Abstract
Background
Lymphatic filariasis is a mosquito-borne disease caused by filarioid nematodes. A comparative understanding of parasite biology and host-parasite interactions can provide information necessary for developing intervention programmes for vector control. Here, to understand such interactions, we choose highly susceptible filariasis vectors (Aedes togoi and Anopheles lesteri) as well as Anopheles paraliae, which has lower susceptibility, infected them with nocturnally subperiodic (NSP) Brugia malayi microfilariae (mf) and studied the exsheathment, migration and innate immune responses among them.
Methods
Mosquito-parasite relationships were systematically investigated from the time mf entered the midgut until they reached their development site in the thoracic musculature (12 time points).
Results
Results showed that exsheathment of B. malayi mf occurred in the midgut of all mosquito species and was completed within 24 h post-blood meal. The migration of B. malayi mf from the midgut to thoracic muscles of the highly susceptible mosquitoes Ae. togoi and An. lesteri was more rapid than in the low susceptibility mosquito, An. paraliae. Melanisation and degeneration, two distinct refractory phenotypes, of mf were found in the midgut, haemocoel and thoracic musculature of all mosquito species. Melanisation is a complex biochemical cascade that results in deposition of melanin pigment on a capsule around the worms. Also, some biological environments in the body are inhospitable to parasite development and cause direct toxicity that results in vacuolated or degenerated worms. Even though Ae. togoi is highly susceptible to B. malayi, melanisation responses against B. malayi mf were first noted in the haemocoel of Ae. togoi, followed by a degeneration process. In contrast, in An. lesteri and An. paraliae, the degeneration process occurred in the haemocoel and thoracic musculature prior to melanisation responses.
Conclusion
This study provides a thorough description of the comparative pathobiology of responses of mosquitoes against the filarial worm B. malayi.
Similar content being viewed by others
Background
Lymphatic filariasis (LF) is a mosquito-borne disease that results in chronic medical conditions in 856 million people in 52 countries worldwide [1]. The causative pathogens are three species of filarial parasite, i.e. Wuchereria bancrofti, Brugia malayi and Brugia timori, which are transmitted primarily by Aedes, Anopheles, Culex and Mansonia mosquitoes. The Global Programme to Eliminate LF (GPELF) was established to eliminate LF by 2020 [1]. Vector control methods can reduce the transmission of the disease by lowering the vector density in areas actively undergoing mass drug administration (MDA) and in post-MDA areas [2]. Vector surveillance also plays a vital role in preventing the occurring of LF recrudescence. The success of control and surveillance for the vector depend on a clear understanding of the vector species involved in transmission [3].
Aedes togoi is a vector of filariasis in China, Japan and Taiwan [4, 5]. In Thailand, Ae. togoi (Chanthaburi strain) has been reported as a highly susceptible vector to nocturnally subperiodic (NSP) B. malayi (Narathiwat strain), W. bancrofti (Tak and Kanchanaburi strains), Brugia pahangi (Malaysia strain) and Dirofilaria immitis (Chiang Mai strain) [6, 7]. Anopheles lesteri is also incriminated as an important vector for malaria parasites in China [8] and is highly susceptible to infection with Plasmodium vivax in Korea [9, 10]. Recently, by performing cross-mating experiments between Anopheles paraliae (Thailand strain) and An. lesteri (Korea strain) and comparing sequence for the internal transcribed spacer (ITS2) and cytochrome c oxidase subunits 1 and 2, Taai et al. [11] showed that the two species are synonymous. However, remarkable differences were seen in their vector competences as An. lesteri was highly susceptible to B. malayi compared to An. paraliae [12].
Within the mosquito, there are physical and biochemical barriers that affect the compatibility of the vector-pathogen association (vector competence), i.e. cibarial and pharyngeal armature (foregut), midgut, haemolymph and haemocoel, and thoracic musculature [13,14,15]. In susceptible vectors, microfilariae (mf) circulate in the peripheral blood of infected hosts and are ingested with a blood meal and move through the midgut lumen prior to crossing the midgut epithelium. Then, mf migrate in the mosquito’s haemolymph to access the thoracic musculature and penetrate into the indirect flight muscles, the site of development of worms to the infective, third-stage larvae (L3s) [16].
The midgut is one of the first tissue barriers preventing pathogens from entering the body cavity. Numerous studies have suggested that the exsheathment of mf either occurred exclusively in the lumen of the midgut [17,18,19] or the haemocoel as well as the midgut lumen [20,21,22]. Likewise, the study by Chen & Shih [23] demonstrated that the exsheathment of B. pahangi microfilariae occurred in the lumen of midgut and haemocoel of susceptible and refractory strains of Aedes aegypti. Jariyapan et al. [22] showed the exsheathment of NSP B. malayi microfilariae happens in the haemocoel of Ae. togoi. However, they neither observed the invasion of mf to the thoracic musculature nor the immune response of mosquitoes against mf during migration from the midgut to the development site in their study. By contrast, melanised mf sheaths of B. pahangi have been found in the haemocoel of Ae. aegypti and Anopheles quadrimaculatus [24]. Melanised mf sheaths of B. malayi were observed in the haemocoel of both strains of An. quadrimaculatus (refractory and susceptible) and the susceptible strain of Ae. aegypti (Black-eyed, Liverpool) [25].
