Abstract
Background
Cerebral blood flow (CBF) quantitation using technetium-99m hexamethylpropyleneamine oxime (99mTc-HMPAO) generally requires assessment of input function by arterial blood sampling, which would be invasive for small animals. We therefore performed chest dynamic planar imaging, instead of arterial blood sampling, to estimate the input function and establish noninvasive quantitation method of rat CBF using the image-derived input function.
Results
Integrated radioactivity concentration in the heart-blood pool on planar images (AUCBlood-planar) was identical to that in arterial blood samples (AUCBlood-sampling). Radioactivity concentration in the brain determined by SPECT imaging (CBrain-SPECT) was identical to that using brain sampling (CBrain-sampling). Noninvasively calculated CBF obtained by dividing CBrain-SPECT by AUCBlood-planar was well correlated with conventionally estimated CBF obtained by dividing CBrain-sampling by AUCBlood-sampling.
Conclusion
Rat CBF could be noninvasively quantitated using 99mTc-HMPAO chest dynamic planar imaging and head SPECT imaging without arterial blood sampling.
Similar content being viewed by others
Background
Cerebral blood flow (CBF) is associated with brain function in acute and chronic brain disorders, such as cerebrovascular disease, dementia, and epilepsy [1]. CBF levels also influence drugs’ therapeutic efficacy because most drug delivery to the brain is dependent on CBF. For studies using small-animal models of these brain disorders, CBF assessment meaningfully enhances the understanding of pathology in model animals and facilitates evaluation of drug efficacy.
Tc-99m hexamethylpropyleneamine oxime (99mTc-exametazime, 99mTc-HMAO) is a single-photon emission computed tomography (SPECT) radiotracer for CBF assessment [2]. Our previous study reported that rat CBF could be quantified using 99mTc-HMPAO [3]. Quantitative assessment of CBF using 99mTc-HMPAO requires input function, which is generally assessed by arterial blood sampling. Arterial cannulation would be invasive for small animals and difficult to repeat in the same animal over the long term [4]. In addition, abundant and frequent blood sampling can affect the physiological condition, including blood pressure, blood cell count, and plasma hormone levels [5, 6]. Although blood sampling systems using microfluidic technologies have been developed to reduce the amount of blood sampling from small animals [4, 7], they cannot entirely obviate the need for arterial cannulation and blood sampling. Therefore, evaluation of the input function without blood sampling would be desirable for CBF quantification in small animals.
Input function assessed by dynamic imaging of a large blood pool is used for quantitative analysis of some SPECT and positron emission tomography (PET) radiotracers in humans [8,9,10] and small rodents [11]. In the present study, we performed dynamic planar imaging of the heart after intravenous administration of 99mTc-HMPAO to assess image-derived input function. Rat CBF was quantified using the input function obtained from dynamic planar images and compared with the CBF conventionally quantified using arterial blood sampling.
Methods
Sodium [99mTc]pertechnetate was eluted from a 99Mo/99mTc generator (Ultra Techne Kow, Fujifilm RI Pharma Co., Ltd., Tokyo, Japan), which was previously eluted within 24 h. The kit formulation of HMPAO was obtained from Nihon Medi-Physics Co., Ltd. (Tokyo, Japan). The animal experiments in this study were performed in accordance with institutional and national guidelines regarding animal care and approved by the Animal Care and Use Committee of the Hamamatsu University School of Medicine. Male SD rats (8 w) supplied by Japan SLC Co. (Hamamatsu, Japan) were housed under light/dark 12-h cycles and had free access to food and water.
Preparation of 99mTc-HMPAO
Approximately 1.11 GBq freshly eluted sodium [99mTc]pertechnetate (5 mL) was added to the reaction vial of the HMPAO kit, and the reaction vial was gently swirled for a few seconds. Radiochemical purity was determined using a paper chromatography strip (Tec-Control Chromatography Strips for Bicisate and Exametazine; Biodex, Shirley, NY, USA) with ethyl acetate used as the mobile phase. Radioactivity in the fraction of the strips was determined with an auto-well gamma counter (1480 WIZARD2 3, Perkin Elmer, Waltham, MA, USA). 99mTc-HMPAO was injected into the rats within 30 min after its preparation. The radiochemical purity of 99mTc-HMPAO just before injection was 90.5% ± 2.2%.
