These latter should ensure a healthy microbial environment in the larval rearing tanks and hence decrease disease and mortality [16]. Various environmentally-friendly prophylactic disease treatments are currently being pinpointed for marine larvae including probiotics [17,18,19], prebiotics [20, 21] and immunostimulants [22], with hitherto no data are available on the use of such treatments including probiotics in Dover sole larvae. In addition, there is a clear paucity of information in our understanding of the mode of action of probiotics and their interaction with the aquatic organism especially in the marine larval stage [20, 23, 24]. To remediate this and to elucidate the mechanism by which these treatments exert their beneficial impact, more knowledge on how the bacterium interacts with its host and causes disease is needed. For that purpose, the availability of reliable experimental infection models is imperative. Only a limited number of studies succeeded in developing such models for marine fish larvae. Significant mortality was noted following challenge of turbot larvae (Scophthalmus maximus L.) with V. anguillarum HI610 [25] and sea bass larvae with V. anguillarum HI610 [26] or V. harveyi [27]. For Dover sole, an experimental multi well plate housing system was pinpointed [28]. However, a reproducible and reliable experimental infection model eliciting vibriosis is non-existing, hampering in-depth research on the interplay between Vibrio and its larval host.
In this respect, the present study aimed at developing the first experimental infection model for vibriosis in Dover sole larvae. In addition, the protective potential of probiotic candidates for Dover sole was evaluated in vitro and subsequently in vivo by means of the pinpointed challenge model.
Materials and methods
All experiments were approved by the Ethical Committee of the Faculty of Veterinary Medicine and Bio-engineering Sciences, Ghent University (No. EC2015/28, EC2015/70 and EC2015/73).
Solea solea larvae
Solea solea eggs were obtained from the Wageningen Marine Research (Ijmuiden, the Netherlands) and Stichting Zeeschelp (Kamperland, the Netherlands). Eggs were naturally spawned overnight and collected the next morning. The dead eggs were removed, whereafter transportation to the research facilities in natural seawater (32 g/L) occurred. Upon arrival, eggs were acclimatized with artificial seawater (ASW) of 34 g/L (Instant Ocean, Aquarium Systems, Mentor, Ohio) and further incubated herein under aeration. One day after arrival, dead eggs were removed and developing Dover sole eggs were disinfected with 1% H2O2 for 3 min [28]. After disinfection, eggs were kept in 400 mL aerated autoclaved artificial seawater (AASW, Instant Ocean) in glass bottles at 16 ± 1 °C, each bottle containing approximately 600 embryos. Housing the larvae was performed as described by [28]. Two days post-hatching (dph), larvae were placed individually in 24-well plates, incubated at 16 ± 1 °C and fed ad libitum with sterile Artemia franciscana nauplii (EG type; INVE Aquaculture NV, Belgium) every other day, starting from 6 dph onwards, except when indicated otherwise. Sterile Artemia cysts and nauplii were obtained through decapsulation [29]. Half of the well water was replaced every other day and all larvae were subjected to a circadian rhythm of 9 h light and 15 h darkness.
Bacterial isolates
Experimental infection model
Three Vibrio strains were adopted. Vibrio anguillarum HI610 was originally isolated from diseased Atlantic cod (Gadus morhua L.) [30]. Vibrio anguillarum WT and V. harveyi WT strains were both procured from a disease outbreak in a French sea bass farm and subjected to minimal in vitro passaging.
In vitro selection of probiotic candidates
A total of 371 isolates retrieved from Dover sole larvae or the intestine of adults (both wild caught individuals and animals that were housed for 2–3 months in experimental facilities) were screened for their antagonism against V. anguillarum HI610, V. anguillarum WT or V. harveyi WT using the Kirby-Bauer disk diffusion method [31] as described in [32]. Briefly, the presence or absence of an inhibition zone surrounding disks immersed in the cultivated broth of the probiotic candidates following incubation, was recorded. The isolates eliciting growth inhibition were tested for their haemolytic activity by inoculating Marine Agar (MA, Scharlab S.L., Sentmenat, Spain) plates supplemented with 5% sheep blood (Oxoid Ltd, Hampshire, UK) with the cultivated broth of the probiotic candidates. Haemolytic activity was examined after 48 h incubation at 18 °C. Probiotic candidates exhibiting inhibition against at least one of the tested Vibrio strains and without haemolytic activity were identified by means of 16S rRNA gene sequencing. Therefore, the genomic DNA was extracted according to [33] and the 16S rRNA gene was amplified [34]. In short, amplification of the 16S rRNA gene was performed using the commercially available Qiagen Taq Mastermix and primers αβ-NOT (5′-TCAAACTAGGACCGAGTC-3′) and ωMB (5′-TACCTTGTTACTTCACCCCA-3′) as described by [35]. PCR products were sequenced using the BigDye Terminator sequencing kit (Applied Biosystems) and primers pD, Gamma*, 3 and O* [36]. Sequences were determined on an automatic DNA sequencer (ABI Prism 3100 Genetic analyser; Applied Biosystems) and identified using the program BLAST and the NCBI/GenBank. Species known to be potentially zoonotic were excluded from further experiments.
Vibrio lentus isolated from clinically healthy seabass larvae (10 dph) and proven to significantly reduce mortality of seabass larvae after challenge with V. harveyi WT [32], was also included as a probiotic candidate. Vibrio lentus showed in vitro inhibition against V. anguillarum HI610 and V. harveyi WT and proved to be non-haemolytic [32]. The inhibitory effect against V. anguillarum WT was tested as described above.
