Abstract
Background
Hard ticks act as arthropod vectors in the transmission of human and animal pathogens and are widely distributed in northern China. The aim of this study is to screen the important tick-borne pathogens (TBPs) carried by hard ticks in Inner Mongolia using metagenomic next-generation sequencing (mNGS) and to estimate the risk of human infection imposed by tick bites.
Methods
The adult Dermacentor nuttalli (n = 203) and Ixodes persulcatus (n = 36) ticks feeding on cattle were collected. The pooled DNA samples prepared from these ticks were sequenced as the templates for mNGS to survey the presence of TBPs at the genus level. Individual tick DNA samples were detected by genus--specific or group-specific nested polymerase chain reaction (PCR) of these TBPs and combined with DNA sequencing assay to confirm the results of mNGS.
Results
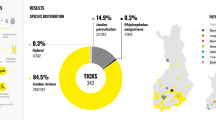
R. raoultii (45.32%, 92/203), Candidatus R. tarasevichiae (5.42%, 11/203), Anaplasma sp. Mongolia (26.60%, 54/203), Coxiella-like endosymbiont (CLE) (53.69%, 109/203), and Babesia venatorum (7.88%, 16/203) were detected in D. nuttalli, while R. raoultii (30.56%, 11/36), Anaplasma sp. Mongolia (27.80%, 10/36), and CLE (27.80%, 10/36) were detected in I. persulcatus. The double- and triple-pathogen/endosymbiont co-infections were detected in 40.39% of D. nuttalli and 13.89% of I. persulcatus, respectively. The dual co-infection with R. raoultii and CLE (14.29%, 29/203) and triple co-infection with R. raoultii, Anaplasma sp. Mongolia, and CLE (13.79%, 28/203) were most frequent in D. nuttalli.
Conclusions
This study provides insight into the microbial diversity of D. nuttalli and I. persulcatus in Inner Mongolia, China, reporting for the first time that Candidatus R. tarasevichiae had been found in D. nuttalli in China, and for the first time in the world that Anaplasma sp. Mongolia has been detected in I. persulcatus. This study proves that various vertically transmitted pathogens co-inhabit D. nuttalli and I. persulcatus, and indicates that cattle in Inner Mongolia are exposed to several TBPs.
Graphical Abstract

Similar content being viewed by others
Background
Hard ticks (Acari: Ixodidae) are obligate blood-sucking parasitic arthropods which can infest mammals, birds, and reptiles, and act as arthropod vectors in the transmission of human and animal pathogens. A wide variety of pathogens can be maintained and transmitted by hard ticks, including Ehrlichia spp., Anaplasma spp., Rickettsia spp., Coxiella spp., Babesia spp., Borrelia spp., etc. [1]. In addition, a variety of endosymbionts, such as Coxiella-like, Rickettsia-like, and Arsenophonus-like endosymbionts, live inside hard ticks [2,3,4]. Therefore, hard ticks are usually considered to be the most important vectors of pathogens, and knowledge of the microbial communities within these ticks will be of benefit for risk assessment of tick-borne diseases.
China covers approximately 9.6 million square kilometers of land area. At least 117 tick species in ten genera of two families have been reported in China [5], with Ixodes persulcatus, Dermacentor nuttalli, Hyalomma asiaticum, Dermacentor marginatus, and Dermacentor niveus being the most common [6]. Increasing numbers of cases of human tick-borne diseases, including spotted fever [7], Q fever [8], anaplasmoses, ehriochioses [9], tick-borne encephalitis [10], and babesiosis [11], have been reported in China due to climate change combined with human movement into tick habitats. Therefore, human co-infection with more than one tick-borne pathogen (TBP) may occur after tick bite [12,13,14].
Mongolia Hulunbuir League, one of the important pastoral regions in Inner Mongolia in China, contains significant amounts of pastures and is an important region for animal production. The eastern part of Hulunbuir, stretching across the primeval forest-covered area in the Daxing'anling Mountains, is an important habitat for hard ticks [15]. Many TBPs including C. burnetii [16], Rickettsia spp. [16,17,18], Anaplasma spp. [19], and tick-borne encephalitis virus [20] have been detected in hard ticks collected here. However, little attention has been given to co-infection with TBPs in ticks, and continued research is needed to fully comprehend the diversity of TBPs. In this study, we investigated the microbial communities in hard ticks collected from cattle in Hulunbuir League to reveal the coexistence of TBPs using metagenomic next-generation sequencing (mNGS) combined with nested polymerase chain reaction (PCR). The results of our study might provide broader knowledge of the microorganisms inside hard ticks in the region, thereby strengthening programs to prevent and control the potential infections caused by TBPs.