Animal preparation
Rats (n = 13) were fasted for 15 h and then administered pentobarbital (50 mg/kg) intraperitoneally. The subsequent experiments were performed under pentobarbital anesthesia. A polyethylene catheter (i.d. 0.5 mm, o.d. 0.8 mm) was inserted into the femoral artery and filled with heparin saline solution (10 IU/mL) for blood sampling. Acetazolamide (0 mg/kg (n = 8), 25 mg/kg (n = 2), or 50 mg/kg (n = 3)) was then injected intravenously 15 min before 99mTc-HMPAO administration.
Experimental protocol
99mTc-HMPAO (~ 185 MBq) was injected intravenously into the rats. Just after the 99mTc-HMPAO injection, dynamic chest coronal planar scans were performed dorsally for 1 min using a small animal PET/SPECT/CT system (FLEX; Gamma Medica Ideas, Northridge, CA, USA) equipped with single pinhole collimators (1 mm). Arterial blood (approximately 100 μL per sample) was collected at 2, 6, 10, 14, 18, 22, 26, 30, 34, 38, 42, 46, 50, 54, and 58 s post-injection of 99mTc-HMPAO. After a CT scan of the head, SPECT scan was performed using the small animal FLEX system equipped with multiple pinhole collimators (1 mm) from 15 to 57 min post-injection of 99mTc-HMPAO, since radioactivity concentration in the brain is steady from 14 s to 60 min post-injection of 99mTc-HMPAO [3]. SPECT data acquisition was performed at 30 s per projection with stepwise rotation of 64 projections over 360°. SPECT images were reconstructed using three-dimensional-ordered subset expectation maximization algorithms with 8 subsets and 10 iterations. After SPECT scanning, the rats were immediately sacrificed by decapitation, and the brain was removed and weighed. 99mTc radioactivity concentrations in the arterial blood samples (CBlood-sampling) and brain (CBrain-sampling) were measured with the auto-well gamma counter.
Phantom studies were performed using a 14.1 mm diameter cylindrical phantom with known 99mTc concentration (~ 11.1 MBq/mL) to mimic the size of rat heart and brain. Planar and SPECT imagings of the phantom were performed under the same conditions as already described rat heart and brain imagings. The region of interest (ROI) on planar images and the volume of interest (VOI) were manually drawn over the whole phantom to cross-calibrate between the radioactivity concentrations obtained from the planar or SPECT images, and auto-well gamma counter.
CBF calculation using arterial blood and brain sampling (conventional procedure)
CBF was calculated using the microsphere method, as previously described [3], with slight modification. Briefly, CBlood-sampling at 14–60 s was fitted to a biexponential curve by the least-squares method to estimate the arterial blood radioactivity concentration at 19 s post-injection according to previous results [3]. CBlood-sampling was integrated using the trapezoidal approximation to obtain the area under the curve (AUC) until 19 s post-injection of 99mTc-HMPAO (AUCBlood-sampling). CBrain-sampling quantified by the auto-well gamma counter was divided by the AUCBlood-sampling value to calculate the CBF using arterial blood and brain sampling (CBF-sampling).
CBF calculation using planar and SPECT imaging
The ROI was manually drawn on the heart in the chest planar image using the radiographic planar image of the same rat as a reference (Fig. 1a), and the mean radioactivity concentration in the ROI was quantified (CBlood-planar). CBlood-planar was integrated using the trapezoidal approximation to obtain the AUC until 19 s post-injection of 99mTc-HMPAO (AUCBlood-planar). Head CT and SPECT images were combined, and the VOI was manually drawn over the whole brain (Fig. 2a). CBrain was quantified as the mean concentration in the VOI (CBrain-SPECT). CBrain-SPECT was divided by AUCBlood-planar to obtain CBF-imaging. CBF-imaging was also compared with the CBF calculated from CBrain-SPECT and AUCBlood-sampling to estimate the effects on CBF calculation between AUCBlood-planar and AUCBlood-sampling.