In vivo experiments
For each experimental trial, Dover sole eggs from one single batch were used and a negative control group was included in which larvae underwent the same physical handling and water exchanges but without the addition of bacterial cells. Each group consisted of 96 larvae at 4 dph, divided over four 24-well plates filled with AASW. At the end of each experiment all remaining larvae were sacrificed by immersion in an overdose of MS 222 (tricaine methanesulfonate, Sigma-Aldrich, Diegem, Belgium).
Bacterial cultivation practices
All bacterial isolates were grown for 48 h at 18 °C on MA, followed by cultivation in tryptic soy broth (TSB, Becton, Dickinson and Company, New Jersey, USA) supplemented with 1.5% NaCl for 24 h at 18 °C. Cells were harvested by centrifugation at 3500 rpm for 10 min. The resulting pellet was washed twice with AASW and subsequently resuspended in AASW. Optical densities were determined using an ATB 1550 densitometer (BioMérieux, Marcy-l’Etoile, France). Bacterial titres were verified by making a tenfold dilution series in triplicate on MA plates, prior to administration.
Development of the experimental infection model
In the first challenge experiment, three groups were challenged with either V. anguillarum HI610, V. anguillarum WT or V. harveyi WT. The Vibrio strains were added to the well water of 10 dph larvae at a final concentration of 1 × 105 colony forming units (CFU)/mL. In the second experiment, the same groups were included but the Vibrio strains were added so as to achieve a final concentration of 1 × 106 CFU/mL. In the third experiment, only one group was challenged with V. anguillarum WT resulting in a final concentration of 1 × 107 CFU/mL.
Six hours following the inoculation with the Vibrio strains, half of the well water was replaced. From the next day onwards, the normal feeding regime with sterile Artemia nauplii and water replacement every other day were started. Larval mortality was monitored daily up to 17 dph.
Assessment of the protective potential of probiotic candidates
Harmfulness of the probiotic candidates to Dover sole larvae
The harmfulness of the resulting probiotic candidate 1 and of V. lentus was tested in two separate experiments. Bacterial cells were added to the well water of larvae at 4, 6 and 8 dph resulting in a final concentration of 1 × 107 CFU/mL. Larval mortality was monitored daily up to 17 dph. The standard body length of all remaining larvae was measured using an Olympus SZX7 stereomicroscope and cell D software (Soft imaging system, Olympus NV).
Protection of Dover sole larvae against challenge with V. anguillarum WT
In the first experiment, the larvae of two experimental groups were provided with probiotic candidate 1 or V. lentus via the well water on 4, 6 and 8 dph in a final concentration of 1 × 107 CFU/mL. Subsequently, the larvae were challenged with V. anguillarum WT at a final concentration of 1 × 107 CFU/mL at 10 dph. A third group (positive control) was inoculated with V. anguillarum WT without being previously administered a probiotic candidate.
In the second experiment the same experimental groups were included but probiotic candidates were supplied via the feed. Therefore, newly hatched sterile Artemia nauplii were incubated at 20 °C for 6 h in a suspension (1 × 107 CFU/mL) of one of the two probiotic candidates. Subsequently, the Artemia nauplii were washed and fed to the Dover sole larvae at 5 and 7 dph. To evaluate the bacterial concentration present on the surface and inside of the Artemia nauplii, a subsample of at least 20 rinsed Artemia nauplii were homogenised and resuspended in 100 µL AASW. Bacterial titres were verified by making a tenfold dilution series of the homogenate on MA. From 9 dph onwards, the normal feeding regime with sterile Artemia nauplii every other day was started.
In both experiments, mortality was monitored daily up to 21 dph.
Statistical analysis
For the experimental infection model, the survival (0–1) at the end of the study was compared between the three Vibrio strains (in different concentrations) and the negative control group using a logistic regression model. In the harmfulness study, the survival (0–1) of the negative control was compared with the probiotic candidates by a logistic regression model. The body length measurements of the larvae exposed to the probiotic candidates in comparison with the negative control group were analyzed within each experiment by a linear fixed effects model. To evaluate the protective potential of the probiotic candidates, administered via the water or the food, the survival (0–1) at the end of the study was compared between the negative control, the positive control and probiotic candidates by a logistic regression model.
All analyses were performed using SAS version 6.4. The global significance level of 5% was used but multiple comparisons significance levels were adjusted based on the Bonferroni correction method in order to compare the outcome of the challenges with the three Vibrio isolates with the negative control and to compare the probiotic candidate treatments, negative control and the positive control group (comparison wise significance level set at 0.05/3 = 0.0167).
Results
Experimental infection model
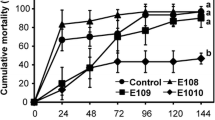
In the first challenge experiment to develop the infection model, no significant difference in survival at 17 dph was observed between the negative control group (survival of 91%) and the larvae inoculated with 105 CFU/mL V. anguillarum HI610 (survival of 86%) (OR = 0.66, 95% CI [0.26;1.66], p = 0.367), V. anguillarum WT (survival of 84%) (OR = 0.56, 95% CI [0.23;1.37], p = 0.195) and V. harveyi (survival of 94%) (OR = 1.55, 95% CI [0.52;4.64], p = 0.423). In the second challenge experiment, no significant difference in survival at 17 dph was noted between the negative control group (survival of 90%) and the larvae inoculated with 106 CFU/mL V. anguillarum HI610 (survival of 93%) (OR = 1.48, 95% CI [0.53;4.16], p = 0.448) or V. harveyi WT (survival of 86%) (OR = 0.90, 95% CI [0.36;2.27], p = 0.817). Following challenge with V. anguillarum WT at 106 CFU/mL, a statistically significant reduction in survival to 61% (OR = 0.19, 95% CI [0.08;0.41], p < 0.001) was discerned. In the third experiment, a significantly reduced survival of 50% (p < 0.001) was detected between the larvae inoculated with V. anguillarum WT at 107 CFU/mL and the negative control group (survival of 92%) [OR = 0.09, 95% CI [0.04;0.21], p < 0.001).