Methods
Collection and identification of ticks
All ticks collected were feeding on cattle in Balin Town (E 122°24′10″, N 48°19′26″; E 122°22′12″, N 48°20′11″; E 122°22′13″, N 48°19′47″; E 122°24′59″, N 48°19′55″; E 122°21′11″, N 48°19′59″; E 122°20′59″, N 48°19′16″), Yake City, Hulunbuir League, Inner Mongolia, from April to October in 2019 (Fig. 1). Tick species were identified based on morphological characterization and by molecular biology methods based on the sequences of species-specific 16S rRNA and mitochondrial cytochrome c oxidase I (COI) genes as previously described [21]. Following identification, the ticks were stored at −80 °C for further analysis.
Tick washing, homogenization, and DNA extraction
To remove environmental contaminants, each tick was surface-sterilized twice with 75% ethanol, followed by phosphate-buffered saline (PBS) twice. Ticks were then individually homogenized in 300 μL of PBS using MagNA Lyser Green Beads (Roche, Mannheim, Germany), and DNA extraction was performed on 200 μL of each tick homogenate using a QIAamp® Fast DNA Tissue Kit (Qiagen, Dusseldorf, Germany) according to the manufacturer’s instructions. The extracted genomic DNA was dissolved in 100 μL ultrapure water and stored at −20 °C for further analysis. For these previous steps, ultrapure water, sterile tubes, and filter tips were used, and all operations were carried out in a biological safety cabinet. Each time DNA extraction was performed, an extraction control (water) was added.
Individual DNA samples were mixed in an equal volume (20 μL) to prepare pooled DNA samples for full microbial genome sequencing using mNGS.
Metagenome assembly, gene prediction, and taxonomy prediction
All pooled DNA samples were paired-end sequenced on the Illumina HiSeq platform (insert size 350 bp, read length 150 bp) by the Beijing Genomics Institute (BGI) (Beijing, China). The reads with more than 40 nt low-quality bases (quality value ≤ 38) were removed. Meanwhile, the reads with more than 10 nt “N” bases were filtered out of the data sets. Lastly, the reads overlapping more than 15 nt bases with the adapters were removed. Reads that aligned to tick genes were also removed using Bowtie 2 (v2.2.4) with the parameters -end-to-end, -sensitive, -I 200, -X 400 [22, 23]. Accordingly, the clean data were obtained.
Then the clean reads were mapped against scaffolds using SOAPdenovo (V2.04) with the parameters -d 1, -M 3, -R, -u, -F, -K 55 [24]. The unused reads from each sample were then assembled using the same parameters. The scaffolds were broken at N into the scaftigs [25], and the scaftigs with the length of ≥ 500 nt were used for further analysis [26]. Open reading frames (ORFs) in the scaftigs (≥ 500 bp) were predicted by MetaGeneMark (V2.10) [23, 27]. A nonredundant gene catalog was obtained after processing by using CD-HIT (V4.5.8) with the parameters -c 0.95, -G 0, -aS 0.9, -g 1, -d 0 [28, 29], and using a sequence identity threshold of 0.95 and a minimum coverage cutoff of 0.9. To determine the gene abundances, the reads were realigned with the gene catalog using Bowtie 2 and the following parameters: -m 200 -× 400 -s 119. Only genes with ≥ 2 mapped reads were deemed to be present in a sample [30]. Relative abundance of genes was calculated based on the number of reads mapped to the genes and the length of the genes as previously described [31,32,33].
To access the taxonomic assignments of genes, genes were aligned to the integrated NR database (version: 2018-01-02) of NCBI using DIAMOND (V0.9.9) and default parameters, with the exception of -k 50 -sensitive -e 0.00001 [34]. For each gene, the significant matches which were defined by e-values ≤ 10 * e-value of the top hit were retained to distinguish taxonomic groups [30]. Then the taxonomical level of each gene was determined by using the lowest common ancestor (LCA)-based algorithm implemented in MEGAN [35]. The results containing the number of genes and the abundance information for each sample, and the relative abundances of each taxonomic group were calculated by adding the relative abundances of genes annotated to the same feature [23, 26, 36].