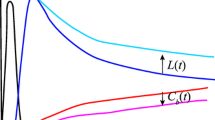
Input function of technetium-99m hexamethylpropyleneamine oxime (99mTc-HMPAO). a Typical chest planar radiographic image (left panel) and 99mTc-HMPAO images (middle and right panels) from the same rat. Region of interest (ROI) was manually surrounded on the 99mTc-HMPAO-planar image by a red circle using the corresponding radiographic image as a reference. b Typical time–activity curves of the radioactivity concentration in arterial blood samples (CBlood-sampling, dashed line) and in the heart on planar images (CBlood-planar, solid line). c Comparison of integrated radioactivity concentrations in arterial blood samples (AUCBlood-sampling) and that in the heart on planar images (AUCBlood-planar) until 19 s post-injection of 99mTc-HMPAO. Correlation between the AUC value determined by arterial blood sampling and that by chest dynamic planar imaging was assessed using Spearman’s rank correlation coefficient
Radioactivity concentration in the brain (CBrain) after 99mTc-HMPAO injection. a Typical single-photon emission computed tomography/computed tomography (SPECT/CT) fusion transversal image of rat brain during 15–57 min post-injection of 99mTc-HMPAO (left panel) and the manually drawn volume of interest (VOI) (red circle, right panel). b Comparison of radioactivity concentration in brain determined by resection just after SPECT (CBrain-sampling) and that by SPECT 15–57 min post-injection of 99mTc-HMPAO (CBrain-SPECT). Correlation between the concentration determined by resection and that by SPECT imaging was assessed using Spearman’s rank correlation coefficient
Statistical analysis
The associations between AUCBlood-sampling and AUCBlood-planar, CBrain-sampling and CBrain-SPECT, and CBF-sampling and CBF-imaging were calculated using Spearman’s rank correlation coefficients.
Results
Input function measured from dynamic planar images vs. blood sampling
The time–activity curves for the heart measured from dynamic chest planar images and in arterial blood of the same rat measured from blood samples are shown in Fig. 1b. Both time–activity curves showed the highest concentration of 99mTc at 5–10 s post-injection and followed time-dependent decreased radioactivity. AUCBlood-planar showed a good correlation with AUCBlood-sampling (Fig. 1c) (n = 13, ρ = 0.868, p < 0.001), and the regression line was nearly y = x (slope = 1.09).
Brain radioactivity measured from SPECT images vs. resected samples
Figure 2b compares CBrain obtained from SPECT images and resected brain samples. CBrain-SPECT was well correlated with CBrain-sampling (n = 13, ρ = 0.929, p < 0.001), and the regression line was nearly y = x (slope = 1.02).
Noninvasively quantified CBF vs. conventionally quantified CBF
Figure 3a shows the correlation between CBF-imaging, which is noninvasively quantified using AUCBlood-planar and CBrain-SPECT, and CBF-sampling, which is conventionally quantified using AUCBlood-sampling and CBrain-sampling. CBF-imaging showed a good correlation with CBF-sampling. (n = 13, ρ = 0.918, p < 0.001). The regression line was nearly y = x (slope = 0.94).
