Abstract
Background
European species of the large genus Phyllodistomum Braun, 1899 had historically been erected based solely on morphological characters. Unfortunately, many of them are still poorly known and inadequately described. Molecular approaches are critical to delineate species which were impossible to differentiate based on morphology alone.
Methods
New samples of adult Phyllodistomum spp. were collected from the urinary bladder and/or ureters of European freshwater fishes and fixed to conduct a light and scanning electron microscopy study, and to obtain sequences of nuclear (ITS2 spacer and 28S rRNA gene), to be analysed in the context of a molecular phylogeny.
Results
Based on new findings, a new species of Phyllodistomum from the urinary bladder of the European perch, Perca fluviatilis, in Volga River basin, Russia, is described. Additionally, new data on the morphology and tegumental surface topography of P. macrocotyle (Lühe, 1909) Odhner, 1911 from ureters of the common rudd, Scardinius erythrophthalmus, is presented. The host range of P. folium, confirmed by DNA analysis, is extended to other cyprinid fish species.
Conclusions
The present study has again shown that species of the genus Phyllodistomum are in dire need of revision based on both molecular analysis and detailed morphological redescriptions of the forms attributed to the genus. Morphologically, P. kupermani n. sp. most closely resembles P. pseudofolium, a highly host-specific parasite of Gymnocephalus cernuus (L.), but molecular phylogenetic analyses based on ITS2 and 28S rDNA sequences showed that these species are distantly related. Phyllodistomum kupermani n. sp. was found to be phylogenetically most closely related to the type-species of Phyllodistomum, P. folium. Phylogenetic analyses revealed that Phyllodistomum kupermani n. sp. and P. folium formed a clade with other freshwater species for which cystocercous cercariae develop in bivalves of the family Sphaeriidae. The micromorphology and tegumental surface topography of P. macrocotyle revealed in the present study provide a valuable taxonomic criterion for congeneric species differentiation.

Similar content being viewed by others
Background
Phyllodistomum Braun, 1899 is one of the most speciose digenean genera, comprised of species parasitizing in the urinary bladder and/or ureters of freshwater and marine fish and, more rarely, amphibians throughout the world [1,2,3,4,5,6], and new species descriptions continue to be published on a regular basis [3, 4, 7, 8]. Species of Phyllodistomum infecting fishes of Europe have been studied for more than two centuries, starting with the description of Phyllodistomum umblae (Fabricius, 1780) (as Fasciola umblae) from the Arctic char, Salvelinus alpinus (L.) in Norway. Despite the relatively common occurrence of Phyllodistomum spp. in European freshwater fishes, the species composition of the genus is under scrutiny and remains controversial. Our recent studies [5, 6, 9] have challenged previous data on species diversity and life-cycles in this presumably well-known group of trematodes. However, there are still serious gaps in our knowledge of the genus Phyllodistomum and a number of unanswered questions concerning the validity and specificity of the nominal species and the identity of unidentified genetic lineages are still awaiting clarification.
Previous molecular phylogenetic analysis revealed that specimens of Phyllodistomum sp. (preliminary identified as P. pseudofolium Nybelin (1926)) obtained from the urinary bladder of the European perch, Perca fluviatilis L., from Volga River basin, Russia, represent distinct genetic lineage (presumably an undescribed species), resolving between P. folium (Olfers, 1816) and P. umblae clades in the ITS2 and 28S phylograms [6]. To validate the independent taxonomic status of this genetic linage, a comparative morphological study of Phyllodistomum species from P. fluviatilis was required. New samples of Phyllodistomum spp. were also obtained from the urinary system of three species of cyprinids, the ide, Leuciscus idus (L.), the common rudd, Scardinius erythrophthalmus (L.) and the common roach, Rutilus rutilus (L.). Morphological characteristics along with sequence data allowed us to identify these samples to species. The present paper provides description of a new species of Phyllodistomum and a detailed redescription of the adult stage of P. macrocotyle (Lühe, 1909) Odhner, 1911 based on both light microscopy (LM) and scanning electron microscopy (SEM). Phyllodistomum macrocotyle has a complex taxonomic history and this name has often not been used properly. The microcercous cercariae of P. macrocotyle develop in sporocysts localised the gills of the intermediate host Dreissena polymorpha. In the past, Phyllodistomum trematodes found in D. polymorpha were also referred to as P. folium sensu Sinitsin, 1905 or P. dogieli Pigulevsky, 1953 but recent molecular evidence has shown that P. macrocotyle is the only valid Phyllodistomum species thus far documented from D. polymorpha [5]. It is notable that Pigulevsky [10], without convincing reasons, named this species P. dogieli; hence, both P. dogieli and P. folium sensu Sinitsin, 1905 should be regarded as synonymous with P. macrocotyle. To avoid further confusion, morphological redescription of adults is required in combination with molecular data. It should be noted that the study of Peribáñez et al. [11] used comparative analysis of ITS1-5.8S-ITS2 sequence data in order to link digeneans (named as P. folium) from the urinary system of three cyprinid species, S. erythrophthalmus, Cyprinus carpio and R. rutilus, and the sporocysts found in zebra mussels, D. polymorpha, in the Ebro River, Spain. However, adult digeneans were not observed by microscopic examination and no morphological characteristics of these specimens are available from this study.
Recent progress shows again that species delimitation will require the use of molecular markers in combination with morphological description to discriminate species and verify validity of problematic nominal species. Continuing with our efforts to survey Phyllodistomum species diversity in European freshwater fishes, we describe the new species with sequences identical to Phyllodistomum sp. previously reported by Stunžėnas et al. [6] from Russia. Also, we provide a detailed morphological description of P. macrocotyle, including microphotographs of the body surface though SEM, which is for the first time linked to ITS2 and 28S rDNA sequences.
Methods
Specimens of Phyllodistomum were recovered from the ureters and urinary bladder of the European perch, Perca fluviatilis, the ide, Leuciscus idus, the common rudd, Scardinius erythrophthalmus, the common roach, Rutilus rutilus from Volga River basin, Russia (Table 1).
Adult trematodes were isolated from the urinary system of naturally infected fish, placed in saline solution (0.65%) and identified in vivo. Specimens selected for DNA extraction were washed in saline and preserved in 96% ethanol. Voucher specimens from the same collecting event used for morphological examination were washed in saline, fixed without pressure in hot 10% formalin. Morphological samples were stained with alum carmine, dehydrated in a graded ethanol series, cleared in clove oil and mounted as permanent slides using Canada balsam. Voucher specimens are deposited in the helminthological collection of I. D. Papanin Institute of Biology of Inland Waters, Russia. Extracted total DNA is deposited in the P. B. Šivickis Laboratory of Parasitology of Nature Research Centre, Vilnius, Lithuania.