Polymerase chain reaction (PCR)
Based on the results of mNGS, genus-/group-specific PCR was performed to confirm the presence of TBPs in individual ticks. PCR was performed using a PCR System 9700 (Applied Biosystems, GeneAmp®, USA). For nested PCR, 1 μL of each individual DNA sample (150–330 ng) was used as template for the first round, and 1 μL of the primary PCR production was used as template for the second round. For the first round, a negative control (water) and an extraction control mentioned above were included in each PCR experiment. Tube strips with individual caps were used in amplification steps to prevent cross-contamination, and all PCR amplifications were carried out using PrimeSTAR® HS (Premix) (TaKaRa, Beijing, China). All operations were carried out in a biological safety cabinet. Amplified products were then electrophoresed in 1.5% agarose gel, and the positive amplicons were sent to TSINGKE Biological Technology (Beijing, China) for sequencing. The PCR primers for the spotted fever group Rickettsia (SFGR) [37], Anaplasma spp. and Ehrlichia spp. [38], Coxiella spp. [39], and Babesia spp. [40] are presented in Table 1.
Phylogenetic analysis
The obtained DNA sequences were compared with those available in GenBank using the National Center for Biotechnology Information (NCBI; Bethesda, MD) Basic Local Alignment Search Tool (BLAST) search engine (http://blast.ncbi.nlm.nih.gov/blast.cgi), and multiple sequence alignment was performed using the ClustalW multiple alignment tool with the default parameters in MEGA 7.0. The phylogenetic analysis of gltA for SFGR, 16S rRNA for Anaplasma spp., 16S rRNA for Coxiella spp., or 18S rRNA for Babesia spp. was performed using the maximum likelihood method based in MEGA 7.0. Bootstrap values were estimated for 1000 replicates [41, 42].
Results
Taxonomic classification
A total of 239 adult hard ticks were identified as D. nuttalli (n = 203) (accession number: MK213083.1) and I. persulcatus (n = 36) (accession number: MH790201.1) based on morphological identifications confirmed by species-specific PCR and sequencing assay. Ten pools of D. nuttalli DNA samples were finally analyzed by mNGS on the Illumina HiSeq platform. Sequencing yielded between 5970 and 7475 million reads per pool, while all were of high quality (Clean_Q20 > 96%) (shown in Additional file 1: Table S1). The construction of a metagenomic library of I. persulcatus DNA samples failed.
The presence of the bacterial genera Rickettsia, Anaplasma, and Coxiella in the pooled tick samples was confirmed by the taxonomic profiles at genus level (Fig. 2; Table 2). Rickettsia spp. were most abundant in sample pools 2–4 and 7–8 and also abundant in other pools. In pools 1, 9, and 10, Anaplasma spp. were abundant. However, Coxiella spp. were abundant only in pool 1. In addition, Pseudomonas spp. were most abundant in pools 5 and 6, and Psychrobacter spp. were most abundant in pool 10 (Table 2).
Prevalence of tick-borne pathogens in individual ticks
By mNGS, the important pathogenic bacterial genera Rickettsia, Anaplasma, and Coxiella were found in the pooled tick samples, and thus each tick was detected by the genus-/group-specific PCR combined with sequencing in order to identify the TBPs carried by it. In addition, Babesia spp. were often detected in ticks, and thus each tick was detected by Babesia-specific PCR.
As a result, R. raoultii (45.32%, 92/203), Candidatus R. tarasevichiae (5.42%, 11/203), Anaplasma sp. Mongolia (26.6%, 54/203), Coxiella-like endosymbiont (CLE) (53.69%, 109/203), and Babesia venatorum (7.88%, 16/203) were detected in D. nuttalli, while R. raoultii (30.56%, 11/36), Anaplasma sp. Mongolia (27.8%, 10/36), and CLE (27.8%, 10/36) were detected in I. persulcatus (Table 3).
Co-infection in individual ticks
In 190 TBP-positive ticks, 87 ticks (45.79%) were found to be co-infected with more than one species identified in the present study (Table 3). The dual- and triple-pathogen/endosymbiont co-infections were detected in 40.39% of D. nuttalli and 13.89% of I. persulcatus. The dual co-infection with R. raoultii and CLE and the triple co-infection with R. raoultii, Anaplasma sp. Mongolia and CLE were most frequent in D. nuttalli (Table 3).
Phylogenetic analysis
By phylogenetic analysis, R. raoultii and Candidatus R. tarasevichiae were placed in a clade with R. raoultii Binxian-91 (MN450399.2) and Candidatus R. tarasevichiae (MN450396.2, MN450397.2), respectively (Fig. 3). Anaplasma sp. Mongolia identified in both D. nuttalli and I. persulcatus were shown to be clustered with Anaplasma sp. Mongolia 6 (LC194132.1) (Fig. 4). Coxiella-like endosymbiont identified in D. silvarum and in I. persulcatus were placed in a clade with Coxiella endosymbiont of D. silvarum (KP994814.1) and in a clade with Coxiellaceae bacterium RFE03 (KM079619.1), respectively (Fig. 5). B. venatorum was most close to Babesia sp. Venatorum strain HLJ371 (KU204792.1) and Babesia sp. YZ-2012 isolate hlj223 (JQ993426.2) (Fig. 6).