Unlike competent vectors, some mosquitoes limit filarial worm infections with various refractory or resistance mechanisms. Melanisation is a robust and potent mechanism that can make a mosquito entirely refractory to filarial worms, as is the case in the mosquito, Armigeres subalbatus, infected with B. malayi [13]. Melanisation is thought to kill pathogens through nutrient starvation and/or direct toxic effects of reaction intermediates and by-products [26]. During the process of melanisation, several free radicals or cytotoxic molecules are generated and released [27]. The direct toxicity effect on B. malayi larvae has been noticed in the first-stage larvae (L1) found in the thoracic muscle fibres of mosquitoes with high and low parasite susceptibility [12, 28].
It is well accepted that a better understanding of parasite biology and host-parasite interactions are essential for the development of effective tools or strategies for vector control programmes, particularly the development of genetically modified mosquitoes that are refractory to filarial worm development. We reasoned that a comparative study of exsheathment and melanisation immune responses against B. malayi, from the time mf enter the midgut until they develop to L3 in the thoracic musculature, would be useful to understand the range of parasite susceptibility and thereby vector competence for three crucial Asian malaria and LF parasite vector species. Therefore, we utilised an interesting host-parasite model based on the highly susceptible strains of Ae. togoi and An. lesteri, the low susceptible strain of An. paraliae and NSP B. malayi, for systematically investigating exsheathment, migration of mf and host immune responses.
Methods
Mosquito species
Aedes togoi, An. lesteri and An. paraliae laboratory mosquito strains were used in this study [29]. All mosquito species were established successfully for many consecutive generations in the insectary of the Department of Parasitology, Faculty of Medicine, Chiang Mai University, Thailand, at 27 ± 2 °C, 70–80% relative humidity, and 12:12 day night ratios adjusted with fluorescent lighting and natural lights coming from the windows [30].
Maintenance of B. malayi and preparation of blood containing B. malayi microfilariae
Mongolian jirds (Meriones unguiculatus) were used for maintaining the NSP B. malayi, that originated from a resident of the Bang Paw district, Narathiwat Province, South Thailand, at the animal house of the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand [31]. We followed the systematic procedures as described by Saeung & Choochote [32] for parasite maintenance. This provides a simple system for maintenance and mass production of B. malayi in a space-constrained laboratory.
Infection of mosquitoes with B. malayi microfilariae
Five-day-old adult female Ae. togoi, An. lesteri and An. paraliae were starved for 24 h and allowed to feed on human blood, which was taken from the principal investigator, containing B. malayi mf (average microfilarial density of 330 mf/20 μl for three experiments) through an artificial membrane [32]. We allowed mosquitoes to feed on the B. malayi-infected blood meal (mixed well) and five fully engorged mosquitoes were randomly selected for dissection. The size of the blood meal did not differ among the three species as seen in our previous study by Dedkhad et al. [29]. We found that the average number of mf per infected midgut dissected immediately after feeding on blood containing B. malayi microfilariae ranged between 1–299 mf for Ae. togoi and 5–245 mf for An. paraliae.
Exsheathment and migration studies of NSP B. malayi mf
Infected adult females of Ae. togoi, An. lesteri, and An. paraliae were dissected after full engorgement and their midguts were removed in normal saline. Five mosquitoes were dissected on glass slides with sodium chloride solution 0.85% (Sigma-Aldrich, Queenstown, Singapore) at different time points (5 min, and 1, 2, 3, 4, 5, 6, 12, 18, 24, 48 and 72 h, n = 60). Each dissected midgut was transferred to a new glass slide. Mosquitoes were discarded if the gut ruptured during dissection. The remaining mosquito tissues (thorax and abdomen with fluids) were dissected separately on a glass slide. These samples were then made into thick blood films, dried, de-hemoglobinized, fixed with absolute methanol, and stained with Giemsa (pH 7.2). The number and percentage of sheathed and exsheathed mf of B. malayi from the midgut (ingested), haemocoel and thoracic muscle fibres of each mosquito species were counted at different time points. Photographs of the mf were taken using a digital camera attached to a compound microscope (BX53, Olympus®, Japan).
