Abstract
Background
Hookworms belonging to the genus Ancylostoma (Dubini, 1843) cause ancylostomiasis, a disease of considerable concern in humans and domestic and wild animals. Molecular and epidemiological data support evidence for the zoonotic potential among species of Ancylostoma where transmission to humans is facilitated by rapid urbanization and increased human-wildlife interactions. It is important to assess and describe these potential zoonotic parasite species in wildlife, especially in hosts that have physiological similarities to humans and share their habitat. Moreover, defining species diversity within parasite groups that can circulate among free-ranging host species and humans also provides a pathway to understanding the distribution of infection and disease. In this study, we describe a previously unrecognized species of hookworm in the genus Ancylostoma in the giant panda, including criteria for morphological and molecular characterization.
Methods
The hookworm specimens were obtained from a wild giant panda that died in the Fengtongzai Natural Reserve in Sichuan Province of China in November 2013. They were microscopically examined and then genetically analyzed by sequencing the nuclear internal transcribed spacer (ITS, ITS1-5.8S-ITS2) and mitochondrial cytochrome c oxidase subunit 1 (cox1) genes in two representative specimens (one female and one male, FTZ1 and FTZ2, respectively).
Results
Ancylostoma ailuropodae n. sp. is proposed for these hookworms. Morphologically the hookworm specimens differ from other congeneric species primarily based on the structure of the buccal capsule in males and females, characterized by 2 pairs of ventrolateral and 2 pairs of dorsolateral teeth; males differ in the structure and shape of the copulatory bursa, where the dorsal ray possesses 2 digitations. Pairwise nuclear and mitochondrial DNA comparisons, genetic distance analysis, and phylogenetic data strongly indicate that A. ailuropodae from giant pandas is a separate species which shared a most recent common ancestor with A. ceylanicum Looss, 1911 in the genus Ancylostoma (family Ancylostomatidae).
Conclusion
Ancylostoma ailuropodae n. sp. is the fourth species of hookworm described from the Ursidae and the fifteenth species assigned to the genus Ancylostoma. A sister-species association with A. ceylanicum and phylogenetic distinctiveness from the monophyletic Uncinaria Frölich, 1789 among ursids and other carnivorans indicate a history of host colonization in the evolutionary radiation among ancylostomatid hookworms. Further, phylogenetic relationships among bears and a history of ecological and geographical isolation for giant pandas may be consistent with two independent events of host colonization in the diversification of Ancylostoma among ursid hosts. A history for host colonization within this assemblage and the relationship for A. ailuropodae n. sp. demonstrate the potential of this species as a zoonotic parasite and as a possible threat to human health. The cumulative morphological, molecular and phylogenetic data presented for A. ailuropodae n. sp. provides a better understanding of the taxonomy, diagnostics and evolutionary biology of the hookworms.
Similar content being viewed by others
Background
Hookworms (Nematoda: Ancylostomatidae) are one of the most common soil-transmitted helminths, causing serious iron-deficiency anemia and protein malnutrition in humans and domestic and wild mammals [1,2,3]. Both major genera Ancylostoma (Dubini, 1843) and Necator Stiles, 1903, relegated to two distinct subfamilies, are responsible for morbidity and socioeconomic burdens [4]. Unlike species in the genus Necator, most Ancylostoma hookworms are considered to be of greater medical and veterinary importance because of distribution, prevalence, and multiple zoonotic species [2]. Currently there are fourteen valid species identified in the genus Ancylostoma that are often considered in the context of the range of hosts that are typically infected. For example, the ‘anthrophilic’ form is limited to Ancylostoma duodenale (Dubini, 1843) which principally infects humans. ‘Anthropozoonotic’ forms, capable of circulating among free-ranging wild hosts, some domestic hosts and humans include Ancylostoma caninum (Ercolani, 1859), Ancylostoma braziliense Gomes de Faria, 1910 and Ancylostoma ceylanicum Looss, 1911. Other species, including most of the recognized diversity in the genus are considered to be primarily of veterinary importance, including Ancylostoma tubaeforme (Zeder, 1800), Ancylostoma malayanum (Alessandrini, 1905), Ancylostoma pluridentatum (Alessandrini, 1905), Ancylostoma paraduodenale Biocca, 1951, Ancylostoma kusimaense Nagayosi, 1955, Ancylostoma buckleyi Le Roux & Biocca, 1957, Ancylostoma taxideae Kalkan & Hansen, 1966, Ancylostoma genettae Macchioni, 1995, Ancylostoma protelesis Macchioni, 1995, and Ancylostoma somaliense Macchioni, 1995 [5, 6]. It is noteworthy that nearly all of these species can also be found in wildlife, such as A. duodenale in Crocuta crocuta (Erxleben); A. caninum and A. braziliense in Acinonyx jubatus (Schreber) and Canis mesomelas Schreber; A. ceylanicum in Canis lupus dingo Meyer; A. paraduodenale in Leptailurus serval (Schreber); A. malayanum in Ursus thibetanus G. Cuvier; A. pluridentatum in Puma concolor coryi (Bangs); A. kusimaense in Nyctereutes procyonoides viverrinus Temminck; A. taxideae in Taxidea taxus taxus (Schreber); A. genettae in Genetta genetta (Linnaeus); A. protelesis in Proteles cristata (Sparrman); and A. somaliense in C. mesomelas [5,6,7,8,9,10,11,12]. Although a diverse assemblage of carnivorans is recognized as hosts for Ancylostoma, only one species had been documented or described previously among the Ursidae [7]; species of the distantly related Uncinaria Frölich, 1789, are considered typical in ursine hosts [13].
Recent molecular-based genetic and epidemiological investigations have shown that among certain wild or domestic animal-derived species of Ancylostoma, A. ceylanicum is becoming the second most common hookworm found to infect and complete its life-cycle in humans [12, 14,15,16,17,18]. Similar transmission and cross-infection cases have been reported for other congeneric species, notably A. caninum [12, 19, 20] and A. braziliense [12]. Such situations highlight the public health significance of hookworm infection and the necessity to assess their prevalence and distribution, and to identify their wildlife hosts. This has become especially important for wildlife hosts that may have recently adapted to the human environment due to rapid urbanization [14, 21] leading to increased interactions with people in conservation centers and zoological gardens constructed for endangered and valuable animals [22]. Regrettably, little attention has been broadly paid to the species of Ancylostoma because of a limited understanding of their diversity, abundance and distribution and the difficulty in etiological and epidemiological sampling in the wild [12, 14].
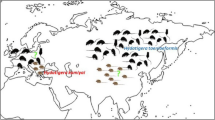
The giant panda, Ailuropoda melanoleuca (David), one of the most endangered and rare species of China, is regarded as one of the preeminent species for wildlife conservation in the world. Higher taxonomic status for these enigmatic carnivorans had been unresolved, until relatively recent decisions that unequivocally placed giant pandas among the Ursidae (e.g. [23,24,25,26]). Wild giant pandas currently inhabit six small mountain ranges of China i.e. Qinling, Minshan, Qionglai, Daxiangling, Xiaoxiangling and Liangshan (Fig. 1), with an estimated population size of ~1,864 [27,28,29,30]. Since the 1950s, numerous natural reserves, conservation centers, research bases and zoological gardens were specifically established by the Chinese government to protect this threatened species [31]. Some of these wild giant pandas have become closely associated with humans as they are housed for artificial breeding and conservation and biological investigations. Also, some pandas have been displayed publically as the ‘messenger of peace and friendship’ around the world [32]. Although ecological, genetic and etiological studies have shown that the panda faces the threat of extinction due to habitat loss, poor reproduction and low resistance to infectious diseases [33, 34], recent surveys strongly indicate that parasitic infections represent the leading health threat to giant pandas of China [35,36,37,38,39,40].