Phylogenetic tree of R. raoultii and Candidatus R. tarasevichiae in ticks based on partial gltA gene sequence similarity. The sequence obtained in this study is indicated with a black dot. Sequences were aligned using the MEGA 7 (version 7.0) software package. Phylogenetic analysis was performed by the maximum likelihood method, and bootstrap values were estimated for 1000 replicates
Phylogenetic tree of Anaplasma sp. Mongolia in ticks based on partial 16S rRNA gene sequence similarity. The sequence obtained in this study is indicated with a black dot. Sequences were aligned using the MEGA 7 (version 7.0) software package. Phylogenetic analysis was performed by the maximum likelihood method, and bootstrap values were estimated for 1000 replicates
Phylogenetic tree of Coxiella-like endosymbionts in ticks based on partial 16S rRNA gene sequence similarity. The sequence obtained in this study is indicated with a black dot. Sequences were aligned using the MEGA 7 (version 7.0) software package. Phylogenetic analysis was performed by the maximum likelihood method, and bootstrap values were estimated for 1000 replicates
Phylogenetic tree of B. venatorum in ticks based on partial 18S rRNA gene sequence similarity. The sequence obtained in this study is indicated with a black dot. Sequences were aligned using the MEGA 7 (version 7.0) software package. Phylogenetic analysis was performed by the maximum likelihood method, and bootstrap values were estimated for 1000 replicates
Discussion
In recent years, much attention has been focused on ticks and TBPs in China. However, although a variety of pathogens have been identified, co-infection with multiple pathogens in hard ticks has rarely been investigated. In this study, we applied mNGS combined with nested PCR to survey TBPs in D. nuttalli and I. persulcatus feeding on cattle in Inner Mongolia, China.
By mNGS, the endosymbionts including Coxiella spp., Rickettsia spp., Francisella spp., and “Candidatus Midichloria mitochondrii” have been recognized as the most abundant bacterial species identified frequently in entirely homogenized ticks [43]. Qiu et al. applied NGS to examine the microbiomes of salivary glands of ticks collected in Japan, revealing a large number of bacterial genera, including 71 I. ovatus, 127 I. persulcatus, and 59 H. flava, and detecting some of the medically important bacteria including Coxiella spp., Ehrlichia spp., and Rickettsia spp. [44].
In the present study, the pooled DNA samples of D. nuttalli and I. persulcatus collected were assayed by mNGS. The result revealed the presence of the bacterial genera Rickettsia, Anaplasma, and Coxiella in these ticks. In order to identify the bacteria at species level, each tick was detected by SFGR-, Anaplasma and Ehrlichia-, and Coxiella-specific PCR as well as Babesia-specific PCR, respectively. After sequencing of the DNA fragments amplified by PCR and the sequence comparison, two Rickettsia species (R. raoultii and Candidatus R. tarasevichiae), one Anaplasma species (Anaplasma sp. Mongolia), B. venatorum, and CLEs were found in D. nuttalli, while R. raoultii, Anaplasma sp. Mongolia, and CLEs were also found in I. persulcatus.
R. raoultii, a species of SFGR, was firstly detected in Dermacentor ticks collected in Russia in 1999 and isolated from Dermacentor ticks and named in 2008 [45]. It is one of the causative agents of tick-borne lymphadenopathy (TIBOLA), which is also known as Dermacentor-borne necrosis erythema and lymphadenopathy (DEBONEL) in humans [46]. R. raoultii has been found to be present in various ticks, including Dermacentor, Haemaphysalis, Rhipicephalus, Hyalomma, and Amblyomma. In the present study, R. raoultii was detected in 45.32% of D. nuttalli and 30.56% of I. persulcatus, suggesting that it was the dominant Rickettsia species prevalent in the hard ticks in Inner Mongolia, and this may have health implications, as humans may suffer from spotted fever after experiencing a tick bite from this region. Phylogenetic analysis showed that the R. raoultii strains in D. nuttalli and I. persulcatus were identical and most related with R. raoultii Binxian-91 from H. longicornis in Shandong Province of China (MN450399.2), suggesting that its geographical distribution is wider in China.