Mosquito immune responses against filarial worms
The number and percentage of normal and abnormal (degenerated and melanised) mf from the midgut, haemocoel and thoracic muscle fibres of each mosquito species, detected on the slide containing normal saline, were counted at different time points in the drop of normal saline. Photographs of the mf were taken using the same microscope system. The mf were counted and scored as “normal” if mf were alive with intact morphology and had normal movement, and as “melanised” if mf had evidence of a melanin capsule and had partial movement or were immotile (in case of completed melanised), and as “degenerated” if mf had vacuolated internal organs without any evidence of melanisation and had sluggish movement or were immotile [33].
Data analysis
The mean total number of normal and abnormal mf recovered from all mosquito species were compared using non-parametric Kruskal-Wallis tests. A post-hoc Dunn’s test was used for multiple comparisons of means. The level of significance was set at 5% (P-value < 0.05). Statistical analyses were conducted using IBM SPSS statistics, version 22 for Windows (Chicago, SPSS Inc.). The level of significance was set at 5% (P-value < 0.05).
Results
Exsheathment and migration of B. malayi mf in Ae. togoi, An. lesteri and An. paraliae
The mean number of total microfilariae (mf), sheathed (Fig. 1a) and exsheathed (Fig. 1b) per body region (midgut, haemocoel and thorax) found in the three mosquito species are given in Tables 1, 2 and 3. Of the 12 time points, the mean total number of mf found in the midgut of Ae. togoi, An. lesteri and An. paraliae ranged between 0.2–43.4, 0.2–99.8 and 0.2–36.2, respectively, for three experiments. The mf penetrated the midgut wall into the haemocoel and invaded the thoracic muscle fibres of Ae. togoi as soon as 5 min after the blood meal, while mf were first seen in the thoracic muscle fibres of An. paraliae and An. lesteri at 1 and 2 h post-feeding, respectively. More than 50% of exsheathed mf were found in the midgut of Ae. togoi at 5 min post-blood meal, whereas 20% of exsheathed mf were found in those of An. lesteri and An. paraliae. All ingested mf were completely exsheathed in the midgut at 12 h for Ae. togoi and An. lesteri, and at 18 h post-blood meal for An. paraliae. Importantly, all mf found in the haemocoel and the thoracic muscle fibres of three species were exsheathed.
Investigation of host immune responses against B. malayi microfilariae
To investigate the effect of host immune response on B. malayi mf, the midgut, haemocoel and thoracic muscle fibres were examined using light microscopy. The mean total number of normal and abnormal (degenerated and melanised) mf recovered from all parts were compared among three mosquito species (Table 4). There were significant differences in mean numbers of normal mf observed at four time points including between Ae. togoi and An. paraliae at 1 h (Dunn’s test, P = 0.033), Ae. togoi and An. lesteri at 18 h (Dunn’s test, P = 0.021) and 24 h (Dunn’s test, P = 0.013), and An. lesteri and An. paraliae (Dunn’s test, P = 0.04) at 72 h. The higher number of abnormal mf (degeneration and melanisation) was observed in the low susceptible mosquito, An. paraliae, than those of both high susceptible mosquitoes. A significant difference in the mean numbers of abnormal mf was found between Ae. togoi and An. paraliae at 12 h (Dunn’s test, P = 0.005), 18 h (Dunn’s test, P = 0.005) and 24 h (Dunn’s test, P = 0.01) (Table 4).
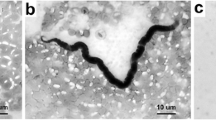
Details of the mean number of normal and abnormal (degenerated or melanised) mf per midgut, haemocoel, and thoracic muscle fibres are shown in Tables 5, 6 and 7. The majority of mf recovered from all mosquito species showed normal morphology. Degenerated mf were found only in the midgut at 4–5 h and thoracic muscle fibres at 48 and 72 h of Ae. togoi. For An. lesteri, degenerating mf were found in all parts with lower numbers but earlier (at 2 h in thoracic muscle fibres) than melanised mf. The earliest time point at which degenerated mf were found in the midgut and haemocoel of An. paraliae was 3 h post-blood meal. In Ae. togoi, melanised mf were observed in the haemocoel and the thoracic muscle fibres at several time points post-blood meal (Fig. 2). Melanised mf were found in the haemocoel and the thoracic muscle fibres of An. lesteri beginning at 5 h post-blood meal, whereas these mf were observed in haemocoel of An. paraliae at 4 h post-blood meal (Fig. 3a). The higher percentages of melanised mf than degenerated mf were found in the haemocoel of An. paraliae at almost all time points (Fig. 3b), except at 12 and 18 h post-blood meal. However, the percentage of degenerated mf found in the thoracic muscle fibres showed higher levels of degenerated mf at all time points. Also, normal (Fig. 4a) and abnormal (Fig. 4b) L1s were also found in the thoracic muscle fibres of An. lesteri at 72 h post-blood meal. The abnormal L1s were found in the thoracic muscle fibres of An. paraliae at 72 h post-blood meal (Fig. 4c, d).