Hookworm parasites have been frequently observed in the intestines of wild dead giant pandas since 1995 [28] and the first record, attributed to a species of Ancylostoma, was reported by Zhang et al. in 2005 [41]. However, detailed morphological descriptions, determination of taxonomic status and indicators of pathogenicity of the Ancylostoma sp. derived from giant panda are lacking. The recent collection of parasites from a wild giant panda that died in the Fengtongzai Natural Reserve in Sichuan Province of China resulted in the recovery of fresh Ancylostoma specimens and provided an opportunity to fill some of these gaps in our knowledge. We have used DNA sequence and morphological analysis, applying clear species criteria established in a phylogenetic context [42], to recognize and describe a previously unknown hookworm species from the giant panda. A putative sister-species relationship with the ‘anthropozoonotic’ A. ceylanicum suggests a possible zoonotic risk for transmission and infection to humans.
Methods
Parasite collection and microscopic examination
In November 2013, a wild female giant panda was found dead in the Fengtongzai Natural Nature Reserve, Sichuan Provence of China (Fig. 1). After a routine necropsy, seventeen hookworm specimens (seven males and ten females) were collected from the small intestine under the Scientific Procedures Premises License for the College of Veterinary Medicine, Sichuan Agricultural University (Sichuan, China). In addition, parasite eggs were isolated from the intestinal content by the centrifuge-flotation method using saturated MgSO4 [43]. After washing in physiological saline, the hookworm specimens were either directly fixed in Berland’s fluid (95% glacial acetic acid and 5% formaldehyde) for morphological analysis or stored in 70% ethanol for subsequent molecular profiling. For morphology, the hookworms were identified to the genus level on the basis of the existing taxonomic keys and descriptions of Ancylostoma spp. (e.g. [44]). In brief, the worms (n = 15; 6 males and 9 females) were prepared as temporary whole mounts in glycerin after clearing in lactophenol and examined under both dissecting and light microscopy at magnifications of 10–40× and 40–200×, respectively; male and female specimens were characterized morphologically including photo-micrographic imaging and morphometrics. Measurements are given in micrometres (μm) unless specified otherwise and presented with the range followed by the mean within parentheses. In addition, some key characteristics of the adults were drawn with the aid of serial photographs for morphological comparison and differentiation from other related species. These specimens including the type-series and vouchers for molecular analyses have been deposited in the Department of Parasitology, Sichuan Agricultural University (accession numbers code GYY-XY).
Molecular profiles and phylogeny
For molecular analysis, two adult specimens of Ancylostoma sp. (one female and one male; sample codes FTZ1 and FTZ2, respectively) preserved in 70% ethanol were air-dried and their mid-body regions (~1 cm) were excised individually for extraction of genomic DNA using the Universal Genomic DNA Extraction Kit (TaKaRa, Dalian, China) according to the manufacturer’s protocol. The cephalic and caudal extremities of each specimen were retained as archived vouchers. The DNA extract was used as template for PCR amplifications at the nuclear internal transcribed spacer ITS1-5.8S-ITS2 region (734 bp) and mitochondrial cytochrome c oxidase subunit 1 (cox1) locus (393 bp) using primer pairs designed based on the alignments of the relatively conserved regions of the congeneric species A. ceylanicum, A. caninum, and A. duodenale in GenBank. Two PCR primer sets were as follows: ITS1-5.8S-ITS2, forward: 5′-GTC GAA GCC TTA TGG TTC CT-3′ and reverse: 5′-TAA CAG AAA CAC CGT TGT CAT ACT A-3′; cox1, forward: 5′-ATT TTA ATT TTG CCT GCT TTT G-3′ and reverse: 5′-ACT AAC AAC ATA ATA GGT ATC ATG TAA-3′. The PCR reactions contained ~20 ng of genomic DNA were performed in 50-μl reaction volumes containing 25 μl 2× Phusion High-Fidelity PCR Master Mix (Finnzymes OY, Espoo, Finland), 3 μl gDNA, 3 μL of each primer and 16 μl of ddH2O. PCR cycling conditions carried out in a Mastercycler Gradient 5331 thermocycler (Eppendorf, Germany) were an initial denaturation at 95 °C for 5 min; then for ITS1-5.8S-ITS2, 35 cycles of 95 °C for 30 s, 39.8 °C for 30 s, and 72 °C for 45 s; but for cox1, 35 cycles at 95 °C for 30 s, 44.1 °C for 30 s, and 72 °C for 30 s; followed by a final step at 72 °C for 10 min. For each amplification, samples without parasite gDNA and host DNA as negative controls were also included. All PCR products were examined on agarose (1%) gels to verify that they represented the target bands. The corrected gel-isolated amplicons were column-purified and sequenced in both directions using terminator-based cycle sequencing with BigDye chemistry (Applied Biosystems, Foster City, CA, USA) on an ABI 3730 DNA sequencer (Applied Biosystems) in TaKaRa Biotechnology Co. Ltd. (Dalian, China). To ensure maximum accuracy, each amplicon was sequenced three times independently. The consensus sequences were utilized for the following bioinformatic analyses and added to GenBank under the accession numbers KP842923 (FTZ1) and KP842924 (FTZ2) for ITS1-5.8S-ITS2 and KP842921 (FTZ1) and KP842922 (FTZ2) for cox1.