For SEM, 10 live specimens of P. macrocotyle from S. erythrophthalmus were fixed in 3% glutaraldehyde in 0.1 m sodium cacodylate buffer (pH 7.2) for 20 days at 5 °C and then dehydrated in a graded ethanol series, with a final change to absolute acetone. They were then critical-point dried with liquid CO2, mounted on stubs, sputter-coated with gold-palladium and examined using a JEOL JMS 6510LV scanning electron microscope operating at 30 kV. Genomic DNA was extracted from ethanol-fixed specimens according to the protocol of Stunžėnas et al. [12] with slight modifications [13]. The internal transcribed spacer 2 region (ITS2) was amplified using the forward primer 3S (5′-CGG TGG ATC ACT CGG CTC GTG-3′) [14] and the reverse primer ITS2.2 (5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′) [15]. A new primer pair, GoJe-F and GoJe-R, was designed for species of the Gorgoderidae. Part of the internal transcribed spacer 1 (ITS1), the complete 5.8S rDNA and ITS2, also a small section at the 5’-end of the 28S gene were amplified using forward primer GoJe-F (5′-CTT GCA ATT GTT CCC CGT GA-3′) and the reverse primer GoJe-R (5′-CTG TTC ACT CGC CGT TAC TG-3′). A fragment at the 5′-end of the 28S rRNA gene was amplified using forward primers Digl2 (5′-AAG CAT ATC ACT AAG CGG-3′) or ZX-1 (5′-ACC CGC TGA ATT TAA GCA TAT-3′) [16] and reverse primers L0 (5′-GCT ATC CTG AG (AG) GAA ACT TCG-3′) [17] or 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′) [18]. The new primer pair, GoJe-F and GoJe-R, were utilised under the following conditions: initial denaturation for 3 min at 96 °C, 38 cycles of 28 s at 95 °C, 38 s at 52 °C, 38 s at 72 °C, and a final extention step for 8 min at 72 °C. The amplification protocols for other primers are as described in our previous study [13]. PCR products were sequenced in both directions at BaseClear B.V. (Leiden, the Netherlands) using PCR primers. Contiguous sequences were assembled using Sequencher 4.7 software (Gene Codes Corporation). The newly generated sequences of P. macrocotyle, P. folium and the new Phyllodistomum species, were deposited on GenBank (see accession numbers in Table 1).
Additional rDNA sequences of gorgoderid species and outgroup taxa (Table 1) were downloaded from GenBank and included in pairwise sequence comparisons and phylogenetic analyses. For the phylogenetic analyses, both the ITS2 and 28S datasets were aligned using ClustalW [19] with an open gap penalty of 15 and gap extension penalty of 6.66. The best-fit model for phylogenetic analysis was estimated using jModeltest v. 0.1.1 software [20]. Ambiguously aligned positions were excluded from phylogenetic analysis. Maximum Likelihood (ML) phylogenetic trees were obtained and analyzed using MEGA v6 [21]. Branch support was estimated by bootstrap analyses with 1000 pseudoreplicates. The ML trees were obtained using the general time reversible model with a gamma distribution rate and a proportion of invariant sites (GTR + G + I) for both the ITS2 and the 28S gene datasets. Gamma shape and the number of invariant sites were estimated from the data. Parsimony analysis based on subtree pruning and regrafting (SPR) was used with default parsimony settings. If two or more sequences belong to one species, they were collapsed into one branch, except those of the new species and P. macrocotyle. Estimates of mean evolutionary divergence over sequence pairs within and between groups were calculated using the MEGA v6 programme.
Results
General results
Perca fluviatilis was rarely infected with Phyllodistomum in the water bodies studied; only nine gravid specimens were recovered form 25 fish examined in 2018. The molecular studies demonstrated that these specimens belong to a new species. Of the 30 individuals of S. erythrophthalmus studied, 30% were infected with 1–7 phyllodistomes per fish. Trematodes recovered from the ureters of the host fishes have been shown using molecular methods to be P. macrocotyle, while specimens inhabiting the urinary bladder were identified as P. folium. Trematodes from the ureters of L. idus and R. rutilus, were identified as P. macrocotyle and P. folium, respectively. It is noteworthy that all specimens inhabiting ureters of the studied cyprinid fishes morphologically closely resembled P. elongatum Nybelin, 1926.
Phylogenetic analysis
Sequence data for two regions of rDNA, the 5.8S-ITS2-28S and 5’-end of the 28S gene were used for species-level comparison among samples of Phyllodistomum spp. and to determine phylogenetic affinities of the different morphotypes from different host species.
Alignment of the ITS2 rDNA and 28S rDNA regions for gorgoderid species yielded 495 and 836 characters for phylogenetic analysis, respectively. New sequences of P. folium were identical to those previously reported from other fish hosts. New rDNA sequences for adult P. macrocotyle and sequences of larval stages from Dreissena polymorpha (Pallas, 1771) from a previous study [5] formed a strongly supported subclade nested in a well-supported monophyletic clade including P. pseudofolium and P. angulatum Linstow, 1907. The sequences of the new species were identical to Phyllodistomum sp. ex P. fluviatilis from our previous study [6] and formed a sister clade with sequences of P. folium in both 28S and ITS2 phylograms (Figs. 1, 2). Notably, in the 28S-based phylogeny, P. parasiluri Yamaguti, 1934 was sister to the clade constituted by P. folium and P. kupermani n. sp. The genetic distance values from the 28S and ITS2 datasets of the new species, when compared with the most closely related species P. folium, were 1.1‒1.3% and 1.2‒1.4%, respectively. The new species differed from P. umblae by 1.8% for 28S and 1.2% for ITS2. With respect to the most morphologically similar species, P. pseudofolium, the divergence was much greater: 13% (28S) and 11% (ITS2). New sequences of adult P. macrocotyle from S. erythrophthalmus and L. idus appeared to be identical to the sequences of larval P. macrocotyle from its first intermediate host D. polymorpha.