Melanised microfilariae recovered from the haemocoel of An. paraliae. a Melanised microfilaria at 4 h post-blood meal (arrow indicates the partial melanin capsule around the cephalic region of the microfilaria). b Completely melanised capsule of the microfilaria at 48 h post-blood meal. Scale-bars: 20 μm
First-stage larvae recovered from thoracic muscle fibres of An. lesteri and An. paraliae at 72 h post-blood meal. a Normal live larva with intact cuticle recovered from An. lesteri. b Larva with abnormal shape recovered from An. lesteri. c Vacuolated larva recovered from An. paraliae. d Larva with abnormal shape recovered from An. paraliae. Scale-bars: 20 μm
Discussion
The parasites that cause LF must undergo exsheathment to develop further within the mosquito vector. In this study, the exsheathment of B. malayi microfilariae took place in the midgut of Ae. togoi, An. lesteri and An. paraliae before penetration of the midgut wall. We found that exsheathment of mf in Ae. togoi started earlier than in the other species and only one sheathed mf was found in the haemocoel of Ae. togoi at five minutes post-blood meal. We also noted time differences among the studied groups: the exsheathment of mf in the midgut of highly susceptible vectors (Ae. togoi and An. lesteri) was around 12 hours, but to complete this process, an additional six hours was required in An. paraliae (low susceptiblility). Our results agree with previous studies of refractory vectors [19]. Intakhan et al. [19] found that B. malayi cast off their sheaths only in the midgut of refractory Ae. aegypti (Thailand strain), beginning within five minutes and continuing over an extended period (48 hours) after the blood meal. Earlier, Nayar & Knight [25] reported that most B. malayi mf exsheathed in the midgut of Ae. aegypti (Black eye, Liverpool strain) but some sheathed mf reached the haemocoel and exsheathed there. However, our results contrast with some other studies that describe that mf exsheathed in the haemocoel [20,21,22, 25, 34, 35]. Christensen & Sutherland [21] used in vitro midgut penetration techniques, light and electron microscopy to show that nearly all B. pahangi microfilariae carried their sheaths into the haemocoel and suggested that their sheaths might break at the anterior end during penetration of the midgut of Ae. aegypti (Black eye, Liverpool strain). Agudelo-Silva & Spielman [20] revealed that microfilariae penetrated the midgut wall of susceptible Ae. aegypti (Black eye, Liverpool strain) while still sheathed, and that the sheath remained protruding from the gut into the haemocoel by using scanning electron microscopy. Nayar & Knight [25] showed that the rapid penetration of microfilariae from the midgut to the haemocoel in both susceptible and refractory strains of An. quadrimaculatus allowed most of the sheathed microfilariae to carry their sheaths into the haemocoel, then exsheath. However, Chen & Shin [23] showed that some B. pahangi microfilariae were exsheathed in the midgut of both susceptible (Liverpool) and refractory (Bora-Bora) strains of Ae. aegypti but that most microfilariae carried their sheaths into the haemocoel within two hours after feeding on infected blood. The activity of chitinase may play a role in the exsheathment of microfilariae. Chitinase of B. malayi is a specific enzyme in microfilariae and is found only in the inner body and pharyngeal thread. The chitinase levels found in the excretory/secretory (ES) products collected from exsheathed microfilariae were higher than from non-exsheathed microfilariae [36]. It is possible that chitinase released from B. malayi microfilariae could degrade the chitin on the sheath thereby aiding microfilariae escape from the sheath. However, the mechanisms by which microfilariae penetrate the midgut epithelium are unclear, possibly involving mechanical, enzymatic or integrated processes. Esslinger [17] suggested that the sharp projection at the anterior of mf, called the “cephalic hook”, might be used to tear the midgut epithelium during penetration into the haemocoel. This differs from the observation of Agudelo-Silva & Spielman [20] that this structure is blunt and even bulbous. Several enzymes, including glycolytic, proteolytic and lipolytic enzymes, might aid in modifying the midgut epithelium to allow mf to penetrate more easily. Shahabuddin et al. [37] showed that the protease released from the midgut has an adverse effect on Plasmodium parasites by increasing at least three-fold the activity of parasitic chitinase which aids in midgut penetration.
Peritrophic matrix (PM) formation, relative to that of mf midgut penetration, has been considered a potential physical barrier to mf penetration in some vector species [17, 38,39,40]. Michalski et al. [41] reported that the mosquito midgut is the barrier for infectivity of Brugia spp. in Culex pipiens pipiens, which inflicts internal and lethal damage to ingested microfilariae. In the present study, the migration of microfilariae to thoracic muscles of both high susceptible mosquitoes, Ae. togoi and An. lesteri, was more rapid than the low B. malayi susceptible An. paraliae. However, during 48–72 hours post-blood meal, all exsheathed microfilariae successfully migrated out of the midgut which supports the belief that PM does not serve as a physical barrier to nocturnally B. malayi in these three mosquito species and corresponds with previous studies [19, 22, 42]. Kato et al. [42] used RNAi to knock-down chitin synthase and demonstrated that PM does not affect the development of B. pahangi or the dissemination of dengue virus as well as infectivity of Plasmodium gallinaceum in Ae. aegypti (Black-eyed Liverpool strain). Also, Jariyapan et al. [22] reported that PM degraded from 24 to 72 hours post-blood meal when digestion was completed.