Sequences of ITS1-5.8S-ITS2 and cox1 of Ancylostoma sp. in the present study were separately aligned with reference sequences from closely related species (Table 1), including the congeneric species A. ceylanicum, A. caninum, A. duodenale, A. braziliense and A. tubaeforme as well as other hookworm species Necator americanus (Stiles, 1902), Uncinaria hamiltoni Baylis, 1933 [45], U. lucasi Stiles & Hassall, 1901, U. stenocephala (Railliet, 1884), U. sanguinis Marcus, Higgins, Slapeta & Gray, 2014 [46], Uncinaria sp., and Bunostomum phlebotomum (Railliet, 1900), using the Clustal X 1.83 program [47]. During the procedure, the nucleotide alignment of cox1 was further adjusted by a codon-guided protein alignment. Given the presence of the ambiguous regions within these alignments, an online version of GBlocks (http://molevol.cmima.csic.es/castresana/Gblocks_server.html) was also introduced here. After refining the alignments using Gblocks, the sequence datasets were used for phylogenetic analyses using both maximum parsimony (MP) (PAUP* 4.10b [48]) and Bayesian inference (BI) methods (MrBayes 3.2 [49]). In the MP analysis, heuristic searches were executed by branch-swapping utilizing tree-bisection-reconnection (TBR) algorithm and 1,000 random-addition sequence replicates with 10 trees held at each step, and finally the optimal topology with bootstrapping frequencies (BF) was obtained using Kishino-Hasegawa, as described previously [50]. For the BI analysis, the nucleotide substitution model GTR + I + G was determined using the Bayesian Information Criteria (BIC) test in jModeltest v. 2.1.6 [51], and the trees were constructed employing the Markov chain Monte Carlo (MCMC) method (chains = 4) over 100,000 (cox1) or 1,000,000 (ITS1-5.8S-ITS2) generations with every 100th (cox1) or 1000th (ITS1-5.8S-ITS2) tree being saved; when the average standard deviation of the split frequencies reduced to less than 0.01, 25% of the first saved trees were discarded as “burn-in” and the consensus (50% majority rule) trees were inferred from all remaining trees and further plotted in TreeviewX (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html), with nodal supports expressed as posterior probabilities (PP). The livestock hookworm B. phlebotomum was used as outgroup reference and included in each phylogenetic analysis. Paralleled to the phylogenies, among the genus Ancylostoma the new hookworm species coupled with A. ceylanicum, A. caninum, A. duodenale and A. tubaeforme was also subjected to detection of synonymous and non-synonymous mutations in the mitochondrial cox1 gene using their corresponding protein sequences, followed by determination of genetic distances between them using a distance matrix based on the maximum composite likelihood model in MEGA [52].
Results
Family Ancylostomatidae Looss, 1905
Genus Ancylostoma (Dubini, 1843)
Ancylostoma ailuropodae Yang, Hoberg & Xie n. sp.
Type-host: Giant panda Ailuropoda melanoleuca (David) (Mammalia: Carnivora: Ursidae).
Type-locality: Fengtongzai Natural Reserve (30°42′12″N, 102°56′14″E), Baoxing, Sichuan Province, China.
Type-material: Holotype, adult male (GYY-XY 1301); allotype, adult female (GYY-XY 1308); paratypes, three adult males (GYY-XY 1302-4) and three females (GYY-XY 1309-11). All materials, together with nine vouchers (three males, GYY-XY1305-7; six females, GYY-XY13012-17) containing one male and one female represented by cephalic and caudal extremities, with the mid-body sub-sampled for DNA sequence analysis, are deposited at the Department of Parasitology in Sichuan Agricultural University, Sichuan, China. Collectors: GY Yang, TF Zhang and Y Xie.
Site in host: Small intestine (most in the duodenum).
Representative DNA sequences: Representative nuclear ribosomal and mitochondrial DNA sequences were deposited in the GenBank database under the accession numbers KP842923–KP842924 (ITS1-5.8S-ITS2) and KP842921–KP842922 (cox1).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN) [53], details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) of the article is urn:lsid:zoobank.org:pub:A2492E99-AA70-4A58-AB70-7FED78E726A3. The LSID for the new name Ancylostoma ailuropodae n. sp. is urn:lsid:zoobank.org:act:2C6B6C1E-5F70-49B7-A303-B4D5AE9C7847.
Etymology: The new species is named for the type-host.
Description
General. Slender, relatively small nematodes of white coloration in life (Fig. 2a). Body cylindrical, tapering toward cephalic and caudal extremities with fine transversely striated cuticle; head oriented dorsally in males and females. Buccal capsule widening posteriorly to prominent oral aperture, possessing two pairs of ventrolateral teeth and two pairs of triangular dorsolateral teeth (Fig. 2b-f). Ventrolateral teeth vary in size and shape, with small, sub-aduncate inner and large triangular outer teeth extending dorsally. Dorsal gland well developed, associated with rod-like oesophagus, slightly swollen posteriorly, terminating in a lobed valve at junction with intestine (Fig. 4a, b). Nerve-ring at midlevel of oesophagus. Cervical papillae well developed, conical, situated posterior to level of nerve-ring. Excretory pore opens at level between cervical papillae and nerve-ring (Fig. 5a1, 2).
Photomicrographs of adults of Ancylostoma ailuropodae n. sp. a Total view of males (top) and females (down); b-f Cephalic extremity: lateral view of mouth (b and c), showing dorsolateral and ventrolateral teeth; dorsoventral view of mouth (d-f), showing dorsolateral (d) and ventrolateral (e and f) teeth with their positions, shapes and sizes. The arrangements of dorsolateral (2 pairs; b-d) and ventrolateral (2 pairs; b, c, e and f) teeth are indicated by red arrows
Male. [Based on the holotype and three males.] Body length 8.60–12.00 (10.30) mm, maximum width at mid-body 500–520 (510). Buccal capsule 180–220 (200) long, 120–160 (140) wide in dorsoventral view; oesophagus 960–1,500 (1,230) long, 150–190 (170) wide; oesophageal length 12% of total body. Cervical papillae 600–750 (680), excretory pore 500–580 (530), nerve-ring 390–520 (425) posterior to cephalic extremity. Copulatory bursa well developed, broader than long; dorsal lobe small with lateral lobes projecting in direction of lateral trunks (Figs. 3a-f, 5a5). Dorsal ray thick, 280–390 (350) in length, 40–60 (52) in maximum width; bifurcating at 270–295 (280) from anterior into 2 branches; each branch further dividing into 2 sub-branches; externodorsal rays arcuate, arising from dorsal ray at same level (Figs. 3e-f, 5a5). Lateral rays slender, tapering, and arcuate with a common stem. Anterolateral ray bending anteriad, with medio- and posterolateral rays projecting in parallel, extending to edge of bursa (Figs. 3a-b, 5a5). Antero- and posteroventral rays merge at base and then divide, continuing parallel deep into cleft (Figs. 3c-d, 5a5). Spicules tawny colored, paired, equal, filiform, 2,000–2,900 (2,450) long (Figs. 4e-f, 5a5). Gubernaculum fusiform, 80–120 (90) long, 12–20 (16) wide (Figs. 4c, 5a5). Cloacal papillae (n = 7) (Figs. 4d, 5a5): 1 pair disposed dorsally, 1 pair laterally, 3 single papillae ventrally.