Phylogenetic tree based on Maximum Likelihood analysis of partial sequences of the 28S nuclear rDNA gene. Bootstrap support values lower than 70% are not shown. The species sequenced in this study are indicated in bold. GenBank accession numbers of the collapsed clades are provided in Table 1
Phylogenetic tree based on Maximum Likelihood analysis of the ITS2 nuclear rDNA region. Bootstrap support values lower than 70% are not shown. The species sequenced in this study are indicated in bold. GenBank accession numbers of the collapsed clades are provided in Table 1
Morphological description
Family Gorgoderidae Looss, 1899
Genus Phyllodistomum Braun, 1899
Phyllodistomum kupermani n. sp.
Type-host: Perca fluviatilis L. (Perciformes: Percidae).
Type-locality: River Ilʼdʼ (58°00ʹ48ʺN, 38°09ʹ54ʺE), Yaroslavl Province, Russia.
Other locality: River Latka (58°06ʹ17ʺN, 38°13ʹ53ʺE), Yaroslavl Province, Russia.
Type-material: The type-specimens are deposited in the Parasite Collection of the Institute for Biology of Inland Waters RAS, Russia: holotype No. 1/13 (1), paratypes: No. 1/13 (2–6).
Site in host: Urinary bladder.
Representative DNA sequences: 28S rDNA (MT875008-MT875009); ITS2 rDNA (MT875012-MT875013).
ZooBank registration: To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN) [22], details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) of the article is urn:lsid:zoobank.org:pub:607C4D81-BB93-4BAC-9C1A-44F63F1A46A5. The LSID for the new name Phyllodistomum kupermani is urn:lsid:zoobank.org:act:1D8C1A16-ADB1-47FD-9678-369906F9C6C6.
Etymology: The new species is named after Professor Boris I. Kuperman (1933–2002), Institute for the Biology of Inland Waters (IBIW) of the Russian Academy of Sciences and San Diego State University, California, in recognition of his great contribution to our knowledge on biology, evolution, ecology, functional morphology and biochemistry of fish parasites.
Description
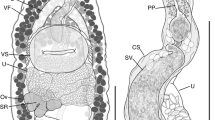
[Based on 10 gravid specimens; Fig. 3] Body pear-shaped in young specimens or scoop-shaped in old specimens, covered with minute spines, 1332–1872 (1515 ± 198) long, maximum width 432–1188 (750 ± 261) at level of middle region of hindbody. No marginal folds present. Body length to width ratio 1.5–3.1 (2.2). Forebody short, 396–666 (495 ± 87) long, representing 28–38% (33%) of total body length. Hindbody leaf-shaped in young specimens and discoid in older ones, 882–1296 (1056 ± 161) long. Oral sucker globular, subterminal or terminal, 144–210 (176 ± 22) long, 132–210 (173 ± 25) wide. Ventral sucker round, larger than oral sucker, 192–300 (236 ± 35) long, 180–300 (235 ± 42) wide. Oral sucker length to width ratio 1:0.69–0.81 (1:0.73). Ventral sucker length to width ratio 1:0.67–0.89 (1:0.74). Oral sucker to ventral sucker distance 198–510 (344 ± 83). Pharynx absent. Oesophagus straight, 102–210 (168 ± 36) long. Intestinal bifurcation 306–426 (357 ± 49) from anterior extremity. Caeca wide, terminating conspicuously far from posterior extremity, of unequal lengths, 255–441 (324 ± 69) from posterior extremity.
Gonads and vitelline masses closely packed. Testes 2, moderately or deeply lobed, oblique. Anterior testis 132–252 (208 ± 38) long, 99–300 (159 ± 63) wide; posterior testis 180–360 (272 ± 71) long, 117–300 (164 ± 61) wide. Anterior testis is situated very closely to vitellarium, posterior testis is situated very closely to ovary, distance between posterior testis and ovary 0–60 (22 ± 20). Seminal vesicle saccular, relatively short, convoluted, 44–110 (85 ± 25) long, 44–77 (58 ± 13) wide. Pars prostatica not observed. Genital pore median, at level of caecal bifurcation, 55–270 (121 ± 73) from anterior margin of ventral sucker.
Ovary irregular, moderately lobed, dextral in 7 specimens, sinistral in 3. Mehlisʼ gland dorsal, posterior to ventral sucker, between vitelline masses. Vitelline masses 2, compact, entire or slightly lobed, round or oval, between ovary and ventral sucker; right vitelline mass 57–156 (100 ± 31) long, 55–150 (93 ± 33) wide; left vitelline mass 84–156 (104 ± 23) long, 48–132 (81 ± 24) wide. Uterus extensively coiled, occupying entire hindbody, inter- and extracaecal. Eggs oval, 29–35 × 20–25 (33 × 22). Excretory vesicle I-shaped, extending to level of caeca end. Excretory pore terminal.
Remarks
The present material most closely resembles P. pseudofolium, a species described from ureters of the Eurasian ruffe, Gymnocephalus cernuus, and strictly specific to its definitive host [6, 10]. However, the new species differs from P. pseudofolium in its hindbody shape (has no body fold or other demarcation), and in having a narrower oral sucker and a larger ventral sucker, deeply lobed testes and a short distance between the posterior testis and ovary.
Our specimens can be distinguished from the other apparently valid European Phyllodistomum spp. (P. folium, P. angulatum, P. umblae, P. macrocotyle and P. elongatum). Phyllodistomum kupermani n. sp. differs from P. folium in the oval shape of the vitelline masses and the closely packed gonads and vitelline masses. The present material differs from P. angulatum by having a smaller body size (P. angulatum is almost 2 times larger), a short forebody and the absence of mid-ventral lateral muscular flaps (a typical diagnostic character for P. angulatum when alive). The new species differs from P. umblae in the body shape and size, caeca length and size of eggs. Phyllodistomum umblae has an elongated shape with the longer foliate hindbody, long caeca, which terminate close to the posterior extremity. Phyllodistomum umblae is a large worm (2990 vs 1515 μm) and has larger eggs (37 × 27 vs 33 × 22 μm). In addition, P. umblae is a parasite of salmonid fishes. The new species differs from P. macrocotyle in its body shape and the arrangement of the genital pore. Phyllodistomum macrocotyle has an elongated form and can be distinguished from P. kupermani n. sp. by its lanceolate hindbody, longer cylindrical forebody representing 36% of total body length, genital pore midway between caecal bifurcation and ventral sucker margin. Phyllodistomum kupermani n. sp. can be clearly distinguished from P. elongatum by its body shape, the arrangement of the genital pore and the shape of vitelline masses. Phyllodistomum elongatum has a more elongated body shape with lanceolate hindbody, a genital pore situated pore more posteriorly (near to ventral sucker margin) and lobed vitelline masses. In addition, P. elongatum and P. macrocotyle are located only in the ureters of the cyprinid fish.