CBF quantification. a Comparison of conventionally quantitated CBF (CBF-sampling) calculated by dividing CBrain-sampling by AUCBlood-sampling and noninvasively quantitated CBF (CBF-imaging) calculated by dividing CBrain-SPECT by AUCBlood-planar. Correlation between CBF-sampling and CBF-imaging was assessed using Spearman’s rank correlation coefficient. b Comparison of CBF (CBF-SPECT/sampling) calculated by dividing CBrain-SPECT by AUCBlood-sampling and noninvasively quantitated CBF (CBF-SPECT/imaging) calculated by dividing CBrain-SPECT by AUCBlood-planar. Correlation between CBF-sampling and CBF-imaging was assessed using Spearman’s rank correlation coefficient
The effects on CBF quantification between dynamic planar images and blood sampling
Figure 3b shows the correlation between CBF-imaging and CBF quantified using AUCBlood-sampling and CBrain-SPECT (CBF-SPECT/sampling). CBF-imaging showed a good correlation with CBF-SPECT/sampling (n = 13, ρ = 0.945, p < 0.001). The regression line was nearly y = x (slope = 0.90).
Discussion
Rat CBF could be quantified by a microsphere method in which CBrain at 5 min post-injection is divided by the integrated arterial blood radioactivity concentration until 19 s post-injection of 99mTc-HMPAO (AUCBlood) [3]. In the present study, AUCBlood and CBrain were noninvasively estimated using planar imaging and SPECT, respectively. Using the noninvasively estimated AUCBlood-planar and CBrain-SPECT, rat CBF was quantified (CBF-imaging) and compared with conventionally quantified rat CBF (CBF-sampling).
CBlood was estimated using dynamic planar imaging of the heart-blood pool. To quantify the radioactivity concentration in arterial blood as an input function via chest dynamic planar images, the ROI should be drawn only on the left ventricle cavity. However, in this study, we drew the ROI over the whole heart to calculate the blood radioactivity concentration in the heart (CBlood-planar) as an input function, because rat left ventricle cavity is too small and too complicated to confirm by two-dimensional planar images. Hence, there could be some differences between CBlood-sampling and CBlood-planar values. In addition, planar imaging could underestimate the radioactivity concentration because of scatter and attenuation of gamma rays [12]. Despite these differences, CBlood-planar was identical to CBlood-sampling until 19 s post-injection of 99mTc-HMPAO (Fig. 1b). AUCBlood-planar was well correlated with AUCBlood-sampling (Fig. 1c), suggesting that dynamic chest planar imaging could estimate the AUCBlood until 19 s post-injection, which is required to quantify the rat CBF using 99mTc-HMPAO [3]. These results indicate that the differences between arterial blood sampling and chest planar imaging might be negligible for estimating AUCBlood until 19 s post-injection under the experimental conditions of the present study.
CBrain was determined by SPECT. Because our previous study showed that CBrain was quite steady from 14 s to 60 min post-injection of 99mTc-HMPAO [3], SPECT would be well suited for quantitative evaluation of CBrain within this period. Figure 2b shows that CBrain-SPECT at 15–57 min post-injection was identical to CBrain-sampling determined just after SPECT imaging. This result suggests that scatter and attenuation of gamma rays would be negligible for CBrain quantitation by SPECT under these conditions, and SPECT could determine CBrain after 99mTc-HMPAO injection. Considering that CBrain is steady from 14 s to 60 min post-injection of 99mTc-HMPAO [3], CBrain-SPECT at 15–57 min post-injection of 99mTc-HMPAO could be equal to CBrain at 5 min post-injection, which is required for quantitation of rat CBF by the 99mTc-HMPAO microsphere method [3].
Thus, rat CBF was calculated using a microsphere method in which CBrain was divided by AUCBlood until 19 s post-injection. CBF-imaging, which is obtained by dividing CBrain-SPECT by AUCBlood-planar, was identical to conventionally estimated CBF-sampling using CBrain-sampling and AUCBlood-sampling, suggesting that rat CBF could be quantitatively estimated using 99mTc-HMPAO-planar and SPECT imaging without sampling arterial blood. Thus, noninvasive quantitation of rat CBF could enhance the repeatable CBF assessment without the physiological damage due to arterial cannulation and blood sampling.