Photomicrographs of Ancylostoma ailuropodae n. sp. male, caudal extremity. a, b Lateral view of bursa showing position of lateral rays and genital cone. c, d Ventral view of bursa showing configuration of antero- and postero-ventral rays. e, f Dorsal view of bursa showing relationships of the dorsal and externodorsal rays; note configuration of the bifurcations of the dorsal ray. Arrows in a, c and e denote the rays which are magnified in panels b, d and f, respectively
Photomicrographs of adults of Ancylostoma ailuropodae n. sp. a Dorsoventral view of anterior region of female, showing buccal capsule and entire oesophagus. b Lobed valves between oesophagus and intestine. c Ventrolateral view of male tail, showing gubernaculum. d Ventral view of male tail, showing cloacal papillae. e, f Ventral and ventrolateral views of male tail, showing spicules from both proximal (e) and distal (f) extremities. g Lateral view of female tail with spine-like point. Arrows indicate some small structures, including cervical papillae (a), lobed valve (b), gubernaculum (c), cloacal papillae (d), spicules (e, f) and spine-like point of female tail (g)
Line drawings of Ancylostoma ailuropodae n. sp. and comparison of dorsal rays among Ancylostoma spp. a Morphological structures of A. ailuropodae n. sp.: 1, dorsoventral view of anterior region; 2, lateral view of anterior region; 3, lateral view of female vulval region; 4, lateral view of female caudal region; 5, dorsal view of male caudal region; 6, egg. b Ten Ancylostoma species for comparison of dorsal rays: 1, A. taxideae [10]; 2, A. duodenale [9]; 3, A. paraduodenale [6]; 4, A. caninum [58]; 5, A. malayanum [7]; 6, A. kusimaense [9]; 7, A. ceylanicum [9, 11]; 8, A. braziliense [9, 11]; 9, A. tubaeforme [58]; 10, A. ailuropodae n. sp
Female. [Based on the allotype and three females.] Body 9.80–16.00 (12.90) mm long, with maximum width at mid-body 560–740 (650); width at anus 270–340 (285). Buccal capsule 170–250 (210) long, 130–190 (160) wide in dorsoventral view; oesophagus 1,280–1,320 (1,300) long, 170–250 (200) in maximum width near base. Cervical papillae 800–1,230 (900), excretory pore 760–950 (820), nerve-ring 600–650 (620) posterior to cephalic extremity. Vulva opens ventrally in posterior third of body, at 2,450–4,686 (3,480) from caudal extremity; vagina relatively short. Female reproductive system amphidelphic, with poorly differentiated vestibule, paired sphincters and infundibula confluent with uterine and ovarian stems (Fig. 5a3). Tail 90–370 (230) long, terminating in acute, spine-like point 9–25 (17) in length (Figs. 4g, 5a4). Eggs oval, 54–71 × 28–38 (62 × 33) (n = 20) (Fig. 5a6).
Remarks
Ancylostoma ailuropodae n. sp. is established based on comparisons to available descriptions among congeners in the global fauna [6, 7, 9, 10, 54,55,56,57,58,59,60,61,62]. Ancylostoma ailuropodae is unequivocally differentiated from congeners by structural characteristics of male and female specimens including body size, arrangement, number and dimensions of buccal teeth and shape of the buccal capsule, and in males by the configuration of the dorsal ray and bursa and lengths of spicules and gubernaculum, respectively (see Table 2 and Fig. 5b). Of note, tooth-number appears to represent one of the key morphological characters separating A. ailuropodae from other species of Ancylostoma. Specifically, (i) A. ailuropodae differs from A. caninum, A. tubaeforme and A. taxideae by the number (2 vs 3 pairs) of ventrolateral teeth; and (ii) from A. ceylanicum, A. braziliense, A. duodenale, A. kusimaense, A. paraduodenale and A. malayanum by the number (2 vs 0/1 pairs) of triangular dorsolateral teeth. Furthermore, the shape of the dorsal rays appears to be another potential species-specific morphological indicator (Fig. 5b). Specimens of A. ailuropodae n. sp. vary from A. tubaeforme by differences in cleft length of two digitations in each branch (Fig. 5b9 and 10) and further from A. taxideae, A. duodenale, A. paraduodenale, A. caninum, A. malayanum, A. kusimaense, A. ceylanicum and A. braziliense by the absence of a third digitation in each branch (Fig. 5b1–8 and 10). Verified specimens of A. genettae, A. protelesis and A. somaliense have not yet been described and these three species were not included in the comparison above. Notably, the adults of both A. pluridentatum and A. buckleyi can be distinguished from the new species by the number of ventrolateral teeth, given that A. pluridentatum has only one pair while A. buckleyi has three pairs according to the original descriptions (e.g. [60, 63]). Based on these morphological attributes, A. ailuropodae is considered to be a previously unrecognized species within the genus Ancylostoma.
Molecular characterization
To further probe the taxonomic position of A. ailuropodae, both nuclear ITS1-5.8S-ITS2 and mitochondrial cox1 sequences from two representative specimens (codes FTZ1 and FTZ2, respectively) were obtained and subjected to sequence characterization and phylogenetic analyses.
Sequence characterization
For ITS1-5.8S-ITS2, the 734 bp sequences from FTZ1 and FTZ2 were identical and had 52.2% A + T content. BLAST analysis revealed that A. ailuropodae shared the highest identity with A. ceylanicum (99.6%), followed by 98.8% identity with A. duodenale, 97.2% with A. tubaeforme, 95.8% with A. caninum, and 92.6% with A. braziliense. Based on the identities, there were a total of 59 variable positions found in the pairwise alignment of ITS1-5.8S-ITS2, including 17 parsimony-informative and 42 singleton sites (data not shown). Within cox1 sequences, same base composition (A = 23.4%; C = 9.7%; G = 22.6%; T = 44.3%) and sequence length (393 bp) were also observed in these two representative individuals of A. ailuropodae, with an A + T content of 67.7%, a typical mitochondrial nucleotide feature in nematodes (towards AT). BLAST search against GenBank/DDBJ/EMBL databases once again showed the highest nucleotide identity existing between the new species and A. ceylanicum (92.6%), followed by 89.2% identity between A. ailuropodae and A. tubaeforme, 88.6% between A. ailuropodae and A. duodenale, and 86.0% between A. ailuropodae and A. caninum, together corresponding to 99.2–100% identities at the amino-acid level. In terms of identity comparisons, there were a total of 78 variable positions in the 378 bp pairwise alignment, including 28 parsimony-informative and 50 singleton sites.
Further, we located these sites and determined if there were non-synonymous substitutions apparent via comparison of their protein sequences, and the results are shown in Fig. 6. Out of 78 variable base sites, 13 were unique for A. ailuropodae (in red); 16 were identical between A. ailuropodae and one of A. ceylanicum, A. duodenale, A. caninum and A. tubaeforme (in orange); and 49 were shared between A. ailuropodae and any two or three of these four congeneric species (in yellow). Among the 49 variable sites, however, the non-synonymous substitutions A/G250 in A. ceylanicum and T/A251 in A. caninum led to their amino acid changes: I (Ilu) → V (Val) in the former and I (Ilu) → N (Asn) in the latter (see Fig. 6). In addition, analysis of genetic distances using maximum composite likelihood estimates placed A. ailuropodae close to A. ceylanicum with the minimum interspecific evolutionary divergence (0.084), compared with 0.121 evolutionary divergence to A. tubaeforme, 0.127 to A. duodenale, and 0.151 to A. caninum (not shown).