During the time microfilariae travel through the haemolymph-filled haemocoel, cellular and humoral responses can attack and restrict parasite development. These include encapsulation, melanisation, and immune system peptide production [13]. Additionally, there are some reports that the microfilarial sheath may activate an immune response [14, 24, 25, 43]. The thoracic musculature is the developmental site for W. bancrofti, B. malayi and B. pahangi, and here microfilariae develop to L3 in susceptible mosquitoes. In contrast, in refractory mosquitoes, microfilariae are killed in the midgut or haemocoel, or migrate to the developmental site, but fail to develop, which may be due to a physiological incompatibility that is independent of active immune responses [21]. Melanin pigment is a product synthesised in mosquitoes by a complex biochemical pathway [44]. Accumulation or capsule formation of melanin is generally very specific to the surface parasites, and this melanised microfilariae might not be able to continue their further development due to a lack of nutrition [45]. Besides melanisation, larvae with degenerated tissues have been reported as another defence reaction in Anopheles spp., and have been explained to result from direct toxicity [12, 28]. In this study, we observed both defence reactions; melanisation and degeneration of microfilariae throughout 12 time points, and we found that the degeneration of microfilariae in the thoracic musculature is the dominant type for An. lesteri and An. paraliae. This event occurred first in the haemocoel and thoracic musculature of these species and was followed by melanisation responses. In contrast, a melanisation response occurred first in the haemocoel of Ae. togoi, as soon as five minutes post-blood meal, and was followed by a degeneration process. Similar results were also observed in the development of B. malayi larvae in the thoracic musculature of Ar. subalbatus [46]. The authors demonstrated that larvae were first melanised in the haemocoel, whereas the degenerated larvae were observed first and then melanin formation of the larvae followed in the thoracic musculature; they suggested that the defence reactions of Ar. subalbatus against this filarial worm differ between the haemocoel and thoracic musculature. Furthermore, although intracellular melanisation occurring in tissues, such as thoracic musculature and Malpighian tubules is not common [26], our study demonstrates that the melanisation of microfilariae does occur in the thoracic musculature of mosquitoes that exhibit both high and low susceptibility to filarial worms.
We hypothesise that the degeneration or direct toxicity to the microfilariae may be due to the effect of some chemical components which are generated during the start of the process of melanisation. Melanisation is a complex reaction of enzymatic and non-enzymatic reactions generating melanin pigment. By-products from this process are cytotoxic molecules, including reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI), such as superoxide anion O2-, hydrogen peroxide (H2O2), nitric oxide (NO) and its derivatives, and hydrochloric acid (HOCl-), through coordinated activities of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, superoxide dismutase (SOD), and inducible nitric oxide synthase (NOS) [27]. During melanogenesis, NO that is generated and released and can react with a ROI such as O2-and H2O2 to form peroxynitrite (ONOO) and hydroxyl radicals (OH) [47]. The role of NO in killing parasites without melanin deposition has been reported in Drosophila infected with a parasitoid wasp [47]. NO could damage both host epithelium and pathogens [48]. Although both in vitro and in vivo studies of inducible NO demonstrated its direct toxicity towards virtually every tested pathogen, from bacteria (Escherichia coli and Micrococcus luteus [49]), Plasmodium spp. [50, 51] to metazoan parasites, including the trematodes Schistosoma spp. [52, 53], no detailed studies on filarial nematodes have been carried out. Further studies are required not only to investigate the role of NO in the host-parasite relationship but also gain fundamental information on mosquito NO. In addition to cytotoxic molecules, other essential factors in the mosquito midgut, such as proteolytic enzymes or pH, may directly damage the cells inside the body of the microfilariae because vacuolated or degenerated worms were observed. Furthermore, Michalski et al. [41] suggested that the mechanism of Cx. p. pipiens-induced midgut damage to Brugia spp. microfilariae is not yet clear, but the differential vital staining and protease sensitivity of intact (Ae. aegypti-derived) and damaged (Cx. p. pipiens-derived) worms indicate that the Cx. p. pipiens midgut environment breaches the microfilaria cuticle, leading to the death of cells inside the worms.