Phyllodistomum macrocotyle (Lühe, 1909) Odhner, 1911
Syns Catoptroides macrocotyle Lühe, 1909; Phyllodistomum folium sensu Sinitsin, 1905 nec Olfers, 1817; Phyllodistomum dogieli Pigulevsky, 1953.
Type-host: Scardinius erythrophthalmus (L.) (Cypriniformes: Cyprinidae).
Other host: Leuciscus idus (L.) (Cypriniformes: Cyprinidae) (present study).
Type-locality: Rybinsk Reservoir (58°02ʹ28ʺN, 38°15ʹ18ʺ E), Yaroslavl Province, Russia.
Voucher material: Eleven voucher specimens ex S. erythrophthalmus on 8 slides [No. 1/12(1–8)] were deposited in the Parasite Collection of the Institute for Biology of Inland Waters RAS, Russia.
Site in host: Ureters.
Representative DNA sequences: 28S rDNA (MT872663-MT872664); ITS2 rDNA (MT875010-MT875011) (see also Table 1).
Redescription
[Based on 11 gravid specimens; Fig. 4]. Body elongate, with smooth lateral margins, 1764–2277 (1943 ± 150) long, 180–720 (442 ± 141) wide. Forebody cylindrical, 621–7773 (707 ± 75) long, 33–41% (36%) of total body length. Hindbody lanceolate, 1026–1404 (1227 ± 126) long, 56–67% (63%) of total body length. Oral sucker subterminal, round or sometimes oval, 176–258 (201 ± 28) long, 138–234 (178 ± 25) wide. Ventral sucker round or oval, 150–306 (189 ± 44) long, 150–306 (191 ± 43) wide. Oral sucker length to width ratio 1:0.78–1.25 (1:1.1). Ventral sucker length to width ratio 1:0.61–1.08 (1:0.95). Oral sucker to ventral sucker distance 462–654 (543 ± 60). Pharynx absent. Oesophagus long, straight, 90–240 (160 ± 44) long. Intestinal bifurcation 258–510 (363 ± 64) from anterior end. Caeca terminating close to the posterior extremity, 42–252 (144 ± 53).
Testes 2, slightly lobed, oblique. Anterior testis 84–162 (132 ± 26) long, 51–132 (106 ± 23) wide; posterior testis 120–204 (164 ± 33) long, 64–156 (116 ± 64) wide. Posterior testis to ovary distance 252–366 (303 ± 33). Seminal vesicle saccular, comparatively short, 37–99 (71 ± 16) long, 29–66 (46 ± 11) wide. Pars prostatica not observed. Genital pore median, midway between caecal bifurcation and ventral sucker margin, 66–180 (127 ± 32) from anterior margin of ventral sucker.
Ovary irregular, faintly lobed, sinistral in 8 specimens and dextral in 3; 117–150 (134 ± 11) long, 75–162 (107 ± 75) wide. Vitelline masses 2, compact, entire, round, oval or irregular, between ovary and ventral sucker, right vitelline mass 84–126 (106 ± 19) long, 48–90 (63 ± 13) wide, left vitelline gland 86–192 (118 ± 37) long, 55–96 (73 ± 13) wide. Uterus extensively coiled, occupying entire hindbody, inter- and extracaecal. Eggs oval, 29–33 × 11–22 (31 × 19). Excretory vesicle I-shaped, extending to level of caeca end.
Remarks
Phyllodistomum macrocotyle morphologically closely resembles P. elongatum. This species can be differentiated from P. elongatum by the lobed nature of the vitelline masses, together with a shorter oesophagus, intercaecal uterus and a more posteriorly situated genital pore (near to ventral sucker margin).
Tegumental topography of Phyllodistomum macrocotyle
SEM analysis shows that there is a distinct irregular transverse folding of the body surface; this is especially clear in unrelaxed worms (Fig. 5a, b). Numerous small, irregular and shallow elevations were apparent on the tegumental surface at a greater magnification (Fig. 5f). A large number of papillae are scattered on the ventral surface of both the forebody and hindbody (Fig. 5a, c, d). These button-like papillae are rounded in shape, unciliate, possess a smooth surface and are 7.5–8.0 µm in diameter. They are distributed randomly on the surface of the forebody and are accumulated mainly on the ventro-lateral surface, with only solitary papillae being observed ventro-medially (Fig. 5a). Large number of these papillae is randomly distributed throughout the surface of the ventral hindbody (Fig. 5c, d).
SEM micrographs of the ventral surface topography of adult Phyllodistomum macrocotyle. a Forebody of an unrelaxed worm, note the genital pore and randomly distributed papillae along the ventro-lateral surface. b Oral sucker showing two surrounding rings of tegument bearing surface papillae in a definite pattern. c Posterior extremity of the worm, note the pore of Laurer’s canal and the posterior notch. d Entire body of a relaxed worm. e Ventral sucker showing two surrounding rings of tegument bearing papillae in a definite pattern. f Button-like surface papillae, note the surrounding small, irregular surface elevations. g Region of the ventral sucker margin showing papillae within sucker cavity. Abbreviations: abp, apical papillae on border of sucker rim; cp, papillae within sucker cavity; fb, forebody; ft, frontal tubercle; ftp, papillae adjacent to frontal tubercle; gp, genital pore; hb, hindbody; ie, irregular elvations; ir, inner ring (rim) of oral sucker; la, pore of Laurer’s canal; lbp, lateral papillae on border of sucker rim; mrp, papillae on inner margin of sucker rim; or, outer ring of the oral and ventral suckers; os, oral sucker; p, papillae; pbp, posterior papillae on border of sucker rim; plp, postero-lateral papillae on outer ring of ventral sucker; pn, posterior notch; por, papillae on outer ring; rp, papillae on outer border of rim ventral sucker; sc, sucker cavity; tf, transverse tegumental folds; vs, ventral sucker
The genital pore is situated ventro-medially in the forebody closer to the ventral sucker than to the oral sucker (Fig. 5a). The surrounding tegument is devoid of papillary aggregations (Fig. 5a). The slit-like pore of Laurerʼs canal is located on the ventral surface, about 2/3 of the distance between the ventral sucker and the posterior extremity (Fig. 5c). The excretory pore opens on a notch situated at the posterior extremity of the body (Fig. 5c).