Simultaneous alignments of nucleotide and amino-acid sequences of mitochondrial cox1 genes from Ancylostoma ailuropodae n. sp. and its congeneric species. For the alignments, the nucleotide sequences of cox1 genes were retrieved from the GenBank database (species and accession numbers are indicated in parentheses): Aai (A. ailuropodae n. sp.; KP842921), Ace (A. ceylanicum; KF896601), Aca (A. caninum; AB751617), Adu (A. duodenale; NC_003415), and Atu (A. tubaeforme; AJ407940). The corresponding protein sequences were deduced based on the Invertebrate Mitochondrial Code. Both nucleotide and amino-acid sequences were aligned with Clustal X 1.83 program. Regions of identity in either nucleotide (*) or amino-acid (#) are indicated. Variable base loci in Aai unique for A. ailuropodae n. sp. are highlighted in red; those shared between A. ailuropodae n. sp. and one of A. ceylanicum, A. duodenale, A. caninum and A. tubaeforme are highlighted in orange; and those shared between A. ailuropodae n. sp. and any two or three of these four congeneric species are highlighted in yellow. The non-synonymous substitutions A/G250 in A. ceylanicum and T/A251 in A. caninum as well as their amino-acid changes: I (Ilu)/V (Val) and I (Ilu)/N (Asn) are noted in red with a red star. Percentages of nucleotide and amino-acid identities with respect to Aai are shown at the end of each sequence
Phylogenetic characterization
Phylogenetic relationships between A. ailuropodae and other species were inferred from the respective sequences of ITS1-5.8S-ITS2 and cox1 using both MP and BI algorithms and their corresponding tree topologies are shown in Fig. 7. Although the two consistent structures (MP/BI) topologically varied from each other due to the different reference species included, both trees provided an identical, robust phylogenetic resolution for A. ailuropodae within the genus Ancylostoma and for the genus Ancylostoma within the family Ancylostomatidae. Specifically, (i) the two A. ailuropodae specimens clustered together as a monophyletic group that was separated from the other Ancylostoma species. (ii) When the congeneric species A. ceylanicum, A. caninum A. duodenale and A. tubaeforme were considered in our cox1-based analysis (Fig. 7a), A. ailuropodae and A. ceylanicum were more closely related to each other than to A. caninum, A. tubaeforme and A. duodenale, with robust support for tree topology (BP = 95 and PP = 0.99). (iii) When another species, A. braziliense, was added to re-construct this phylogenetic relationship using the ITS1-5.8S-ITS2 data (Fig. 7b), A. ailuropodae remained as the putative sister of A. ceylanicum, regardless of isolate origins (one from the UK and another from India; see Table 1), with high statistical support (BP = 89 and PP = 0.91), which was in agreement with the inferences from the cox1 gene analysis (see Fig. 7a). (iv) The inter-relationships of A. ailuropodae, A. ceylanicum, A. caninum, A. duodenale, A. braziliense and A. tubaeforme in the genus Ancylostoma; U. sanguinis, U. hamiltoni, U. lucasi, U. stenocephala and Uncinaria sp. in the genus Uncinaria; and N. americanus in the genus Necator, demonstrated phylogenetic stability of these monophyletic groups, with the current analyses being consistent with previously proposed molecular phylogenies of the hookworms based on the nuclear ribosomal and mitochondrial DNA data [64,65,66,67,68,69,70,71,72,73,74,75].
Phylogenetic relationships of hookworms isolated from the giant panda with the related hookworms in the family Ancylostomatidae. Phylogeny was inferred on the basis of mitochondrial cox1 (a) and nuclear ITS1-5.8S-ITS2 (b) sequences using both maximum parsimony (MP) and Bayesian inference (BI) methods. The livestock hookworm Bunostomum phlebotomum represented the outgroup species. Taxa belonging to the three major genera including Ancylostoma, Uncinaria and Necator in the family Ancylostomatidae are indicated by differently colored rectangles and shown in both phylogenetic topologies. The numbers along the branches indicate bootstrap values resulting from different analyses in the order MP/BI; values less than 50% are shown as “-”
Discussion
Hookworms in the genus Ancylostoma cause significant medical and veterinary disease (ancylostomiasis) in various hosts including humans and domestic and wild mammals [2, 71]. Recent epidemiological surveys revealed that some wild animal-derived species of Ancylostoma are emerging as important helminthic zoonotic agents because of rapid urbanization and increased human-wildlife interactions [11, 13,14,15,16,17,18,19,20,21]. The giant panda, for example, is an endangered and rare wild species in China that has been artificially protected and even partially housed for decades due to habitat loss [33]. Clinically unidentified specimens of Ancylostoma in giant pandas had been confirmed by veterinarians and wildlife biologists since the last century, but their potential zoonotic importance remains to be defined [41]. In the present study, A. ailuropodae n. sp. was isolated from the giant panda, morphologically characterized and demonstrated to be closely related to the anthropozoonotic A. ceylanicum by molecular analysis.
In general, morphological identification is a conventional and authoritative approach to define a new nematode parasite species. Concerning the genus Ancylostoma, several common species can be morphologically differentiated by key characters such as body size, teeth of the buccal capsule and shape of bursal rays (see Table 2 and Fig. 5b; cf. [9]). Similarly, specimens of A. ailuropodae from giant pandas are separated from other hookworms on the basis of either ventrolateral and dorsolateral teeth or dorsal rays, supporting the previous conclusions that teeth and rays were reliable morphological indicators in the differential diagnosis of Ancylostoma spp. [55, 56]. Among this assemblage, it is important to note that A. ailuropodae is clearly structurally distinct from A. malayanum, the only other species of Ancylostoma known in ursid hosts (e.g. Ursus thibetanus) (Table 2), with the implication that each of these species endemic to China may be more closely related to other congeners within the genus. Specimens upon which the description and differentiation of A. ailuropodae n. sp. was based were restricted to fully developed adults and eggs. Further work, using a combined laboratory-egg cultivation and Baermann technique, to describe the morphology of developmentally advanced larval stages is needed to complement morphological characteristics of the new species, and to provide valuable information assisting in species identification and differentiation in this genus [55, 76].
Following our morphological evidence, A. ailuropodae from giant pandas was further confirmed as an independent species by molecular analysis. For example, the internal transcribed spacer region (ITS1-5.8S-ITS2) of the nuclear ribosomal DNA is regarded as an appropriate genetic marker to resolve nematode relationships at the species level [77]. Pairwise comparisons of ITS1-5.8S-ITS2 in A. ailuropodae with congeneric species available in the GenBank database revealed a species-specific sequence feature (containing 59 variable informative sites) and overall identity of 92.6–99.6% among A. ceylanicum, A. tubaeforme, A. caninum and A. braziliense. Furthermore, high bootstrap support was evident, based on phylogenetic analysis of ITS1-5.8S-ITS2 that demonstrated monophyly of A. ailuropodae as the putative sister of A. ceylanicum (see Fig. 7b).
Critically, similar conclusions were reinforced by analysis of the mitochondrial cox1 gene. It should also be noted that cox1 analysis was included because recent studies of the substitution patterns for nematode mitochondrial genes (e.g. cox1 and nad4) revealed that they have utility in identifying and differentiating novel or cryptic species among closely related taxa due to assumed faster evolutionary rates than nuclear genes, features of maternal inheritance and absence of recombination [78,79,80]. Compared to the nuclear ITS, the cox1 of A. ailuropodae appeared to have more variable informative sites (n = 78, including 13 unique loci). Nevertheless, results based on cox1 were consistent with inference from ITS, in revealing a sister-species relationship with A. ceylanicum among a broader assemblage of congeners in the genus. Phylogenetic analysis of cox1 data (Fig. 7a) also supported the contention that A. ailuropodae n. sp. is an independent species which is clearly differentiated from A. ceylanicum, A. caninum, A. tubaeforme and A. duodenale.