Assessment of the protective potential of probiotic candidates
In vitro selection of probiotic candidates
None of the isolates retrieved from adult Dover sole displayed in vitro inhibition against one of the tested Vibrio strains. Four probiotic candidates recovered from Dover sole larvae were selected based on the presence of in vitro inhibition against the three tested Vibrio strains and the absence of haemolytic activity. Following 16S rRNA sequencing, three probiotic candidates were identified as V. parahaemolyticus with 99% sequence homology and hence excluded from further assays due to their potential zoonotic character. The remaining probiotic candidate was identified as V. proteolyticus with 99% sequence homology and further analysed in vivo for its protective potential.
Vibrio lentus isolated from seabass larvae demonstrated in vitro inhibition against V. anguillarum WT.
Harmfulness of the probiotic candidates to Dover sole larvae
In the first experiment, no significant difference in survival at 17 dph was observed between the negative control group (survival of 76%) and the larvae inoculated with V. proteolyticus (survival of 74%) (OR = 0.89 [95% CI 0.46;1.74], p = 0.739). Also in the second experiment, no significant difference in survival at 17 dph was observed between the negative control group (survival of 89%) and the larvae inoculated with V. lentus (survival of 88%) (OR = 0.89 [95% CI 0.46;1.74], p = 0.824). In both experiments the estimated odds ratios were close to one. No significant difference in standard body length was observed at 17 dph between the control group and the group inoculated with V. proteolyticus (p = 0.3223). By 17 dph, the larvae inoculated with V. lentus had a mean standard body length of 5570.49 µm (SD = 749.13 µm), which is significantly higher than what was noted in the larvae of the control group, 5000.06 µm (SD = 421.73 µm) (p = 0.006).
Protection of Dover sole larvae against challenge with V. anguillarum WT
In the first experiment, no significant differences in survival at 21 dph were observed between the positive control group (survival of 35%) and the larvae inoculated before challenge via the water with V. proteolyticus (survival of 37%) (OR = 1.05 [95% CI 0.57;1.91], p = 0.880) or with V. lentus (survival of 34%) (OR = 0.96, 95% CI [0.52;1.75], p = 0.880). A significant difference in survival at 21 dph was discerned between the negative control group (survival of 80%) and the larvae inoculated with V. proteolyticus (OR = 0.14 [95% CI 0.07;0.26], p < 0.001) or with V. lentus (OR = 0.14 [95% CI 0.07;0.26], p < 0.001) prior to challenge.
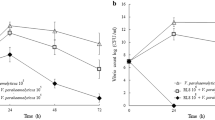
In the second experiment, no significant differences in survival at 21 dph were noted between the positive control group (survival of 52%) and the larvae that were inoculated via the feed before being challenged with V. proteolyticus (survival of 45%) (OR = 0.75 [95% CI 0.42;1.33], p = 0.312) or with V. lentus (survival of 49%) (OR = 0.88, 95% CI [0.50;1.57], p = 665). A significant difference in survival at 21 dph was noticed between the negative control group (survival of 72%) and the larvae inoculated with V. proteolyticus (OR = 0.32 [95% CI 0.17;0.59], p < 0.001) or with V. lentus (OR = 0.38 [95% CI 0.20;0.69], p = 0.001) before challenge. The bacterial concentration of the Artemia nauplii ranged between 1.5 × 107 and 5 × 107 CFU/mL for V. proteolyticus and between 2.5 × 107 and 2 × 108 CFU/mL for V. lentus.
Discussion
Infectious diseases (e.g. vibriosis) are a major cause of marine larval mortality and various environmentally-friendly prophylactic treatments are currently being pinpointed including the use of pro- and prebiotics. However, very limited data on these alternative treatments are available for Dover sole. In the present study, an experimental challenge model using V. anguillarum WT was developed to reproduce vibriosis in Dover sole and therefore provide a building block to move forward in the assessment of novel therapies and the elucidation of the pathogenesis of vibriosis. This model was then used for evaluating the protective potential of two probiotic candidates, selected using in vitro criteria.
Only a limited number of studies focus on the development of challenge models in marine fish larvae. These may be performed by bioencapsulation of the pathogen in life feed [25, 37, 38] or challenge via the water. When pathogen delivery is performed via the water, the challenge experiments are mostly conducted in multiwell plate systems [27, 39,40,41]. In a minority of studies, larvae are housed in small groups in vials [26, 42]. However, in these vials an increase virulence of V. anguillarum depending on the number of dead larvae was observed [42]. This indicates that (remnants of) death larvae can have an impact on living animals or the pathogen, increasing variability between replicates. The challenge model as proposed in the current study draws on a multi well housing system that was developed previously [28]. Housing the larvae individually offers the advantage that the possible death of one larva has no effect on the other larvae, rendering these experiments more reproducible. Furthermore, the health status and behaviour of individually housed larvae may be monitored more easily. In the present study, the pathogenicity of three bacterial strains was evaluated and the strain and concentration eliciting around 50% mortality within 6 days following challenge selected. Indeed, the induced mortality needs to be sufficiently high to enable the investigation of the protective effect of prophylactic or curative treatments. On the other hand, a too severe challenge model is not appropriate neither as this would hinder detection of a possible protective capacity.