Conclusions
The exsheathment, migration and innate immune responses of Ae. togoi, An. lesteri and An. paraliae against infection with NSP B. malayi were systematically investigated for the first time. The exsheathment of microfilariae occurred in the midgut of all mosquito species. All ingested microfilariae were exsheathed entirely in the midgut before 24 hours post-blood meal. We found that the midgut did not function as a barrier to microfilariae migration from this site to the thoracic musculature for all mosquito species. Two defence reactions; melanisation and degeneration of microfilariae were found in the midgut, haemocoel and thoracic muscle fibres of all mosquito species, regardless of their overall susceptibility. In the mosquito with the lowest susceptibility to B. malayi, we observed the highest number of abnormal microfilariae; the mechanism for this observation merits additional study.
Abbreviations
- AL:
-
Anopheles lesteri
- AP:
-
Anopheles paraliae
- AT:
-
Aedes togoi
- LF:
-
lymphatic filariasis
- Mf:
-
microfilariae
- PI:
-
post-infection
References
World Health Organization. Global programme to eliminate lymphatic filariasis: progress report, 2016. Wkly Epidemiol Rec. 2017;91:589–608.
Brady MA, Stelmach R, Davide-Smith M, Johnson J, Pou B, Koroma J, Frimpong K, Weaver A. Costs of transmission assessment surveys to provide evidence for the elimination of lymphatic filariasis. PLoS Negl Trop Dis. 2017;11:e0005097.
Erickson SM, Thomsen EK, Keven JB, Vincent N, Koimbu G, Siba PM, et al. Mosquito-parasite interactions can shape filariasis transmission dynamics and impact elimination programs. PLoS Negl Trop Dis. 2013;7:e2433.
Ramachandran CP, Wharton RH, Dunn FL, Kershaw WE. Aedes (Finlaya) togoi Theobald, a useful laboratory vector in studies of filariasis. Ann Trop Med Parasitol. 1963;57:443–5.
Cheun H-I, Cho S-H, Lee H-I, Shin EH, Lee J-S, Kim T-S, Lee W-J. Seasonal prevalence of mosquitoes, including vectors of brugian filariasis, in Southern Islands of the Republic of Korea. Korean J Parasitol. 2011;49:59–64.
Choochote W, Sucharit S, Abeyewickreme W. A note on adaptation of Anopheles annularis Van Der Wulp, Kanchanaburi, Thailand to free mating in a 30 × 30 × 30 cm cage. Southeast Asian J Trop Med Public Health. 1983;14:559–60.
Choochote W, Keha P, Sukhavat K, Khamboonruang C, Sukontason K. Aedes (Finlaya) togoi Theobald 1907, Chanthaburi strain, a laboratory vector in studies of filariasis in Thailand. Southeast Asian J Trop Med Public Health. 1987;18:259–60.
Liu C. Comparative studies on the role of Anopheles anthropophagus and Anopheles sinensis in malaria transmission in China. Zhonghua Liu Xing Bing Xue Za Zhi. 1990;11:360–3.
Joshi D, Choochote W, Park MH, Kim JY, Kim TS, Suwonkerd W, Min GS. The susceptibility of Anopheles lesteri to infection with Korean strain of Plasmodium vivax. Malar J. 2009;8:42.
Joshi D, Kim JY, Choochote W, Park MH, Min GS. Preliminary vivax malaria vector competence for three members of the Anopheles hyrcanus group in the Republic of Korea. J Am Mosq Control Assoc. 2011;27:312–4.
Taai K, Baimai V, Saeung A, Thongsahuan S, Min GS, Otsuka Y, et al. Genetic compatibility between Anopheles lesteri from Korea and Anopheles paraliae from Thailand. Mem Inst Oswaldo Cruz. 2013;108:312–20.
Saeung A, Hempolchom C, Baimai V, Thongsahuan S, Taai K, Jariyapan N, et al. Susceptibility of eight species members in the Anopheles hyrcanus group to nocturnally subperiodic Brugia malayi. Parasit Vectors. 2013;6:5.
Beerntsen BT, James AA, Christensen BM. Genetics of mosquito vector competence. Microbiol Mol Biol Rev. 2000;64:115–37.
Bartholomay LC, Christensen BM. Vector-parasite interactions in mosquito-borne filariasis. In: Klei TR, Rajan TV, editors. World Class Parasites: Volume 5. The Filaria. 1 ed. Boston: Springer; 2000. p. 9–19.
Bartholomay LC, Michel K. Mosquito immunobiology: the intersection of vector health and vector competence. Annu Rev Entomol. 2018;63:145–67.
Erickson SM, Xi Z, Mayhew GF, Ramirez JL, Aliota MT, Christensen BM, Dimopoulos G. Mosquito infection responses to developing filarial worms. PLoS Negl Trop Dis. 2009;3:e529.
Esslinger JH. Behavior of microfilariae of Brugia pahangi in Anopheles quadrimaculatus. Am J Trop Med Hyg. 1962;11:749–58.