Candidatus R. tarasevichiae, an emerging tick-borne pathogen, is also a species of SFGR. It was first detected in I. persulcatus collected from the southern Urals and Siberia in 2003 [47] and then found in Haemaphysalis ticks in Far East regions in Russia [48]. Human cases caused by Candidatus R. tarasevichiae have been found in China and Russia [49, 50]. In this study, Candidatus R. tarasevichiae was detected in 5.42% of D. nuttalli, which was most related to the Candidatus R. tarasevichiae Mulan-11 strain (MN450396.2) and Bayan-68 strain (MN450397.2) from I. persulcatus in China in phylogenetic analysis. This is the first time that Candidatus R. tarasevichiae has been detected in D. nuttalli.
Anaplasma sp. Mongolia was firstly detected in D. nuttalli [51] and bovine blood in Mongolia [51, 52], demonstrating that the Anaplasma species is an important cattle pathogen. In this study, Anaplasma sp. Mongolia was detected in 26.6% of D. nuttalli and in 27.8% of I. persulcatus. This study is thus the first in the world to report the presence of Anaplasma sp. Mongolia in I. persulcatus.
CLEs are relatively common in the microbiota of various tick species around the world, forming multiple subclusters in the cluster of the genus Coxiella in phylogenetic analysis [39, 53]. The presence of these symbiotic bacteria in ticks confers crucial and diverse benefits to the host, affecting its development, nutrition, chemical defense, or reproduction [53,54,55]. The prevalence of CLEs is from 6.25% in Rhipicephalus sanguineus to 100% in Amblyomma americanum in North America and Europe [53]. In the present study, CLEs were detected in 53.69% of D. nuttalli and 27.78% of I. persulcatus. Phylogenetic analysis suggested that the CLE strain of D. nuttalli, which was mostly related to that (KP994814.1) from D. silvarum in France, was different from that of I. persulcatus, which was mostly related to that (KM079619.1) of Haemaphysalis concinna from Russia.
Babesia spp. are the pathogenic agents of babesiosis in humans and animals. In the present study, B. venatorum was detected in 7.88% of D. nuttall. However, Babesia spp. was not found in the pooled tick samples by mNGS assay, which might be caused by the extremely low abundance of Babesia spp. in the pooled samples. According to phylogenetic analysis, B. venatorum of D. nuttalli was mostly related to B. venatorum strains YZ-2012 (JQ993426.2) and HLJ371 (KU204792.1) detected in I. persulcatus in Heilongjiang Province of China. B. venatorum can cause human infection masquerading as hemophagocytic syndrome [56].
In the present study, the multiple pathogen/endosymbiont co-infections were detected in 40.39% of D. nuttalli and 13.89% of I. persulcatus. Ticks may acquire multiple pathogenic species during blood feeding on their vertebrate hosts, and the hosts may also be infected by the pathogens carried by ticks. Due to the development of molecular diagnostic methods, more and more cases with co-infection of multiple TBPs have been reported [57]. The co-infection may be the result of a single tick bite by the tick carrying more than one pathogen or the result of multiple bites by ticks carrying different pathogens. Therefore, the prevalence of co-infection with TBPs in people living in the area close to the natural focus of TBPs should be investigated in the future.
Conclusions
This study proves that various vertically transmitted pathogens co-inhabit D. nuttalli and I. persulcatus, and is the first to report that Candidatus R. tarasevichiae has been found in D. nuttalli in China, and the first in the world to report that Anaplasma sp. Mongolia has been detected in I. persulcatus. This study provides insight into the microbial diversity of D. nuttalli and I. persulcatus in Inner Mongolia, China, and indicates that cattle in Inner Mongolia are exposed to several TBPs.
Availability of data and materials
All data supporting the conclusions of this article are included in the article.
Abbreviations
- mNGS:
-
Metagenomic next-generation sequencing
- CLE:
-
Coxiella-like endosymbiont
- TBP:
-
Tick-borne pathogen
- PCR:
-
Polymerase chain reaction
- rRNA:
-
Ribosomal RNA
- PBS:
-
Phosphate-buffered saline
- COI:
-
Mitochondrial cytochrome c oxidase I
- NCBI:
-
National Center for Biotechnology Information
- BLAST:
-
Basic Local Alignment Search Tool
- SFGR:
-
Spotted fever group Rickettsia
- ORF:
-
Open reading frame
- LCA:
-
Lowest common ancestor
Reference
Liu XY, Bonnet SI. Hard tick factors implicated in pathogen transmission. PLoS Negl Trop Dis. 2014;8(1):e2566. https://doi.org/10.1371/journal.pntd.0002566.