The oral sucker is directed antero-ventrally (Fig. 5a, d). There are two superficial rings of tegument surrounding its aperture, both of which are characterised by radially directed surface corrugations of differing lengths and widths (Fig. 5b). The inner ring (rim) of the oral sucker is about 14 µm in width and possesses tighter corrugations when compared with the outer wider ring (~ 25 µm) which has larger corrugations. Twenty-one distinct, button-like regular papillae occur on the rings of the oral sucker and within its cavity. Within the sucker cavity there are two papillae (cp) localised antero-dorsally (Fig. 5b). Four papillae (mrp) (two on each side of the rim) are distributed laterally on the inner margin of the rim (Fig. 5b); 11 papillae occur close to the border between the inner and outer rings, two (abp) of which are localised apically juxtaposed to the papillae adjacent to the frontal tubercle (see below), six (lbp) are situated laterally (three on each side) and three (pbp) occur posteriorly (Fig. 5b); and four (por) are present posteriorly on the surface of the outer ring (Fig. 5b). Slightly antero-dorsal to the outer ring of the oral sucker is a distinct frontal tubercle with a single lateral papilla on each side (Fig. 5b).
The ventral sucker is also surrounded by two rings characterised by morphologically distinct surface layers (Fig. 5e). The inner ring (rim) (~10–12 µm in width) borders the sucker cavity and bears radially oriented corrugations, whereas the surface of the outer ring (~ 28–35 µm in width) has a densely packed, cuboidal structure (Fig. 5e). This sucker bears 14 papillae aggregated in a definite pattern (Fig. 5e): six uniformly distributed papillae (rp) are radially arranged and situated on the outer border of the rim (Fig. 5e); four of the same morphology and size (plp) occur on the outer ring arranged in two symmetrical pairs postero-laterally (Fig. 5e); and four similar papillae are randomly distributed within the sucker cavity (Fig. 5g).
Discussion
Molecular comparison of specimens of Phyllodistomum, parasitizing P. fluviatilis, with respect to other congeners revealed significant genetic differences and reinforces the establishment of the new species. The parasite fauna of perch in Europe is relatively well studied, but Phyllodistomum spp. are rarely reported from this fish. As far as we know, the European perch hosts P. pseudofolium, P. folium and P. angulatum (see [23,24,25,26]). Unfortunately, identification of species of Phyllodistomum based solely on morphological characteristics has been unreliable and many of the records of these species from other fish hosts are questionable. The validity of five European Phyllodistomum spp., i.e. P. folium, P. umblae, P. angulatum, P. pseudofolium and P. macrocotyle, has been confirmed based on molecular markers. According to comparative molecular data P. simile Nybelin, 1926, a parasite of bull-head, showed no differences from P. folium, and P. megalorchis is to be regarded as a synonym of P. angulatum. Most species of Phyllodistomum have their own characteristic host associations and the levels of their host specificity are distinct. The type-species of the genus Phyllodistomum, P. folium, is euryxenous and its host specificity appeared the lowest among the known Phyllodistomum species [6]. This low host specificity was confirmed on the basis of molecular markers for P. folium specimens obtained from several fish hosts. This species was detected in Esox lucius (Esociformes, Esociformes), Rutilus rutilus, Aspius aspius, Abramis ballerus, A. brama (Cypriniformes, Cyprinidae), Gymnocephalus cernuus (Perciformes, Percidae), Cottus gobio (Scorpaeniformes, Cottidae), Gasterosteus aculeatus (Gasterosteiformes, Gasterosteidae) [5, 6]. Our results expand the host range of this species to another cyprinid fish, S. erythrophthalmus.
The present material permits comparisons between P. elongatum-like specimens recovered from different fish species. In our previous molecularly-based study, trematodes from ureters of A. bramae identified as P. elongatum, showed no differences from P. folium [5]. Here, based on sequence data we show that specimens of Phyllodistomum spp. recovered from ureters and urinary bladder of S. erythrophthalmus represents two different species, P. macrocotyle and P. folium, respectively. Phyllodistomum from ureters of L. idus proved to be P. macrocotyle, while Phyllodistomum from ureters of R. rutilus were identified as P. folium. Thus, it is evidently that P. elogatum-like trematodes localized in ureters of cyprinid fishes, can represent multiple species.
Phyllodistomum spp. are known for their considerable variation in features, such as egg size, sucker ratio, and body shape, features considered as diagnostic in other genera. Many of these features are severely affected by fixation techniques, flattening, and the condition of the worm at fixation (see [27]). According to Kudinova [1] the body shape of the Phyllodistomum species depends on the fish host species and on the morphology of its excretory system (form and size of the urinary bladder and ureters). Based on morphometric analysis of abundant material from different fish hosts Kudinova [1] concluded that the validity of only three Phyllodistomum species can be confirmed, namely, P. conostomum (syn. P. umblae), P. folium and P. angulatum, while P. pseudofolium, P. simile, P. macrocotyle (usually localised in ureters, rarely in the urinary bladder) and P. dogieli are just morphotypes of P. folium. The study of Namuleno & Scholz [28] revealed a great intraspecific variability of P. folium obtained from the urinary bladder of its type-host Esox lucius; only the diameter of suckers and their ratio and egg size appeared to be relatively stable characteristics. The shape of the body of P. folium differed markedly and quite different morphological types were found, from relatively slender, elongate or lanceolate flukes to broadly pyriform, with distinctly separated fore- and hindbody. Bakke [27] reported polymorphism in P. umblae from coregonid fish and recognized three different basic types of body shape.
The main consequence of the intraspecific phenotypic variation is an unclear view of the Phyllodistomum diversity in the European populations of fish. Earlier keys to European species of Phyllodistomum are uncritical and the authors approach to the diversity and validity of species is very different. Dawes [29] has stated that in the ultimate analysis all the European species of Phyllodistomum may become synonyms of P. folium. On the contrary, Pigulevsky [10] distinguished the nominal species, many of which are poorly known and inadequately described, by features that tend to be susceptible to treatment during or prior to fixation or to ontogenetic variation. He divided the genus Phyllodistomum into a number of subgenera; however, none of the subgenera proposed have received general acceptance [2]. Evaluating more than 1500 specimens of P. folium-like specimens, he concluded that the nominal species P. folium (Olfers, 1816) comprises four independent species, P. folium, P. dogieli Pigulevsky, 1953, P. bychowskii Pigulevsky, 1953 and P. pseudofolium; flukes from perciform fishes he attributed to P. pseudofolium. The validity of the new taxa erected by Pigulevsky [10] has been questioned by various authors. According to Bykchovskaya & Kulakova [23] descriptions of P. dogieli, P. bychowskii, P. baueri Pigulevski, 1953, P. massino Pigulevski, 1953 and P. zachwatkin Pigulevsky, 1953 (these species are listed in the database of Fauna Europaea [30]) are incomplete and their validity is doubtful.