Based on the results from integrated molecular and morphological comparisons, we propose that A. ailuropodae of giant pandas is a previously unrecognized and separate species that is closely related to the anthropozoonotic A. ceylanicum within the genus Ancylostoma. Additional information regarding the ultrastructure and genomics of this species and other related hookworms is still required. Broader taxonomic comparisons can provide an increasingly precise morphological and molecular basis for species recognition among hookworms. In addition, there were two non-synonymous base substitutions detected in cox1 genes of A. ceylanicum (A/G250) and A. caninum (T/A251) (Fig. 6) that were confirmed to be fixed and species-specific after homologous comparisons with other A. ceylanicum or A. caninum isolates from two sites in the same geographic area.
Ancylostoma ailuropodae identified here is the fourth hookworm to be described from the Ursidae. Previously, the hookworm Uncinaria yukonensis (Wolfgang, 1956) was characterized in black bears and Uncinaria rauschi (Olsen, 1968) in grizzly and black bears [81, 82]. On the basis of comparisons of morphometric and distribution data of ursine hookworms as well as the historical biogeography of bears, Catalano et al. [13] proposed that there was a relatively recent host-switching event of U. rauschi from black bears to grizzly bears.
The occurrence of A. ailuropodae appears consistent with speciation following a host colonization event to giant pandas apparently from a carnivoran source in sympatry, and further indicates a history of independent association with ursine hosts for the broader ancylostomatid hookworm assemblage. The timing and geographic source for these hookworms cannot be elucidated based on the currently available data and the reduced and relictual distribution for pandas, but a history of host colonization is compatible with the current tree topology (for parasites and hosts) and distribution of carnivore hosts for other species of Ancylostoma (e.g. [24]). We suggest that acquisition of Ancylostoma by giant pandas likely occurred prior to 7 million years ago (MYA) when a shift from an omnivorous diet to one dominated strictly by bamboo (by 2.4 MYA) was underway [25].
Divergence of A. ailuropodae appears to have occurred prior to acquisition of A. ceylanicum by humans in Southeast Asia, and prior to the intense bottlenecking of giant panda populations that has characterized the past century (cf. [33, 83] for details about the history of giant pandas). This interpretation is significant, as it would relate to the historical independence of A. ailuropodae and A. ceylanicum before the current intensified conservation campaign for maintaining giant pandas, and the potential for cross-transmission of both hookworm species when infected humans are in contact. The unique niche and specialized bamboo-feeding habits of giant pandas suggest that colonization in ecological time, related to the source or origin of A. ailuropodae, was unlikely given relative isolation with respect to a sympatric assemblage of carnivorans or other mammals that may serve as hosts for species of hookworms [25, 26, 33]. Parasitological inventory among potential carnivoran hosts in Sichuan and nearby regions remains necessary to demonstrate that A. ailuropodae has a narrow host range and may now be limited to the giant panda [84]; apparent narrow host range, however, does not preclude the potential or capacity for contemporary host switches to humans as a zoonotic parasite given opportunity due to permissive ecological circumstances [85,86,87,88].
Phylogenetic and historical isolation of giant pandas from the broader assemblage of ursids and ursine bears (e.g. [23, 24]) in conjunction with apparent structural divergence (e.g. teeth and configuration of the dorsal ray; Table 2 and Fig. 5b5 and 10) of A. ailuropodae and A. malayanum suggests that independent events of host colonization, separated in space and time, were essential in the process of speciation for these hookworms; molecular data, particularly from A. malayanum, is still needed to explore this hypothesis. Moreover, phylogenetic hypotheses for the Ursidae have placed giant pandas distantly from species of Ursus (and other ursines) near the base of an extensive radiation for bears that unfolded across the late Miocene and Pliocene [24]. Among ursine hosts for Ancylostoma, U. thibetanus (Asiatic black bear) is regarded as the sister of U. americanus (American black bear) and placed among crown species in ursid phylogenies [23, 24]. These relationships alone would serve to refute a coevolutionary hypothesis for Ancylostoma hookworms among bears, conversely supporting a history of independent events of host colonization that have structured this fauna.
Unlike U. yukonensis and U. rauschi in bears, the hookworm from giant panda is genetically similar to other Ancylostoma species (Fig. 7). These respective genera are referred to two independent subfamilies within the Ancylostomatide, namely Ancylostomatinae Looss, 1905 for Ancylostoma and Bunostominae Looss, 1911 for Uncinaria, consistent with extended evolutionary trajectories for these taxa among the hookworms. This suggests the independent origin of A. ailuropodae, supporting monophyly of A. ailuropodae and congeneric species A. ceylanicum, A. duodenale, A. tubaeforme, A. caninum and A. braziliense, and strengthens the close relationship between the giant panda hookworm and A. ceylanicum within the clade. Concurrently it suggests that Uncinaria spp. from pinnipeds and ursids are a distinct monophyletic group in the family Ancylostomatidae [70]. The apparent genetic differences of A. ailuropodae n. sp. in pandas and U. rauschi and U. yukonensis in bears, coupled with their divergent biogeographic and ecological histories suggest this system as a good model for exploring the complexities of diversification and faunal assembly in the evolution of host range and associations among hookworms (e.g. [85,86,87,88]).
The potential for genetic partitioning among possible disjunct populations of hookworms in giant pandas should be considered, as it will reflect information about the timing of colonization to giant pandas and the duration of the history of association. Further, the history of fragmentation and isolation for giant pandas across now isolated mountain systems in southwestern China suggests a complex relationship among hosts and hookworms in this region. Such history could be explored through fecal-based approaches in conjunction with molecular diagnostics to examine occurrence and the extent of genetic diversity and distribution for hookworm parasites among populations and subspecies of giant pandas.
Conclusions
This study is the first to describe and define a new member of the genus Ancylostoma, A. ailuropodae, in the wild giant panda using morphological and molecular criteria. Morphological characters (e.g. ventrolateral (two pairs) and dorsolateral (two pairs) teeth and dorsal rays) distinctly separate A. ailuropodae n. sp. from other congeneric species in the genus Ancylostoma. Further, nuclear ITS1-5.8S-ITS2 and mitochondrial cox1-based genetic distance analysis and phylogenies supported the assertion that A. ailuropodae is independent and shares a sister-species relationship with the anthropozoonotic A. ceylanicum. Although additional molecular evidence is warranted, this finding should enhance public awareness of parasitic hookworms in giant pandas, especially in captive populations that have frequent contact with breeders, veterinarians and even tourists. Moreover, the morphological and molecular data presented here enhances the information on species within the genera Ancylostoma, Uncinaria, and Necator and contributes to a more complete understanding of the taxonomy, diagnostics and evolutionary biology of hookworms.
Abbreviations
- BF:
-
Bootstrapping frequencies
- BI:
-
Bayesian inference
- BIC:
-
Bayesian information criteria
- cox1:
-
Cytochrome c oxidase subunit 1
- ITS:
-
Internal transcribed spacer (ITS1-5.8S-ITS2)
- MCMC:
-
Markov chain Monte Carlo
- MP:
-
Maximum parsimony
- MYA:
-
Million years ago
- PP:
-
Posterior probabilities
- SD:
-
Standard deviation
- TBR:
-
Tree-bisection-reconnection
References
Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–807.
Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197–288.
Schad GA, Warren KS. Hookworm disease: current status and new directions. London: Taylor & Francis; 1990.
Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32.
Macchioni G. Ancylostoma genettae, A. protelesis, A. somaliense: three new species from wild Carnivora in the Somali republic. Parassitologia. 1995;37:219–28.
Biocca E. On Ancylostoma paraduodenale, a new species from felines, closely related to A. duodenale. J Helminthol. 1951;25:11–8.
Wu J, Zhang DH, Huang H, Hu HG, Zhao GL. [Morphological description on Ancylostoma malayanum from Selenarctos thibetanus in Sichuan.] Chin J Zoonoses. 1986;5:30–2 (In Chinese).
Van Heerden J, Mills M, Van Vuuren M, Kelly P, Dreyer M. An investigation into the health status and diseases of wild dogs (Lycaon pictus) in the Kruger National Park. J S Afr Vet Assoc. 1995;66:18–27.
Yoshida Y. Ancylostoma kusimaense from a dog in Japan and comparative morphology of related ancylostomes. J Parasitol. 1965;51:631–5.
Kalkan A, Hansen M. Ancylostoma taxideae sp. n. from the American badger, Taxidea taxus taxus. J Parasitol. 1966;52:291–4.
Biocca E. On Ancylostoma braziliense (de Faria, 1910) and its morphological differentiation from A. ceylanicum. J Helminthol. 1951;25:1–10.
Smout FA, Thompson RA, Skerratt LF. First report of Ancylostoma ceylanicum in wild canids. Int J Parasitol Parasites Wildl. 2013;2:173–7.
Catalano S, Lejeune M, van Paridon B, Pagan CA, Wasmuth JD, Tizzani P, et al. Morphological variability and molecular identification of Uncinaria spp. (Nematoda: Ancylostomatidae) from grizzly and black Bears: new species or phenotypic plasticity? J Parasitol. 2015;101:182–92.
Mackenstedt U, Jenkins D, Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int J Parasitol Parasites Wildl. 2015;4:71–9.
Ngui R, Mahdy MA, Chua KH, Traub R, Lim YA. Genetic characterization of the partial mitochondrial cytochrome oxidase c subunit I (cox 1) gene of the zoonotic parasitic nematode, Ancylostoma ceylanicum from humans, dogs and cats. Acta Trop. 2013;128:154–7.
Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, et al. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg Infect Dis. 2014;20:976–82.
Ngui R, Lim Y, Traub R, Mahmud R, Mistam MS. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl Trop Dis. 2012;6:e1522.
Hotez PJ. Hookworm disease in children. Pediatr Infect Dis J. 1989;8:516–20.
Brown B, Copeman D. Zoonotic importance of parasites in wild dogs caught in the vicinity of Townsville. Aust Vet J. 2003;81:700–2.
Jenkins D, Allen L, Goullet M. Encroachment of Echinococcus granulosus into urban areas in eastern Queensland, Australia. Aust Vet J. 2008;86:294–300.
Bradley CA, Altizer S. Urbanization and the ecology of wildlife diseases. Trends Ecol Evol. 2007;22:95–102.
Fryxell JM, Sinclair AR, Caughley G. Wildlife ecology, conservation and management. New York: John Wiley & Sons; 2014.
Wozencraft W. Carnivora: Caniformia: Ursidae. In: Wilson DE, Reeder DM, editors. Mammal species of the world a taxonomic and geographic reference. Baltimore: Johns Hopkins University Press; 2005. p. 586–90.
Yu L, Li Q, Ryder OA, Zhang Y. Phylogeny of the bears (Ursidae) based on nuclear and mitochondrial genes. Mol Phylogenet Evol. 2004;32:480–94.
Krause J, Unger T, Noçon A, Malaspinas AS, Kolokotronis SO, Stiller M, et al. Mitochondrial genomes reveal an explosive radiation of extinct and extant bears near the Miocene-Pliocene boundary. BMC Evol Biol. 2008;8:220.
Xue Z, Zhang W, Wang L, Hou R, Zhang M, Fei L, et al. The bamboo-eating giant panda harbors a carnivore-like gut microbiota, with excessive seasonal variations. MBio. 2015;6:e00022–00015.
Wei F, Hu Y, Zhu L, Bruford MW, Zhan X, Zhang L. Black and white and read all over: the past, present and future of giant panda genetics. Mol Ecol. 2012;21:5660–74.
Yang GY, Zhang ZH. Parasitic diseases of wildlife. Beijing: Science Press; 2013.
State Forestry Administration. [The fourth national survey report on giant panda in China.] Fores Chi. 2015;832:18–23 (In Chinese).
Yang B, Busch J, Zhang L, Ran J, Gu X, Zhang W, Du B, Mittermeier RA. Eco-compensation for giant panda habitat. Science. 2013;339:521.
Reid DG, Jien G. Giant panda conservation action plan. In: Servheen C, Herrero S, Peyton B, editors. Bear status survey and conservation action plan, IUCN/SSC bear and polar bear specialist groups, IUCN. Gland: Switzerland and Cambridge; 1999. p. 241–54.
Hartig F. Panda diplomacy: the cutest part of China’s public diplomacy. Hague J Diplomacy. 2013;8:49–78.
Zhang JS, Daszak P, Huang HL, Yang GY, Kilpatrick AM, Zhang S. Parasite threat to panda conservation. Ecohealth. 2008;5:6–9.
Wildt DE, Zhang AJ, Zhang HM, Janssen DL, Ellis S. Giant pandas: biology, veterinary medicine and management. Cambridge: Cambridge University Press; 2006.
Loeffler K, Montali RJ, Rideout BA. Diseases and pathology of giant pandas. In: Wildt DE, Zhang ZA, Zhang HM, Janssen DL, Ellis S, editors. Giant Pandas: Biology, Veterinary Medicine and Management. Cambridge: Cambridge University Press; 2006. p. 377–409.
Zhang W, Yie S, Yue B, Zhou J, An R, Yang J, et al. Determination of Baylisascaris schroederi infection in wild giant pandas by an accurate and sensitive PCR/CE-SSCP method. PLoS One. 2012;7:e41995.
Zhang L, Yang X, Wu H, Gu X, Hu Y, Wei F. The parasites of giant pandas: individual-based measurement in wild animals. J Wildl Dis. 2011;47:164–71.
Cheng WY, Zhao GH, Jia YQ, Bian QQ, Du SZ, Fang YQ, et al. Characterization of Haemaphysalis flava (Acari: Ixodidae) from qingling subspecies of giant panda (Ailuropoda melanoleuca qinlingensis) in qinling mountains (central China) by morphology and molecular markers. PLoS One. 2013;8:e69793.
Liu X, He T, Zhong Z, Zhang H, Wang R, Dong H, et al. A new genotype of Cryptosporidium from giant panda (Ailuropoda melanoleuca) in China. Parasitol Int. 2013;62:454–8.
Wang T, Chen ZQ, Xie Y, Hou R, Wu QD, Gu XB, et al. Prevalence and molecular characterization of Cryptosporidium in giant panda (Ailuropoda melanoleuca) in Sichuan province. China Parasit Vectors. 2015;8:344.