Duan DY, Liu GH, Cheng TY. Microbiome analysis of the saliva and midgut from partially or fully engorged female adult Dermacentor silvarum ticks in China. Exp Appl Acarol. 2020;80(4):543–58. https://doi.org/10.1007/s10493-020-00478-2.
Papa A, Tsioka K, Kontana A, Papadopoulos C, Giadinis N. Bacterial pathogens and endosymbionts in ticks. Ticks Tick Borne Dis. 2017;8(1):31–5. https://doi.org/10.1016/j.ttbdis.2016.09.011.
Ahantarig A, Trinachartvanit W, Baimai V, Grubhoffer L. Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol (Praha). 2013;58(5):419–28. https://doi.org/10.1007/s12223-013-0222-1.
Li LH, Zhang Y, Wang JZ, Li XS, Yin SQ, Zhu D, et al. High genetic diversity in hard ticks from a China-Myanmar border county. Parasit Vectors. 2018;11(1):469. https://doi.org/10.1186/s13071-018-3048-5.
Sheng J, Jiang M, Yang M, Bo X, Zhao S, Zhang Y, et al. Tick distribution in border regions of Northwestern China. Ticks Tick Borne Dis. 2019;10(3):665–9. https://doi.org/10.1016/j.ttbdis.2019.02.011.
Fan MY, Wang JG, Jiang YX, Zong DG, Lenz B, Walker DH. Isolation of a spotted fever group rickettsia from a patient and related ecologic investigations in Xinjiang Uygur Autonomous Region of China. J Clin Microbiol. 1987;25(4):628–32. https://doi.org/10.1128/JCM.25.4.628-632.1987.
Wu XB, Na RH, Wei SS, Zhu JS, Peng HJ. Distribution of tick-borne diseases in China. Parasit Vectors. 2013;6:119. https://doi.org/10.1186/1756-3305-6-119.
Zhang L, Liu Y, Ni D, Li Q, Yu Y, Yu XJ, et al. Nosocomial transmission of human granulocytic anaplasmosis in China. JAMA. 2008;300(19):2263–70. https://doi.org/10.1001/jama.2008.626.
Lu Z, Broker M, Liang G. Tick-borne encephalitis in mainland China. Vector Borne Zoonotic Dis. 2008;8(5):713–20. https://doi.org/10.1089/vbz.2008.0028.
Qi C, Zhou D, Liu J, Cheng Z, Zhang L, Wang L, et al. Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol Res. 2011;109(1):241–5. https://doi.org/10.1007/s00436-011-2382-8.
Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016;32(1):30–42. https://doi.org/10.1016/j.pt.2015.09.008.
Chisu V, Loi F, Foxi C, Chessa G, Masu G, Rolesu S, et al. Coexistence of tick-borne pathogens in ticks collected from their hosts in Sardinia: an update. Acta Parasitol. 2020;65(4):999–1004. https://doi.org/10.1007/s11686-020-00240-z.
Cutler SJ, Vayssier-Taussat M, Estrada-Pena A, Potkonjak A, Mihalca AD, Zeller H. Tick-borne diseases and co-infection: current considerations. Ticks Tick Borne Dis. 2021;12(1):101607. https://doi.org/10.1016/j.ttbdis.2020.101607.
Guo DH, Zhang Y, Fu X, Gao Y, Liu YT, Qiu JH, et al. Complete mitochondrial genomes of Dermacentor silvarum and comparative analyses with another hard tick Dermacentor nitens. Exp Parasitol. 2016;169:22–7. https://doi.org/10.1016/j.exppara.2016.07.004.
Batu N, Wang Y, Liu Z, Huang T, Bao W, He H, et al. Molecular epidemiology of Rickettsia sp. and Coxiella burnetii collected from Hyalomma asiaticum in Bactrian camels (Camelus bactrianus) in inner Mongolia of China. Ticks Tick Borne Dis. 2020;11(6):101548. https://doi.org/10.1016/j.ttbdis.2020.101548.
Yin X, Guo S, Ding C, Cao M, Kawabata H, Sato K, et al. Spotted fever group rickettsiae in Inner Mongolia, China, 2015–2016. Emerg Infect Dis. 2018;24(11):2105–7. https://doi.org/10.3201/eid2411.162094.
Feng J, Wu M, Huang T, Zhang J, Renbatu N, et al. Identification of two genotypes of Argas persicus and associated rickettsia-specific genes from different regions of Inner Mongolia. J Parasitol. 2019;105(1):92–101.
Yin H, Luo J. Ticks of small ruminants in China. Parasitol Res. 2007;101(Suppl 2):S187–9. https://doi.org/10.1007/s00436-007-0688-3.