Conclusions
We propose a noninvasive procedure for quantitating rat CBF using 99mTc-HMPAO. Rat CBF values, which were noninvasively quantified using chest dynamic planar imaging and head SPECT, were nearly identical to conventionally determined rat CBF using arterial blood and brain sampling. These findings indicate that rat CBF could be noninvasively and quantitatively assessed by combining 99mTc-HMPAO-planar imaging and SPECT, thereby avoiding arterial blood sampling.
References
Catafau AM. Brain SPECT in clinical practice. Part I: perfusion. J Nucl Med. 2001;42:259–71.
Andersen AR. 99mTc-D,L-hexamethylene-propyleneamine oxime (99mTc-HMPAO): basic kinetic studies of a tracer of cerebral blood flow. Cerebrovasc Brain Metab Rev. 1989;1:288–318.
Suzuki C, Kimura S, Kosugi M, Magata Y. Quantitation of rat cerebral blood flow using 99mTc-HMPAO. Nucl Med Biol. 2017;47:19–22.
Huang CC, Wu CH, Huang YY, Tzen KY, Chen SF, Tsai ML, et al. Performing repeated quantitative small-animal PET with an arterial input function is routinely feasible in rats. J Nucl Med. 2017;58:611–6.
Hoff J. Methods of blood collection in the mouse. Lab Animal. 2000;29:47–53.
McGuill MW, Rowan AN. Biological effects of blood loss: implications for sampling volumes and techniques. ILAR J. 1989;31:5–20.
Kimura Y, Seki C, Hashizume N, Yamada T, Wakizaka H, Nishimoto T, et al. Novel system using microliter order sample volume for measuring arterial radioactivity concentrations in whole blood and plasma for mouse PET dynamic study. Phys Med Biol. 2013;58:7889–903.
Zanotti-Fregonara P, Chen K, Liow JS, Fujita M, Innis RB. Image-derived input function for brain PET studies: many challenges and few opportunities. J Cerebr Blood F Met. 2011;31:1986–98.
Iida H, Miura S, Shoji Y, Ogawa T, Kado H, Narita Y, et al. Noninvasive quantitation of cerebral blood flow using oxygen-15-water and a dual-PET system. J Nucl Med. 1998;39:1789–98.
Yonekura Y, Sugihara H, Taniguchi Y, Aoki E, Furuichi K, Miyazaki Y. Quantification of brain perfusion SPECT with N-isopropyl-p-iodoamphetamine using noninvasive microsphere method: estimation of arterial input by dynamic imaging. Kaku igaku Japanese J Nucl Med. 1997;34:901–8.
Fang YHD, Muzic RF. Spillover and partial-volume correction for image-derived input functions for small-animal F-18-FDG PET studies. J Nucl Med. 2008;49:606–14.
Andrew BH, Benjamin LF, Grant TG, Bruce HH. Assessment of the sources of error affecting the quantitative accuracy of SPECT imaging in small animals. Phys Med Biol. 2008;53:2233.
Acknowledgements
We thank Nancy Schatken, BS, MT(ASCP), from Edanz Group (http://www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
This work was supported in part by KAKENHI grants (26670553 and 25293626) from the Japan Society for the Promotion of Science (JSPS) and a grant from the Smoking Research Foundation.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Contributions
YM conceived and designed the present study. CS and MK acquired and analyzed the data. CS created the figures and drafted the article. YM supervised, and oversaw the quality control, the overall project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The animal experiments in this study were performed in accordance with institutional and national guidelines regarding animal care and approved by the Animal Care and Use Committee of the Hamamatsu University School of Medicine. This article does not describe any studies with human participants performed by any of the authors.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Suzuki, C., Kosugi, M. & Magata, Y. Noninvasive quantitation of rat cerebral blood flow using 99mTc-HMPAO—assessment of input function with dynamic chest planar imaging. EJNMMI Res 8, 21 (2018). https://doi.org/10.1186/s13550-018-0375-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13550-018-0375-7