Vibriosis is reported as a cause of disease/mortality in as many as at least 48 species of marine fish [43]. The importance of pathogenic Vibrio species in hatcheries and their potential to cause disease is stressed by various authors [9,10,11]. Vibrio anguillarum is described as the causative agent of vibriosis in the young life stages of at least 12 fish species. The number of disease case reports in S. solea culture is limited and involves adults [44] and juveniles [45]. This scarce information on Dover sole health management is largely rooted in the fact that Dover sole aquaculture was initiated only relatively recently, underscoring the need for research on health and disease in this alternative aquaculture species. A V. anguillarum isolate not originally retrieved from sole was included that induced larval mortality in other marine species. Indeed, although fish species-specific virulence was described for different Vibrio species, several strains originating from a disease outbreak in (larvae of) one fish species were able to cause mortality in another [41, 46, 47]. To exemplify this, although V. anguillarum strain 87-9-117 was originally isolated from rainbow trout (Oncorhynchus mykiss Walbaum), it caused high mortality in sea bass larvae, indicating that there is no stringent host-specificity for vibriosis [47]. In the present study, normal survival was obtained for Dover sole larvae subsequent to inoculation with V. harveyi WT retrieved from diseased sea bass and pathogenic to sea bass larvae [27]. Indeed, in a previous study challenge with V. harveyi resulted in 70% mortality in sea bass larvae following administration at 1 × 105 CFU/mL [32]. The latter is also a well-known causative agent of vibriosis in adults of the closely related Senegalese sole (Solea senegalensis Kaup) [48, 49]. Secondly, no increased mortality was observed following challenge of the Dover sole larvae with V. anguillarum HI610 at concentrations up to 1 × 106 CFU/mL. This bacterial strain originating from diseased Atlantic cod larvae [30], elicited high mortality rates in several experimental challenge tests including yolk sac larvae of turbot [41, 47], halibut (Hippoglossus hippoglossus L.) [41, 47], Atlantic cod [41, 49] and seabass [26, 27]. Thirdly, challenge with V. anguillarum WT resulted in significant mortality in Dover sole larvae with an increased death rate noted following inoculation with a higher concentration [39% (1 × 106 CFU/mL) vs 50% (1 × 107 CFU/mL)]. Although retrieved from a different fish species that is sea bass, adequate virulence hence was established. These results again underscore the complexity of fish species-specific virulence of V. anguillarum, impeding extrapolation of experimental challenge models across species and warranting further research.
Although efforts were and are still being made to understand the physiological changes during stress events [50], research concerning welfare and pain awareness parameters in fish larvae remains practically nonexistent. Studies on predictive behavioural traits indicating severe suffering or imminent mortality are imperative and should allow to delineate humane endpoints for fish larvae. The individual housing of the larvae as is the case in the current experimental set-up, may be regarded as an aid in this research journey. In order to reduce the number of experimental animals used, a subsequent experiment with an increasing bacterial concentration was only performed when the lower bacterial titer did not generate sufficient mortality. In the third experiment, V. anguillarum WT was administered at a higher dose (1 × 107 CFU/mL) since only this strain showed pathogenic potential when administered at a lower concentration (1 × 106 CFU/mL). As no increased larval mortality was observed after inoculation with V. anguillarum HI610 and V. harveyi WT at 1 × 106 CFU/mL, both isolates were omitted in the third experiment.
Once the standardized challenge model was developed, the protective potential of two probiotic candidates was evaluated. Probiotics are usually defined as products which contain viable non-pathogenic micro-organisms able to confer health benefits to the host [3]. The effectiveness of probiotic in terms of protection against bacterial pathogens especially was tested in juveniles and adult fish (reviewed in [18, 19]) but also a small number of studies on marine fish larvae were performed. The protective potential of various probiotic candidates involving turbot larvae was evaluated in bottles [51] or tanks [52, 53]. To our knowledge, only one study tested the protective potential of probiotic candidates in a challenge experiment using multi well plates (based on sea bass larvae, [32]), hereby highlighting the significance of the present study on Dover sole larvae.