Denham DA, McGreevy PB. Brugian filariasis: epidemiological and experimental studies. Adv Parasitol. 1977;15:243–309.
Intakhan N, Jariyapan N, Sor-Suwan S, Phattanawiboon B, Taai K, Chanmol W, et al. Exsheathment and midgut invasion of nocturnally subperiodic Brugia malayi microfilariae in a refractory vector, Aedes aegypti (Thailand strain). Parasitol Res. 2014;113:4141–9.
Agudelo-Silva F, Spielman A. Penetration of mosquito midgut wall by sheathed microfilariae. J Invertebr Pathol. 1985;45:117–9.
Christensen BM, Sutherland DR. Brugia pahangi: exsheathment and midgut penetration in Aedes aegypti. Trans Am Microsc Soc. 1984:423–33.
Jariyapan N, Saeung A, Intakhan N, Chanmol W, Sor-suwan S, Phattanawiboon B, et al. Peritrophic matrix formation and Brugia malayi microfilaria invasion of the midgut of a susceptible vector, Ochlerotatus togoi (Diptera: Culicidae). Parasitol Res. 2013;112:2431–40.
Chen CC, Shih CM. Exsheathment of microfilariae of Brugia pahangi in the susceptible and refractory strains of Aedes aegypti. Ann Trop Med Parasitol. 1988;82:201–6.
Chen CC, Laurence BR. The encapsulation of the sheaths of microfilariae of Brugia pahangi in the haemocoel of mosquitoes. J Parasitol. 1985;71:834–6.
Nayar JK, Knight JW. Comparison of migration and encapsulation of Brugia malayi microfilariae from the midgut to the haemocoel between Anopheles quadrimaculatus and Aedes aegypti. J Invertebr Pathol. 1995;65:295–9.
Christensen BM, Li J, Chen CC, Nappi AJ. Melanization immune responses in mosquito vectors. Trends Parasitol. 2005;21:192–9.
Nappi AJ, Christensen BM. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem Mol Biol. 2005;35:443–59.
Saeung A, Min GS, Thongsahuan S, Taai K, Songsawatkiat S, Choochote W. Susceptibility of five species members of the Korean Hyrcanus Group to Brugia malayi, and hybridization between B. malayi-susceptible and -refractory Anopheles sinensis strains. Southeast Asian J Trop Med Public Health. 2014;45:588–97.
Dedkhad W, Bartholomay LC, Christensen BM, Joshi D, Taai K, Hempolchom C, Saeung A. Effects of cross-mating on susceptibility of synonymous mosquitoes, Anopheles paraliae and Anopheles lesteri to infection with nocturnally subperiodic Brugia malayi. Acta Trop. 2018;187:65–71.
Choochote W, Saeung A. Systematic techniques for the recognition of Anopheles species complexes. In: Manguin S, editor. Anopheles Mosquitoes - New Insights into Malaria Vectors. Rijeka: InTech; 2013. p. 57–79.
Choochote W, Sukhavat K, Somboon P, Khamboonruang C, Maleewong W, Suwanpanit P. The susceptibility of small laboratory animals to nocturnally superiodic Brugia malayi in Thailand. J Parasitol Trop Med Assoc Thailand. 1986;9:35–7.
Saeung A, Choochote W. Development of a facile system for mass production of Brugia malayi in a small-space laboratory. Parasitol Res. 2013;112:3259–65.
Song C, Gallup JM, Day TA, Bartholomay LC, Kimber MJ. Development of an in vivo RNAi protocol to investigate gene function in the filarial nematode, Brugia malayi. PLoS Pathog. 2010;6:e1001239.
Yamamoto H, Ogura N, Kobayashi M, Chigusa Y. Studies on filariasis II: exsheathment of the microfilariae of B. pahangi in Armigeres subalbatus. Jap J Parasitol. 1983;32:287–92.
Perrone JB, Spielman A. Microfilarial perforation of the midgut of a mosquito. J Parasitol. 1986;72:723–7.
Wu Y, Preston G, Bianco AE. Chitinase is stored and secreted from the inner body of microfilariae and has a role in exsheathment in the parasitic nematode Brugia malayi. Mol Biochem Parasitol. 2008;161:55–62.
Shahabuddin M, Toyoshima T, Aikawa M, Kaslow DC. Transmission-blocking activity of a chitinase inhibitor and activation of malarial parasite chitinase by mosquito protease. Proc Natl Acad Sci USA. 1993;90:4266–70.
Ewert A. Comparative migration of microfilariae and development of Brugia pahangi in various mosquitoes. Am J Trop Med Hyg. 1965;14:254–9.
Duke BO, Lewis DJ, Moore PJ. Onchocerca-Simulium complexes. I. Transmission of forest and Sudan-savanna strains of Onchocerca volvulus, from Cameroon, by Simulium damnosum from various west African bioclimatic zones. Ann Trop Med Parasitol. 1966;60:318–26.