Species of Phyllodistomum are relatively unusual among trematodes in having different types of cercariae which utilize highly diverse bivalve families, indicating that host extensions have featured in their histories [31,32,33]. However, there is no obvious morphological basis for distinguishing adults of Phyllodistomum spp. which develop from different cercariae, but it was presumed that these cercarial groups reflect phylogenetic distinctions [33]. Phylogenetic analyses of the family showed the genus Phyllodistomum to be paraphyletic and distinct clades correspond variously to the identity of the first intermediate host and the type of cercariae [34]. Recently Phyllodistomum sp. from unionids and common carp were recorded in Japan, the first record of rhopalocercariae (with comparatively short, club-shaped tail and absence of stylet and pharynx) in this country. Interestingly, it was noted that the morphology of the adult specimens resembles that of P. elongatum recorded in Europe [35]. It is worth noting that experimental studies on the life-cycle of European cercaria of rhopalocercous type Cercaria duplicata von Baer, 1827 from freshwater mussels yielded conflicting results; the study of Orechia et al. [36] has demonstrated that it is the larval form of P. elongatum, while Ivantsiv & Kurandina [37] showed that it is P. angulatum. However, molecular data revealed no match between C. duplicata and any species of Phyllodistomum [5].
The present phylogenetic analyses demonstrated that the new species, P. kupermani n. sp., is a member of a clade containing freshwater species with cystocercous cercariae developing in bivalves of the family Sphaeriidae. Morphologically, the new species most closely resembles P. pseudofolium, a highly host-specific parasite of Gymnocephalus cernuus. Our phylogenetic analyses showed that that P. kupermani n. sp. is genetically distantly related to P. pseudofolium, which produces macrocercous (but not cystocercous) cercariae developing in sphaeriid bivalve Pisidium amnicum. It should be noted that P. pseudofolium, P. angulatum and P. macrocotyle formed a highly supported clade despite the fact that these species appear to be associated with distinct patterns of first intermediate host identity and cercarial morphology [6].
Comparative analysis of the available data on the detailed tegumental topography of Phyllodistomum spp. clearly indicates that the SEM is a powerful tool in the discrimination and identification of closely related species. The surface topography of P. macrocotyle revealed in the present study differs from that described for congeneric species in the number and arrangement of papillae on both of the suckers and the surface of the body. Only one papillary type, button-like unciliated papillae, was observed. Large numbers of such papillae are randomly scattered along ventro-lateral regions of the forebody and on the entire ventral hindbody with no tendency to be concentrated in longitudinal rows or form other regular patterns. This differs from most other species of Phyllodistomum studied using SEM, i.e. P. umblae, P. folium, P. inecoli Razo-Mendivil, Pérez-Ponce de León, Rubio-Godoy, 2013, P. spinopapillatum Pérez-Ponce de León, Pinacho-Pinacho, Mendoza-Garfias & García-Varela, 2015, P. pseudofolium and P. angulatum, where one or two paired longitudinal rows of regular papillae are arranged on the surface of the forebody between the suckers [6, 8, 38,39,40,41]. However, as in P. macrocotyle, randomly distributed ventro-lateral papillae have been recorded for a recently described species, P. wallacei Pérez-Ponce de León, Martinez-Aquino & Mendoza-Garfias, 2015, a parasite of cyprinodontiform freshwater fish in central Mexico [4], although other papillary patterns in these two species differ. It is worth noting that irregularly arranged papillae have been described on the hindbody of all Phyllodistomum species, but their number varies significantly among the different species, from a few in P. umblae and P. folium (see [38, 40]), to a large number in P. macrocotyle and P. wallacei (see [4]; present study).
A great diversity in the patterns of papillae associated with the oral sucker has been described in species of Phyllodistomum. In the present SEM investigation of P. macrocotyle, 21 regular papillae were revealed in two rings around the oral sucker, which is a different papillary topography to that found around the oral sucker in all other species of Phyllodistomum examined to date. For example, a total of 16 papillae occur on the inner, upper, middle and lower parts of the oral sucker in P. spinopapillatum (see [8]); a consistent, bilaterally symmetrical arrangement of 18 papillae was noted on the rim and within the oral sucker of P. umblae (see [38]); in P. wallacei seven pairs of papillae have been described on the oral sucker (see [4]), in P. angulatum this figure was 20 papillae and in P. pseudofolium 16 [6].
There is a diversity in the location and number of papillae found on the surface of the ventral sucker of different Phyllodistomum species. In the present study, 14 papillae are aggregated in a definite pattern in P. macrocotyle, whereas in P. folium, P. umblae, P. angulatum there are 10 regular papillae [6, 38,39,40], in P. cribbi Pérez-Ponce de León, Martinez-Aquino & Mendoza-Garfias, 2015 and P. wallacei six papillae [4] and in P. spinopapillatum 18 regular papillae are associated with this sucker [8]. Nevertheless, despite differences in the overall number of papillae, in most species of this genus, there are six papillae on the rim of the ventral sucker and four papillae within the sucker [6, 8, 38,39,40].
Conclusions
The present study illustrates the challenge of identifying closely related parasites that have poor morphological distinguishing features and had historically been described based solely on morphological characters, and emphasising the need to use molecular tools for accurate species identification and to provide insights into the evolution and radiation of such parasites. The molecular markers showed that the European perch, P. fluviatilis, hosts a new species of Phyllodistomum, P. kupermani n. sp., morphologically closely resembling P. pseudofolium. However, phylogenetic analysis shows that the new species is most closely related to the type-species of the genus, P. folium. The identity of other Phyllodistomum spp., reported in this fish, should be confirmed on the basis of molecular markers. Comparative molecular studies have also revealed that P. elongatum-like trematodes, recovered from ureters S. erythrophthalmus and L. idus represents P. macrocotyle, while Phyllodistomum specimens from ureters of R. rutilus were P. folium.