Different probiotics were tested for flatfish species, mainly focusing on turbot, with only a limited number of studies involving fish larvae (turbot [25, 54,55,56], California halibut (Paralichthys californicus Ayres) [57], Dover sole [58], Senegalese sole [5, 59]). Considering sole species, Senegalese sole is widely studied and one probiotic Shewanella putrefaciens was put forward. Beneficial effects on growth, stress levels, onset of metamorphosis, intestinal flora and resistance against infection with Photobacterium damselae were found (reviewed in [60]). For Dover sole, only one probiotic was described (Enterococcus faecium, [58]), modulating amongst others growth and cortisol levels [61]. However, the protective potential of this probiotic against challenge with a pathogenic agent was not evaluated. In this study V. lentus and V. proteolyticus were evaluated as probiotic candidates. Vibrio lentus was described as the causative agent of skin lesions and mortality in wild octopus (Octopus vulgaris Cuvier) [62] but no pathogenic properties were observed in fish, following both intraperitoneal injection [62] or challenge via Artemia nauplii [63]. Also in the present study no decreased survival of the larvae was observed, but due to the large width of the 95% CI, a negative impact on fish larval health may not be fully excluded. However, at 17 dph a significant increase in standard body length of the larvae inoculated with V. lentus compared with the control group was noted. A beneficial growth effect has been correlated with the administration of probiotics via bioencapsulation to the life feed in fish larvae and juveniles [52, 64] but no such effect has been described in marine larvae after probiotic treatment via the water. Increased growth rates due to probiotic administration were linked to the production of beneficial dietary compounds or digestive enzymes [24]. The probiotic potential of V. lentus was previously tested in gnotobiotic sea bass larvae against V. harveyi WT, showing significantly decreased mortality rates [32]. In the present study, however, no such properties were seen in Dover sole larvae after challenge with V. anguillarum WT. No length measurements were performed by [32]. Vibrio proteolyticus can induce mortality in Artemia cultures [19] but no such characteristics have been reported in fish. Also in this study, no increased mortality nor impaired larval growth was observed after administration of this isolate. Beneficial effects of diet supplementation with V. proteolyticus on protein degradation were noted [65]. However, the potential beneficial role of V. proteolyticus as a probiotic agent has not yet been described in aquatic organisms. This renders the present study the first in its kind although no positive effects, observed as an increased survival after challenge with the pathogen or increased standard body length, were discerned.
To test the protective effect of the probiotic candidates during V. anguillarum WT infection, two routes of delivery, via the well water and via attachment to live food (Artemia nauplii), were tested. Adding the probiotic candidate through the rearing water maximizes the exposure of the larvae before the start of exogenous feeding and during the first days, when the food intake is limited [24, 66]. Furthermore, bath challenge may maximize the competitive advantage of added probiotics, as bacteria colonizing the intestines before first feeding may be able to persist amongst the autochthonous microflora [64, 67]. Exogenous feeding was started at 6 dph in Dover sole larvae and also the delivery of the probiotics via Artemia nauplii was studied. Delivery through bioencapsulation may be preferred in hatcheries where water exchange rates are high [24]. For bioencapsulation, lower amounts of probiotic components are needed compared to when these are added to the water, rendering this practice more feasible and economically more interesting. One may presume that delivery through the feed results in the probiotic residing longer in the intestine and hence may increase the probiotic protective potential. In addition, it was described that colonization of the gut increases during exogenous feeding, hereby resembling the microflora of the livefood [68]. Most probiotic studies focus only on one route of delivery, via life feed (e.g. [51]) or via the water (e.g. [5]), underscoring the completeness of this study.
In addition to its possible value for many other applications, this experimental infection model for vibriosis constitutes a firm basis to evaluate the impact of (a)biotic components on larval health. The model is also to be regarded as a powerful tool for investigating the pathogenesis of V. anguillarum infections in Dover sole larvae, evaluating curative or preventive treatments and elucidating their mode(s) of action. In this study, probiotic candidates were selected in vitro and assessed for their protective potential against V. anguillarum challenge in vivo but also prebiotic treatments may be evaluated by means of this model. Indeed, although immunostimulating properties are allocated to prebiotics [21], limited research on the potential protective effect of prebiotics against challenge with a known pathogen has been performed in marine fish larvae [69]. Next to aquaculture related research, this model also renders biological research concerning the impact of environmental components (e.g. microplastics or algal toxins released during harmful algal blooms) on the health of Dover sole larvae possible by evaluating their susceptibility to disease agents.