Obiamiwe BA, Macdonald WW. 1. The effect of heparin on the migration of Brugia pahangi microfilariae Culex pipiens. 2. The uptake of B. pahangi microfilariae in C. pipiens and the infectivity of C. pipiens in relation to microfilarial densities. 3. Evidence of a sex-linked recessive gene, sb, controlling susceptibility C. pipiens to B. pahangi. Trans R Soc Trop Med Hyg. 1973;67:32–3.
Michalski ML, Erickson SM, Bartholomay LC, Christensen BM. Midgut barrier imparts selective resistance to filarial worm infection in Culex pipiens pipiens. PLoS Negl Trop Dis. 2010;4:e875.
Kato N, Mueller CR, Fuchs JF, McElroy K, Wessely V, Higgs S, Christensen BM. Evaluation of the function of a type I peritrophic matrix as a physical barrier for midgut epithelium invasion by mosquito-borne pathogens in Aedes aegypti. Vector Borne Zoonotic Dis. 2008;8:701–12.
Sutherland DR, Christensen BM, Forton KF. Defence reaction of mosquitoes to filarial worms: role of the microfilarial sheath in the response of mosquitoes to inoculated Brugia pahangi microfilariae. J Invertebr Pathol. 1984;44:275–81.
Zhao X, Ferdig MT, Li J, Christensen BM. Biochemical pathway of melanotic encapsulation of Brugia malayi in the mosquito, Armigeres subalbatus. Dev Comp Immunol. 1995;19:205–15.
Chen CC, Chen CS. Brugia bahangi: effects of melanization on the uptake of nutrients by microfilariae in vitro. Exp Parasitol. 1995;81:72–8.
Kobayashi M, Ogura N, Yamamoto H. Studies on filariasis VIII: histological observation on the abortive development of Brugia malayi larvae in the thoracic muscles of the mosquitoes, Armigeres subalbatus. Jpn J Sanit Zool. 1986;37:127–32.
Nappi AJ, Vass E, Frey F, Carton Y. Nitric oxide involvement in Drosophila immunity. Nitric Oxide. 2000;4:423–30.
Han YS, Thompson J, Kafatos FC, Barillas-Mury C. Molecular interactions between Anopheles stephensi midgut cells and Plasmodium berghei: the time bomb theory of ookinete invasion of mosquitoes. EMBO J. 2000;19:6030–40.
Hillyer JF, Estévez-Lao TY. Nitric oxide is an essential component of the hemocyte-mediated mosquito immune response against bacteria. Dev Comp Immunol. 2010;34:141–9.
Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci USA. 1998;95:5700–5.
Lim J, Gowda DC, Krishnegowda G, Luckhart S. Induction of nitric oxide synthase in Anopheles stephensi by Plasmodium falciparum: mechanism of signaling and the role of parasite glycosylphosphatidylinositols. Infect Immun. 2005;73:2778–89.
Colasanti M, Gradoni L, Mattu M, Persichini T, Salvati L, Venturini G, Ascenzi P. Molecular bases for the anti-parasitic effect of NO (Review). Int J Mol Med. 2002;9:131–4.
Rivero A. Nitric oxide: an antiparasitic molecule of invertebrates. Trends Parasitol. 2006;22:219–25.
Acknowledgements
We would like to thank Professor Gi-Sik Min, Department of Biological Sciences, Inha University, South Korea, who kindly provided An. lesteri used in this study.
Funding
This research was supported by grants from the Royal Golden Jubilee (RGJ) PhD Programme, Thailand Research Fund (TRF) (grant number PHD/0066/2555 to AS and WD), the Diamond Research Grant (PAR-2560-04663) and the Faculty of Medicine Research Fund (PAR-2558-03170), Faculty of Medicine, Chiang Mai University, and the Bio & Medical Technology Development Programme of the National Research Foundation (NRF) funded by the Ministry of Science & ICT, South Korea (grant number: 2017M3A9E4070707) to AS.
Availability of data and materials
The data supporting the conclusions of this article are included within the article.
Author information
Authors and Affiliations
Contributions
AS conceived and designed the study. WD and AS performed field and laboratory experiments, analysed the data, and wrote the manuscript. BMC and LCB participated in data analysis and revised the draft manuscript. DJ helped to revise the draft manuscript. CH participated in laboratory experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocols were approved by the Animal Ethics Committee of the Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand (Approval Protocol Number: PAR-2558-03170, No. 05/2015).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Dedkhad, W., Christensen, B.M., Bartholomay, L.C. et al. Immune responses of Aedes togoi, Anopheles paraliae and Anopheles lesteri against nocturnally subperiodic Brugia malayi microfilariae during migration from the midgut to the site of development. Parasites Vectors 11, 528 (2018). https://doi.org/10.1186/s13071-018-3120-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-018-3120-1