Availability of data and materials
Phyllodistomum kupermani n. sp. specimens were deposited in Parasite Collection of the Institute for Biology of Inland Waters RAS, Russia, holotype No. 1/13 (1), paratypes: No. 1/13 (2–6). Eleven voucher specimens of P. macrocotyle ex S. erythrophthalmus on 8 slides [No. 1/12(1–8)] were deposited in the Parasite Collection of the Institute for Biology of Inland Waters RAS, Russia. Nucleotide sequences obtained in the present study have been deposited into the GenBank database under accession numbers listed in Table 1.
Abbreviations
- GTR + G:
-
general time reversible model with gamma distributed rate variation among sites
- ITS2:
-
internal transcribed spacer 2
- ML:
-
maximum likelihood
- LM:
-
light microscopy
- SEM:
-
scanning electron microscopy
- SPR:
-
subtree pruning and regrafting
References
Kudinova MA. On the revision of system of the trematode genus Phyllodistomum Braun, 1899 (Gorgoderidae). In: Shulman SS, editor. Ecological Parasitology. Petrozavodsk, Russia: Karelian Research Center RAS; 1994. p. 96–112 (In Russian).
Campbell RA. Family Gorgoderidae looss, 1899. In: Bray RA, Gibson DI, Jones A, editors. Keys to the Trematoda, vol. 3. Wallingford: CABI Publishing and the Natural History Museum; 2008. p. 191–213.
Nakao M. Phyllodistomum kanae sp nov (Trematoda: Gorgoderidae), a bladder fluke from the Ezo salamander Hynobius retardatus. Parasitol Int. 2015;64:314–8.
Pérez-Ponce de León G, Martinez-Aquino A, Mendoza-Garfias B. Two new species of Phyllodistomum Braun, 1899 (Digenea: Gorgoderidae), from freshwater fishes (Cypriniformes: Goodeinae) in central Mexico: An integrative taxonomy approach using morphology, ultrastructure and molecular phylogenetics. Zootaxa. 2015;4013:87–99.
Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G, Zhokhov AE. European Phyllodistomum (Digenea, Gorgoderidae) and phylogenetic affinities of Cercaria duplicata based on rDNA and karyotypes. Zool Scripta. 2015;44:191–202.
Stunžėnas V, Petkevičiūtė R, Poddubnaya L, Stanevičiūtė G, Zhokhov A. Host specificity, molecular phylogeny and morphological differences of Phyllodistomum pseudofolium Nybelin, 1926 and Phyllodistomum angulatum Linstow, 1907 (Trematoda: Gorgoderidae) with notes on Eurasian ruffe as final host for Phyllodistomum spp. Parasit Vectors. 2017;10:286.
Ho HW, Bray RA, Cutmore SC, Ward S, Cribb TH. Two new species of Phyllodistomum Braun, 1899 (Trematoda: Gorgoderidae looss, 1899) from great barrier reef fishes. Zootaxa. 2014;3779:551–62.
Pérez-Ponce de León G, Pinacho-Pinacho CD, Mendoza-Garfias B, García-Varela M. Phyllodistomum spinopapillatum sp nov (Digenea: Gorgoderidae), from the Oaxaca killifish Profundulus balsanus (Osteichthyes: Profundulidae) in Mexico, with new host and locality records of P. inecoli: morphology, ultrastructure and molecular evidence. Acta Parasitol. 2015;60:298–307.
Petkevičiūtė R, Kudlai O, Stunžėnas V, Stanevičiūtė G. Molecular and karyological identification and morphological description of cystocercous cercariae of Phyllodistomum umblae and Phyllodistomum folium (Digenea, Gorgoderidae) developing in European sphaeriid bivalves. Parasitol Int. 2015;64:441–7.
Pigulevsky SW. Family Gorgoderidae Looss, 1901. In: Skryabin KI, editor. Trematodes of animals and man, vol. 8. Moscow: Izdatel’stvo Akademii Nauk SSSR; 1953. p. 253–615 (In Russian).
Peribáñez MA, Ordovás L, Benito J, Benejam L, Gracia MJ, Rodellar C. Prevalence and sequence comparison of Phyllodistomum folium from zebra mussel and from freshwater fish in the Ebro River. Parasitol Int. 2011;60:59–63.
Stunžėnas V, Petkevičiūtė R, Stanevičiūtė G. Phylogeny of Sphaerium solidum (Bivalvia) based on karyotype and sequences of 16S and ITS1 rDNA. Cent Eur J Biol. 2011;6:105–17.
Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G. Differentiation of European freshwater bucephalids (Digenea: Bucephalidae) based on karyotypes and DNA sequences. Syst Parasitol. 2014;87:199–212.
Bowles J, Blair D, McManus DP. A molecular phylogeny of the human schistosomes. Mol Phylogenet Evol. 1995;4:103–9.
Cribb TH, Anderson GR, Adlard RD, Bray RA. A DNA-based demonstration of a three-host lifecycle for the Bivesiculidae (Platyhelminthes: Digenea). Int J Parasitol. 1998;28:1791–5.
Scholz T, De Chambrier A, Kuchta R, Littlewood DTJ, Waeschenbach A. Macrobothriotaenia ficta (Cestoda: Proteocephalidea), a parasite of sunbeam snake (Xenopeltis unicolor): example of convergent evolution. Zootaxa. 2013;3640:485–99.
Tkach V, Grabda-Kazubska B, Pawlowski J, Swiderski Z. Molecular and morphological evidences for close phylogenetic affinities of the genera Macrodera, Leptophallus, Metaleptophallus, and Paralepoderma (Digenea, Plagiorchioidea). Acta Parasitol. 1999;44:170–9.
Olson PD, Cribb TH, Tkach VV, Bray RA, Littlewood DTJ. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int J Parasitol. 2003;33:733–55.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80.
Posada D. JModelTest: phylogenetic modelling averaging. Mol Biol Evol. 2008;25:1253–6.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;24:2731–9.
ICZN. International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bull Zool Nomencl. 2012;69:161–169.
Bykhovskaya-Pavlovskaya IE, Kulakova AP. Trematoda. In: Bauer ON, editor. Key to parasites of freshwater fish of USSR, vol. 3. Leningrad: Nauka; 1987. p. 77–198 (In Russian).
Rauckis E. Fish parasites in Lithuanian waters. Vilnius: Mokslas; 1988. (In Russian).
Niewiadomska K. Fish parasites in Poland. Digenea.Warszawa: Polskie Towarzystwo Parazytologiczne; 2003. (In Polish).
Kirjušina M, Vismanis K. Checklist of the parasites of fishes of Latvia. FAO Fisheries Technical Paper No. 369/3. Rome: FAO; 2007.