Yu Z, Wang H, Wang T, Sun W, Yang X, Liu J. Tick-borne pathogens and the vector potential of ticks in China. Parasit Vectors. 2015;8:24. https://doi.org/10.1186/s13071-014-0628-x.
Chitimia L, Lin RQ, Cosoroaba I, Wu XY, Song HQ, Yuan ZG, et al. Genetic characterization of ticks from southwestern Romania by sequences of mitochondrial cox1 and nad5 genes. Exp Appl Acarol. 2010;52(3):305–11. https://doi.org/10.1007/s10493-010-9365-9.
Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. https://doi.org/10.1038/nature12198.
Karlsson FH, Fak F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. https://doi.org/10.1038/ncomms2266.
Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, et al. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1(1):18. https://doi.org/10.1186/2047-217X-1-18.
Nielsen HB, Almeida M, Juncker AS, Rasmussen S, Li J, Sunagawa S, et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat Biotechnol. 2014;32(8):822–8. https://doi.org/10.1038/nbt.2939.
Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32(8):834–41. https://doi.org/10.1038/nbt.2942.
Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. https://doi.org/10.1038/nature13568.
Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22(13):1658–9. https://doi.org/10.1093/bioinformatics/btl158.
Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28(23):3150–2. https://doi.org/10.1093/bioinformatics/bts565.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. https://doi.org/10.1038/nature08821.
Villar E, Farrant GK, Follows M, Garczarek L, Speich S, Audic S, et al. Ocean plankton. Environmental characteristics of Agulhas rings affect interocean plankton transport. Science. 2015;348(6237):1261447. https://doi.org/10.1126/science.1261447.
Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–8. https://doi.org/10.1038/nature12480.
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–6. https://doi.org/10.1038/nature12506.
Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12(1):59–60. https://doi.org/10.1038/nmeth.3176.
Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17(3):377–86. https://doi.org/10.1101/gr.5969107.
Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528. https://doi.org/10.1038/ncomms7528.
Cheng C, Fu W, Ju W, Yang L, Xu N, Wang YM, et al. Diversity of spotted fever group Rickettsia infection in hard ticks from Suifenhe, Chinese-Russian border. Ticks Tick Borne Dis. 2016;7(5):715–9. https://doi.org/10.1016/j.ttbdis.2016.02.023.
Qin XR, Han FJ, Luo LM, Zhao FM, Han HJ, Zhang ZT, et al. Anaplasma species detected in Haemaphysalis longicornis tick from China. Ticks Tick Borne Dis. 2018;9(4):840–3. https://doi.org/10.1016/j.ttbdis.2018.03.014.
Duron O, Noel V, McCoy KD, Bonazzi M, Sidi-Boumedine K, Morel O, et al. The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen, Coxiella burnetii. PLoS Pathog. 2015;11(5):e1004892. https://doi.org/10.1371/journal.ppat.1004892.
Hamsikova Z, Kazimirova M, Harustiakova D, Mahrikova L, Slovak M, Berthova L, et al. Babesia spp. in ticks and wildlife in different habitat types of Slovakia. Parasit Vectors. 2016;9(1):292. https://doi.org/10.1186/s13071-016-1560-z.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–4. https://doi.org/10.1093/molbev/msw054.
Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 2013;30(5):1229–35. https://doi.org/10.1093/molbev/mst012.
Greay TL, Gofton AW, Paparini A, Ryan UM, Oskam CL, Irwin PJ. Recent insights into the tick microbiome gained through next-generation sequencing. Parasit Vectors. 2018;11(1):12. https://doi.org/10.1186/s13071-017-2550-5.
Qiu Y, Nakao R, Ohnuma A, Kawamori F, Sugimoto C. Microbial population analysis of the salivary glands of ticks; a possible strategy for the surveillance of bacterial pathogens. PLoS ONE. 2014;9(8):e103961. https://doi.org/10.1371/journal.pone.0103961.
Mediannikov O, Matsumoto K, Samoylenko I, Drancourt M, Roux V, Rydkina E, et al. Rickettsia raoultii sp. Nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int J Syst Evol Microbiol. 2008;58(Pt 7):1635–9. https://doi.org/10.1099/ijs.0.64952-0.
Wijnveld M, Schotta AM, Pinter A, Stockinger H, Stanek G. Novel Rickettsia raoultii strain isolated and propagated from Austrian Dermacentor reticulatus ticks. Parasit Vectors. 2016;9(1):567. https://doi.org/10.1186/s13071-016-1858-x.