Abbreviations
- AASW:
-
autoclaved artificial seawater
- ASW:
-
artificial seawater
- CFU:
-
colony forming units
- dph:
-
days post-hatching
- MA:
-
marine agar
- TSB:
-
tryptic soy broth
References
Bjorndal T, Guillen J, Imsland A (2016) The potential of aquaculture sole production in Europe: production costs and markets. Aquac Econ Manag 20:109–129
Rodgers PE, Read A, Meun G (2005) Benefits of responsible fishing: the impact of an innovative trial of voluntary restraint. Report for the EU Directorate-General for Fisheries, Contract No. 2003/C 115/08-17, CFER, Lincoln
FAO (2016) http://www.fao.org. Accessed 4 May 2017
Ferraresso S, Bonaldo A, Parma L, Cinotti S, Massi P, Bargelloni L, Gatta PP (2013) Exploring the larval transcriptome of the common sole (Solea solea L.). BMC Genomics 14:315
Makridis P, Martins S, Reis J, Dinis MT (2008) Use of probiotic bacteria in the rearing of Senegalese sole (Solea senegalensis) larvae. Aquac Res 39:627–634
Austin B, Austin DA (2012) Bacterial fish pathogens: disease of farmed and wild fish, 5th edn. Springer, Dordrecht. ISBN 978-94-007-4883-5
Novriadi R (2016) Vibriosis in aquaculture. Omni-Akuatika 12:1–12
Thompson FL, Iida T, Swings J (2004) Biodiversity of vibrios. Microbiol Mol Biol Rev 68:403–431
D’Alvise PW, Lillebo S, Wergeland HI, Gram L, Bergh O (2013) Protection of cod larvae from vibriosis by Phaeobacter spp.: a comparison of strains and introduction times. Aquaculture 384:82–86
Touraki M, Niopas I, Karagiannis V (2012) Treatment of vibriosis in European sea bass larvae, Dicentrarchus labrax L., with oxolinic acid administered by bath or through medicated nauplii of Artemia franciscana (Kellogg): efficacy and residual kinetics. J Fish Dis 35:513–522
Silva YJ, Costa L, Pereira C, Mateus C, Cunha A, Calado R, Gomes NCM, Pardo MA, Hernandez I, Almeida A (2014) Phage therapy as an approach to prevent Vibrio anguillarum infections in fish larvae production. PLoS ONE 9:e114197
Toranzo AE, Magarinos B, Romalde JL (2005) A review of the main bacterial fish diseases in mariculture systems. Aquaculture 246:37–61
Cabello FC (2006) Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environ Microbiol 8:1137–1144
Romero J, Feijoo CG, Navarrete P (2012) Antibiotics in aquaculture—use, abuse and alternatives. In: Carvalho ED, David GS, Silva RJ (eds) Health and environment in aquaculture. InTech, Rijeka, pp 159–198. ISBN 978-953-51-0497-1
Willis C (2000) Antibiotics in the food chain: their impact on the consumer. Rev Med Microbiol 11:153–160
Diaz-Rosales P, Arijo S, Chabrillon M, Alarcon FJ, Tapia-Paniagua ST, Martinez-Manzanares E, Balebona MC, Morinigo MA (2009) Effects of two closely related probiotics on respiratory burst activity of Senegalese sole (Solea senegalensis, Kaup) phagocytes, and protection against Photobacterium damselae subsp. piscicida. Aquaculture 293:16–21
De BC, Meena DK, Behera BK, Das P, Das Mohapatra PK, Sharma AP (2014) Probiotics in fish and shellfish culture: immunomodulatory and ecophysiological responses. Fish Physiol Biochem 40:921–971
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14
Verschuere L, Heang H, Criel G, Sorgeloos P, Verstraete W (2000) Selected bacterial strains protect Artemia spp. from the pathogenic effects of Vibrio proteolyticus CW8T2. Appl Environ Microb 66:1139–1146
RingØ E, Olsen RE, Gifstad TO, Dalmo RA, Amlund H, Hemre GI, Bakke AM (2010) Prebiotics in aquaculture: a review. Aquac Nutr 16:117–136
Bricknell I, Dalmo RA (2005) The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol 19:457–472
Ringø E, Olsen RE, Vecino JLG, Wadsworth S, Song SK (2012) Use of immunostimulants and nucleotides in aquaculture: a review. J Mar Sci Res Dev 2:104
Tinh NTN, Dierckens K, Sorgeloos P, Bossier P (2008) A review of the functionality of probiotics in the larviculture food chain. Mar Biotechnol 10:1–12
Vine NG, Leukes WD, Kaiser H (2006) Probiotics in marine larviculture. FEMS Microbiol Rev 30:404–427
Planas M, Perez-Lorenzo M, Vazquez JA, Pintado J (2005) A model for experimental infections with Vibrio (Listonella) anguillarum in first feeding turbot (Scophthalmus maximus L.) larvae under hatchery conditions. Aquaculture 250:232–243
Dierckens K, Rekecki A, Laureau S, Sorgeloos P, Boon N, Van Den Broeck W, Bossier P (2009) Development of a bacterial challenge test for gnotobiotic sea bass (Dicentrarchus labrax) larvae. Environ Microbiol 11:526–533
Schaeck M, De Swaef E, Van Den Broeck W, Van Nevel S, Boon N, De Geyter N, Morent R, Demeestere K, Duchateau L, Coulombet C, Haesebrouck F, Decostere A (2016) Germ-free sea bass Dicentrarchus labrax larval model: a valuable tool in the study of host-microbe interactions. Dis Aquat Organ 117:177–185
De Swaef E, Demeestere K, Boon N, Van den Broeck W, Haesebrouck F, Decostere A (2017) Development of a reliable experimental set-up for Dover sole larvae Solea solea L. and exploring the possibility of implementing this housing system in a gnotobiotic model. Res Vet Sci 115:418–424