Bakke TA. Morphology of adult Phyllodistomum umblae (Fabricius) (Platyhelminthes, Gorgoderidae): the effect of preparation, killing and fixation procedures. Zool Scripta. 1988;17:1–13.
Namuleno G, Scholz T. Biometrical and morphological variability of Phyllodistomum folium (Olfers, 1816) (Trematoda: Gorgoderidae), a parasite of pike (Esox lucius). Helminthologia. 1994;31:175–84.
Dawes B. The Trematoda. Cambridge UK: Cambridge University Press; 1946.
Gibson DI. Fauna Europaea: Gorgoderidae, Phyllodistomum. In: Fauna Europaea version 2020.06. https://fauna-eu.org/cdm_dataportal/taxon/57108945-7276-463d-9e1b-f2b6d218a87d. Accessed 15 Jun 2020.
Goodchild CG. The life history of Phyllodistomum solidum Rankin, 1937, with observations on the morphology, development and taxonomy of the Gorgoderinae (Trematoda). Biol Bull. 1943;84:59–86.
Fischthal JH. Rhopalocercariae in the trematode subfamily Gorgoderinae. Am Midl Nat. 1951;46:395–443.
Cribb TH. A new species of Phyllodistomum (Digenea: Gorgoderidae) from Australian and New Zealand freshwater fishes with notes on the taxonomy of Phyllodistomum Braun, 1899. J Nat Hist. 1987;21:1525–38.
Cutmore SC, Miller TL, Curran SS, Bennett MB, Cribb TH. Phylogenetic relationships of the Gorgoderidae (Platyhelminthes: Trematoda), including the proposal of a new subfamily (Degeneriinae n subfam). Parasitol Res. 2013;112:3063–74.
Urabe M, Ishibashi R, Uehara K. The life cycle and molecular phylogeny of a gorgoderid trematode recorded from the mussel Nodularia douglasiae in the Yodo River. Japan. Parasitol Int. 2015;64:26–32.
Orecchia P, Paggi L, Castagnolo L, Della Seta G, Minervini R. Ricerche sperimentali sul ciclo biologico di Phyllodistomum elongatum Nybelin, 1926 (Digenea: Gorgoderidae Looss, 1901). Parassitologia. 1975;17:95–101.
Ivantsiv VV, Kurandina DP. Life cycle of Phyllodistomum angulatum (Trematoda, Phyllodistomidae). Vest Zool. 1985;1:73–5 (In Russian).
Bakke TA. A redescription of adult Phyllodistomum umblae (Fabricius) (Digenea, Gorgoderidae) from Salvelinus alpinus (L) in Norway. Zool Scripta. 1984;13:89–99.
Bakke TA. Phyllodistomum conostomum (Olsson, 1876) (Digenea, Gorgoderidae): a junior subjective synonym for P umblae (Fabricius, 1780). Zool Scripta. 1985;14:161–8.
Bakke TA, Ždárská Z. Tegumental microtopography and arrangement of papillae in adult Phyllodistomum folium (Olfers, 1816) (Digenea: Gorgoderidae) from pikes (Esox lucius L). Folia Parasitol. 1985;32:43–9.
Razo-Mendivil U, Pérez-PoncedeLeón G, Rubio-Godoy M. Integrative taxonomy identifies a new species of Phyllodistomum (Digenea: Gorgoderidae) from the twospot livebearer, Heterandria bimaculata (Teleostei: Poeciliidae), in Central Veracruz, Mexico. Parasitol Res. 2013;112:4137–50.
Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G. Cytogenetic and sequence comparison of adult Phyllodistomum (Digenea: Gorgoderidae) from the three-spined stickleback with larvae from two bivalves. Parasitology. 2004;129:771–8.
Stunžėnas V, Cryan JR, Molloy DP. Comparison of rDNA sequences from colchicine treated and untreated tissues. Parasitol Int. 2004;53:223–8.
Rosas-Valdez R, Choudhury A, Perez-Ponce de Leon G. Molecular prospecting for cryptic species in Phyllodistomum lacustri (Platyhelminthes, Gorgoderidae). Zool Scripta. 2011;40:296–305.
Cutmore SC, Cribb TH. Two species of Phyllodistomum Braun, 1899 (Trematoda: Gorgoderidae) from Moreton Bay. Australia. Syst Parasitol. 2018;95:325–36.
Tkach V, Pawlowski J, Mariaux J. Phylogenetic analysis of the suborder Plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences Int J Parasitol. 2000;30:83–93.
Bolek MG, Snyder SD, Janovy JJ. Alternative life cycle strategies and colonization of young anurans by Gorgoderina attenuata in Nebraska. J Parasitol. 2009;9:604–16.
Petkevičiūtė R, Stunžėnas V, Stanevičiūtė G. Clarification of the systematic position of Cercariaeum crassum Wesenberg-Lund, 1934 (Digenea), based on karyological analysis and DNA sequences. J Helminthol. 2012;86:293–301.
Galaktionov KV, Blasco-Costa I, Olson PD. Life cycles, molecular phylogeny and historical biogeography of the ‘pygmaeus’ microphallids (Digenea: Microphallidae): widespread parasites of marine and coastal birds in the Holarctic. Parasitology. 2012;139:1346–60.
Acknowledgements
Part of this research was performed in the framework of program (no. AAAA-A18-118012690100-5) of I. D. Papanin Institute for Biology of Inland Waters, RAS, to L. Poddubnaya and A. Zhokhov. The authors are grateful to Dr David Gibson for improving the style and grammar of part of the MS (SEM based micromorphology).
Funding
This research was funded by the Research Council of Lithuania (grant number MIP-43/2015).
Author information
Authors and Affiliations
Contributions
RP designed the study. RP, VS and GS performed the laboratory research and analyzed data. AEZ conducted field collections and carried out morphological research. LGP performed micromorphological research. VS and GS extracted DNR for PGR and sequencing. Molecular analyses were carried out by VS. All authors actively contributed to the interpretation of the findings and development of the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Petkevičiūtė, R., Zhokhov, A.E., Stunžėnas, V. et al. Phyllodistomum kupermani n. sp. from the European perch, Perca fluviatilis L. (Perciformes: Percidae), and redescription of Phyllodistomum macrocotyle (Lühe, 1909) with notes on the species diversity and host specificity in the European Phyllodistomum spp. (Trematoda: Gorgoderidae). Parasites Vectors 13, 561 (2020). https://doi.org/10.1186/s13071-020-04434-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-020-04434-2