Yuan TT, Ma L, Jiang BG, Fu WM, Sun Y, Jia N, et al. First Confirmed Infection Of Candidatus Rickettsia Tarasevichiae in rodents collected from Northeastern China. Vector Borne Zoonotic Dis. 2020;20(2):88–92. https://doi.org/10.1089/vbz.2019.2443.
Eremeeva ME, Oliveira A, Moriarity J, Robinson JB, Tokarevich NK, Antyukova LP, et al. Detection and identification of bacterial agents in Ixodes persulcatus Schulze ticks from the north western region of Russia. Vector Borne Zoonotic Dis. 2007;7(3):426–36. https://doi.org/10.1089/vbz.2007.0112.
Rudakov N, Samoylenko I, Shtrek S, Igolkina Y, Rar V, Zhirakovskaia E, et al. A fatal case of tick-borne rickettsiosis caused by mixed Rickettsia sibirica subsp. sibirica and Candidatus Rickettsia tarasevichiae infection in Russia. Ticks Tick Borne Dis. 2019;10(6):101278. https://doi.org/10.1016/j.ttbdis.2019.101278.
Jia N, Zheng YC, Jiang JF, Ma L, Cao WC. Human infection with Candidatus Rickettsia tarasevichiae. N Engl J Med. 2013;369(12):1178–80. https://doi.org/10.1056/NEJMc1303004.
Fischer T, Myalkhaa M, Krucken J, Battsetseg G, Batsukh Z, Baumann MPO, et al. Molecular detection of tick-borne pathogens in bovine blood and ticks from Khentii, Mangolia. Transbound Emerg Dis. 2020;67(Suppl 2):111–8. https://doi.org/10.1111/tbed.13315.
Moore TC, Pulscher LA, Caddell L, von Fricken ME, Anderson BD, Gonchigoo B, et al. Evidence for transovarial transmission of tick-borne rickettsiae circulating in Northern Mongolia. PLoS Negl Trop Dis. 2018;12(8):e0006696. https://doi.org/10.1371/journal.pntd.0006696.
Zhong J. Coxiella-like endosymbionts. Adv Exp Med Biol. 2012;984:365–79. https://doi.org/10.1007/978-94-007-4315-1_18.
Ben-Yosef M, Rot A, Mahagna M, Kapri E, Behar A, Gottlieb Y. Coxiella-like endosymbiont of Rhipicephalus sanguineus is required for physiological processes during ontogeny. Front Microbiol. 2020;11:493. https://doi.org/10.3389/fmicb.2020.00493.
Smith TA, Driscoll T, Gillespie JJ, Raghavan R. A Coxiella-like endosymbiont is a potential vitamin source for the Lone Star tick. Genome Biol Evol. 2015;7(3):831–8. https://doi.org/10.1093/gbe/evv016.
Blackberg J, Lazarevic VL, Hunfeld KP, Persson KEM. Low-virulent Babesia venatorum infection masquerading as hemophagocytic syndrome. Ann Hematol. 2018;97(4):731–3. https://doi.org/10.1007/s00277-017-3220-6.
Moniuszko A, Dunaj J, Swiecicka I, Zambrowski G, Chmielewska-Badora J, Zukiewicz-Sobczak W, et al. Co-infections with Borrelia species, Anaplasma phagocytophilum and Babesia spp. in patients with tick-borne encephalitis. Eur J Clin Microbiol Infect Dis. 2014;33(10):1835–41. https://doi.org/10.1007/s10096-014-2134-7.
Acknowledgements
Not applicable.
Funding
This work was supported by the Beijing Natural Science Foundation [grant number 5204039], the National Key Research and Development Program of China [no. 2019YFC1200500], and the National Natural Science Foundation of China [grant numbers 31970178, 32000139, and 32000140].
Author information
Authors and Affiliations
Contributions
This study was conceived and designed by Jun Jiao and Xiaolu Xiong. Sample collection, tick species identification, and laboratory work were performed by Jun Jiao, Zhiyu Lu, and Yonghui Yu. Experimental data analysis was performed by Mengjiao Fu, Yangxuan Ou, Nier Wu, Mingliang Zhao, and Yan Liu. The manuscript was drafted by Jun Jiao and Xiaolu Xiong, and edited by Yi Sun, Bohai Wen, and Dongsheng Zhou.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Pooling strategy for metagenomic next-generation sequencing.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiao, J., Lu, Z., Yu, Y. et al. Identification of tick-borne pathogens by metagenomic next-generation sequencing in Dermacentor nuttalli and Ixodes persulcatus in Inner Mongolia, China. Parasites Vectors 14, 287 (2021). https://doi.org/10.1186/s13071-021-04740-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-021-04740-3