Sorgeloos P, Lavens P, Leger P, Tackaert W, Versichele D (1986) Manuel for the use of Brine Shrimp Artemia in Aquaculture. Artemia Reference center, State University of Ghent, Faculty of Agriculture, Belgium, p 319
Samuelsen OB, Bergh O, Ervik A (2003) Pharmacokinetics of florfenicol in cod Gadus morhua and in vitro antibacterial activity against Vibrio anguillarum. Dis Aquat Organ 56:127–133
National Committee for Clinical Laboratory Standards (1997) Performance standards for analysis antimicrobial disk susceptibility tests. Approved standard M2-A6, vol 19, no 18. Wayne, pp 1–7
Schaeck M, Duchateau L, Van Den Broeck W, Van Trappen S, De Vos P, Coulombet C, Boon N, Haesebrouck F, Decostere A (2016) Vibrio lentus protects gnotobiotic sea bass (Dicentrarchus labrax L.) larvae against challenge with Vibrio harveyi. Vet Microbiol 185:41–48
Declercq AM, Boyen F, Van Den Broeck W, Bossier P, Karsi A, Haesebrouck F, Decostere A (2013) Antimicrobial susceptibility pattern of Flavobacterium columnare isolates collected worldwide from 17 fish species. J Fish Dis 3:45–55
Smet A, Flahou B, D’herde K, Vandamme P, Cleenwerck I, Ducatelle R, Pasmans F, Haesebrouck F (2012) Helicobacter heilmannii sp. nov., isolated from feline gastric mucosa. Int J Syst Evol Microbiol 62:299–306
Baele M, Devriese LA, Haesebrouck F (2001) Lactobacillus agilis is an important component of the pigeon crop flora. J Appl Microbiol 91:488–491
Coenye T, Falsen E, Vancanneyt M, Hoste B, Govan JRW, Kersters K, Vandamme P (1999) Classification of Alcaligenes faecalis-like isolates from the environment and human clinical samples as Ralstonia gilardii sp. nov. Int J Syst Evol Microbiol 49:405–413
Muroga K, Yasunobu H, Okada N, Hasumura K (1990) Bacterial enteritis of cultured flounder, Paralichthys olivaceus larvae. Dis Aquat Organ 9:121–125
Munro PD, Barbour A, Birkbeck TH (1995) Comparison of the growth and survival of larval turbot in the absence of culturable bacteria with those in the presence of Vibrio anguillarum, Vibrio alginolyticus, or a Marine aeromonas sp. Appl Environ Microbiol 61:4425–4428
Bergh O, Hjeltnes B, Skiftesvik AB (1997) Experimental infection of turbot Scophthalmus maximus and halibut Hippoglossus hippoglossus yolk sac larvae with Aeromonas salmonicida subsp. salmonicida. Dis Aquat Organ 29:13–20
Sandlund N, Bergh O (2008) Screening and characterisation of potentially pathogenic bacteria associated with Atlantic cod Gadus morhua larvae: bath challenge trials using a multidish system. Dis Aquat Organ 81:203–217
Sandlund N, Rodseth OM, Knappskog DH, Fiksdal IU, Bergh O (2010) Comparative susceptibility of turbot, halibut, and cod yolk-sac larvae to challenge with Vibrio spp. Dis Aquat Organ 89:29–37
Li X, Defoirdt T, Yang Q, Laureau S, Bossier P, Dierckens K (2014) Host-induced increase in larval sea bass mortality in a gnotobiotic challenge test with Vibrio anguillarum. Dis Aquat Organ 108:211–216
Austin B, Austin DA (2007) Bacterial fish pathogens: disease of farmed and wild fish, 4th edn. Praxis Publishing Ltd, Chichester, p 552. ISBN 978-1-4020-6068-7
Manfrin A, Doimi M, Antonetti P, Delgado Montero ML, Qualtieri K, Rampazzo E, Vascellari M, Mutinelli F, Melchiotti E, Bozza MA, Selli L, Bovo G (2003) Primo isolamento di Vibrio anguillarum sierotipo O 1 da sogliola (Solea solea, L.) in Itali/First isolation of Vibrio anguillarum O1 serotype from sole (Solea solea) in Italy. Boll Soc It Patol Ittica 37:12–17
Paolini A, Magi GE, Gennari L, Egidi C, Torresi M, Vallerani M, Berti M, Pavone A, Giorgetti G (2010) Severe mortality in common sole (Solea solea) at post-weaning stage following environmental stress. Bull Eur Assoc Fish Pathol 30:160–169
Frans I, Dierckens K, Crauwels S, Van Assche A, Leisner J, Larsen MH, Michiels CW, Willems KA, Lievens B, Bossier P, Rediers H (2013) Does virulence assessment of Vibrio anguillarum using sea bass (Dicentrarchus labrax) larvae correspond with genotypic and phenotypic characterization? PLoS One 8:e70477
Rønneseth A, Castillo D, D’Alvise P, Tønnesen Ø, Haugland G, Grotkjær T, Engell-Sørensen K, Nørremark L, Bergh Ø, Wergeland HI, Gram L (2017) Comparative assessment of Vibrio virulence in marine fish larvae. J Fish Dis 40:1373–1385
Arijo S, Chabrillon M, Diaz-Rosales P, Rico RM, Martinez-Manzanares E, Balebona MC, Toranzo AE, Morinigo MA (2005) Bacteria isolated from outbreaks affecting cultured sole, Solea senegalensis (Kaup). Bull Eur Assoc Fish Pathol 25:148–154
Seljestokken B, Bergh O, Melingen GO, Rudra H, Olsen RH, Samuelsen OB (2006) Treating experimentally induced vibriosis (Listonella anguillarum) in cod, Gadus morhua L., with florfenicol. J Fish Dis 29:737–742
Pederzoli A, Mola L (2016) The early stress responses in fish larvae. Acta Histochem 118:443–449
Gatesoupe FJ (1994) Lactic-acid bacteria increase the resistance of turbot larvae, Scophthalmus maximus, against pathogenic Vibrio. Aquat Living Resour 7:277–282
Gatesoupe FJ (1997) Siderophore production and probiotic effect of Vibrio sp. associated with turbot larvae, Scophthalmus maximus. Aquat Living Resour 10:239–246
Garcia de la Banda I, Chereguini O, Rasines I (1992) Influence of lactic bacterial additives on turbot (Scophthalmus maximus L.) larvae culture. Bol Inst Esp Oceanogr 8:247–254
Huys L, Dhert P, Robles R, Ollevier F, Sorgeloos P, Swings J (2001) Search for beneficial bacterial strains for turbot (Scophthalmus maximus L.) larviculture. Aquaculture 193:25–37
Munoz-Atienza E, Araujo C, Magadan S, Hernandez PE, Herranz C, Santos Y, Cintas LM (2014) In vitro and in vivo evaluation of lactic acid bacteria of aquatic origin as probiotics for turbot (Scophthalmus maximus L.) farming. Fish Shellfish Immunol 41:570–580
Hjelm M, Bergh O, Riaza A, Nielsen J, Melchiorsen J, Jensen S, Duncan H, Ahrens P, Birkbeck H, Gram L (2004) Selection and identification of autochthonous potential probiotic bacteria from turbot larvae (Scophthalmus maximus) rearing units. Syst Appl Microbiol 27:360–371
Zorrilla I, Arijo S, Chabrillon M, Diaz P, Martinez-Manzanares E, Balebona MC, Morinigo MA (2003) Vibrio species isolated from diseased farmed sole, Solea senegalensis (Kaup), and evaluation of the potential virulence role of their extracellular products. J Fish Dis 26:103–108
Avella MA, Olivotto I, Silvi S, Ribecco C, Cresci A, Palermo F, Polzonetti A, Carnevali O (2011) Use of Enterococcus faecium to improve common sole (Solea solea) larviculture. Aquaculture 315:384–393
Lobo C, Moreno-Ventas X, Tapia-Paniagua S, Rodriguez C, Morinigo MA, De La Banda IG (2014) Dietary probiotic supplementation (Shewanella putrefaciens Pdp11) modulates gut microbiota and promotes growth and condition in Senegalese sole larviculture. Fish Physiol Biochem 40:295–309
Tapia-Paniagua ST, Diaz-Rosales P, Leon-Rubio JM, De La Banda IG, Lobo C, Alarcon FJ, Chabrillon M, Rosas-Ledesma P, Varela JL, Ruiz-Jarabo I, Arijo S, Esteban MA, Martinez-Manzanares E, Mancera JM, Balebona MC, Morinigo MA (2012) Use of the probiotic Shewanella putrefaciens Pdp11 on the culture of Senegalese sole (Solea senegalensis, Kaup 1858) and gilthead seabream (Sparus aurata L.). Aquac Int 20:1025–1039
Palermo FA, Mosconi G, Avella MA, Carnevali O, Verdenelli MC, Cecchini C, Polzonetti-Magni AM (2011) Modulation of cortisol levels, endocannabinoid receptor 1A, proopiomelanocortin and thyroid hormone receptor alpha mRNA expressions by probiotics during sole (Solea solea) larval development. Gen Comp Endocrinol 171:293–300
Farto R, Armada SP, Montes M, Guisande JA, Perez MJ, Nieto TP (2003) Vibrio lentus associated with diseased wild octopus (Octopus vulgaris). J Invertebr Pathol 83:149–156
Austin B, Austin D, Sutherland R, Thompson F, Swings J (2005) Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ Microbiol 7:1488–1495
Carnevali O, de Vivo L, Sulpizio R, Gioacchini G, Olivotto I, Silvi S, Cresci A (2006) Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax, L.), with particular attention to IGF-1, myostatin and cortisol gene expression. Aquaculture 258:430–438
De Schrijver R, Ollevier F (2000) Protein digestion in juvenile turbot (Scophthalmus maximus) and effects of dietary administration of Vibrio proteolyticus. Aquaculture 186:107–116
Reitan KI, Natvik CM, Vadstein O (1998) Drinking rate, uptake of bacteria and microalgae in turbot larvae. J Fish Biol 53:1145–1154
Hansen GH, Olafsen JA (1999) Bacterial interactions in early life stages of marine cold water fish. Microb Ecol 38:1–26
Munro PD, Barbour A, Birkbeck TH (1994) Comparison of the gut bacterial-flora of start-feeding larval turbot reared under different conditions. J Appl Bacteriol 77:560–566
Skjermo J, Bergh O (2004) High-M alginate immunostimulation of Atlantic halibut (Hippoglossus hippoglossus L.) larvae using Artemia for delivery, increases resistance against vibriosis. Aquaculture 238:107–113
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EDS designed the study, performed the experiments, drafted and wrote the manuscript. MV contributed to the experiments and interpretation. LD contributed to the experimental design and performed the statistical analyses. FH critically revised the manuscript. AD conceived of the study, participated in the design and coordination of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Prof. Dr P. Simoens is gratefully acknowledged for critically reviewing the manuscript. Furthermore, the authors would like to thank Alain Le Breton for kindly providing the V. anguillarum WT and V. harveyi WT isolates and the Laboratory of Aquaculture & Artemia Reference Center (UGent) for V. anguillarum HI610. Wageningen Marine Research (the Netherlands) and Stichting Zeeschelp (the Netherlands) are gratefully acknowledged for providing the S. solea eggs. Marleen Foubert is thanked for her assistance in the bacterial strain identification based on 16S rRNA gene sequencing.
Ethics approval and consent to participate
All experiments were approved by the Ethical Committee of the Faculty of Veterinary Medicine and Bio-engineering Sciences, Ghent University (No. EC2015/28, EC2015/70 and EC2015/73).
Funding
This research was supported by the Special Research Grant (Bijzonder Onderzoeksfonds, BOF12/GOA/022 and BOF12/BAS/070) Ghent University, Belgium.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
De Swaef, E., Vercauteren, M., Duchateau, L. et al. Experimental infection model for vibriosis in Dover sole (Solea solea) larvae as an aid in studying its pathogenesis and alternative treatments. Vet Res 49, 24 (2018). https://doi.org/10.1186/s13567-018-0520-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13567-018-0520-3