Zhang TF, Yang GY, Lu MK, Lai CL, Sha GR, Yang ML. Hookworms found in animals of Sichuan province. Sichuan J Zool. 2005;24:177–9.
Brooks DR, McLennan DA. The nature of diversity: an evolutionary voyage of discovery. Chicago: University of Chicago Press; 2002.
Markovics A, Medinski B. Improved diagnosis of low intensity Spirocerca lupi infection by the sugar flotation method. J Vet Diagn Invest. 1996;8:400–1.
Lichtenfels JR. Keys to the genera of the superfamiles Ancylostomatoidea and Diaphanocephaloidea. In: Anderson RC, Chabaud AG, Wilmot S, editors. CIH keys to the nematode parasites of vertebrates. Farnham Royal: Commonwealth Agricultural Bureaux; 1980. p. 1–26.
Baylis HA. A new species of the nematode genus Uncinaria from a sea-lion, with some observations on related species. Parasitology. 1933;25:308–16.
Marcus AD, Higgins DP, Slapeta J, Gray R. Uncinaria sanguinis n. sp. (Nematoda: Ancylostomatidae) from the endangered Australian sea lion, Neophoca cinerea (Carnivora: Otariidae). Folia Parasitol. 2014;61:255–65.
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82.
Swofford DL. PAUP*: Phylogenetic analysis using parsimony (and other methods). Sunderland: Sinauer Associates; 2002.
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–42.
Xie Y, Zhang ZH, Niu LL, Wang Q, Wang CD, Lan JC, et al. The mitochondrial genome of Baylisascaris procyonis. PLoS One. 2011;6:e27066.
Santorum JM, Darriba D, Taboada GL, Posada D. jmodeltest.org: selection of nucleotide substitution models on the cloud. Bioinformatics. 2014;30:1310–1.
Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A. 2004;101:11030–5.
International Commission on Zoological Nomenclature. Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Zootaxa. 2012;3450:1–7.
Yoshida Y, Kondo K, Kurimoto H, Fukutome S, Shirasaka S. Comparative studies on Ancylostoma braziliense and Ancylostoma ceylanicum. III. Life history in the definitive host. J Parasitol. 1974;60:636–41.
Yoshida Y. Morphological differences between Ancylostoma duodenale and Necator americanus in the fourth larval stage. J Parasitol. 1966;52:122–6.
Yoshida Y. Comparative studies on Ancylostoma braziliense and Ancylostoma ceylanicum. I. The adult stage. J Parasitol. 1971;57:983–9.
Yoshida Y, Kondo K, Okada S, Okamoto K, Kurimoto H, Oda K, Shimada Y. Morphology and life history of Ancylostoma kusimaense Nagayosi, 1955. Jap J Parasitol. 1974;23:187–200.
Burrows RB. Comparative morphology of Ancylostoma tubaeforme (Zeder, 1800) and Ancylostoma caninum (Ercolani, 1859). J Parasitol. 1962;48:715–8.
Norris DE. Morphology of a North American strain of Ancylostoma braziliense Gomes de Faria, 1910. J Parasitol. 1971;57:993–7.
Setasuban P. Morphology of Ancylostoma buckleyi Le Roux and Biocca, 1957 in dogs from Cairns, North Queensland, Australia. Southeast Asian J Trop Med Public Health. 1976;7:45–9.
Setasuban P. Comparative morphology of genital cones of genus Ancylostoma Dubini, 1843. Southeast Asian J Trop Med Public Health. 1975;6:230–4.
Setasuban P. Studies on the life history and morphology of Ancylostoma tubaeforme (Zeder, 1800) with comparative studies on Ancylostoma caninum (Ercolani, 1859). PhD thesis. Queensland: The University of Queensland; 1980.
Schwartz B. Description of Ancylostoma paraduodenale, a hookworm of carnivores, and a review of the genus Ancylostoma. Proc US Nat Mus. 1927;72:1–9.
e Silva LM, Miranda RR, Santos HA, Rabelo EM. Differential diagnosis of dog hookworms based on PCR-RFLP from the ITS region of their rDNA. Vet Parasitol. 2006;140:373–7.
Jex AR, Waeschenbach A, Hu M, van Wyk JA, Beveridge I, Littlewood DT, et al. The mitochondrial genomes of Ancylostoma caninum and Bunostomum phlebotomum - two hookworms of animal health and zoonotic importance. BMC Genomics. 2009;10:79.
Traub RJ, Hobbs RP, Adams PJ, Behnke JM, Harris PD, Thompson RC. A case of mistaken identity - reappraisal of the species of canid and felid hookworms (Ancylostoma) present in Australia and India. Parasitology. 2007;134:113–9.
Hu M, Chilton NB, Zhu XQ, Gasser RB. Single-strand conformation polymorphism-based analysis of mitochondrial cytochrome c oxidase subunit 1 reveals significant substructuring in hookworm populations. Electrophoresis. 2002;23:27–34.
Hu M, Chilton NB, Gasser RB. The mitochondrial genomes of the human hookworms, Ancylostoma duodenale and Necator americanus (Nematoda: Secernentea). Int J Parasitol. 2002;32:145–58.
Lucio-Forster A, Liotta JL, Yaros JP, Briggs KR, Mohammed HO, Bowman DD. Morphological differentiation of eggs of Ancylostoma caninum, Ancylostoma tubaeforme, and Ancylostoma braziliense from dogs and cats in the United States. J Parasitol. 2012;98:1041–4.
Nadler SA, Lyons ET, Pagan C, Hyman D, Lewis EE, Beckmen K, et al. Molecular systematics of pinniped hookworms (Nematoda: Uncinaria): species delimitation, host associations and host-induced morphometric variation. Int J Parasitol. 2013;43:1119–32.
Haynes BT, Marcus AD, Higgins DP, Gongora J, Gray R, Slapeta J. Unexpected absence of genetic separation of a highly diverse population of hookworms from geographically isolated hosts. Infect Genet Evol. 2014;28:192–200.
Hu M, Chilton NB, El-Osta YGA, Gasser RB. Comparative analysis of mitochondrial genome data for Necator americanus from two endemic regions reveals substantial genetic variation. Int J Parasitol. 2003;33:955–63.
Nadler SA, Adams BJ, Lyons ET, DeLong RL, Melin SR. Molecular and morphometric evidence for separate species of Uncinaria (Nematoda: Ancylostomatidae) in California sea lions and northern fur seals: hypothesis testing supplants verification. J Parasitol. 2000;86:1099–106.
Hasegawa H, Modry D, Kitagawa M, Shutt KA, Todd A, Kalousova B, et al. Humans and great apes cohabiting the forest ecosystem in Central African Republic harbour the same hookworms. PLoS Negl Trop Dis. 2014;8:E2715.
Wang CR, Gao JF, Zhu XQ, Zhao Q. Characterization of Bunostomum trigonocephalum and Bunostomum phlebotomum from sheep and cattle by internal transcribed spacers of nuclear ribosomal DNA. Res Vet Sci. 2012;92:99–102.
Svensson RM, Kessel JF. Morphological differences between Necator and Ancylostoma larvae. J Parasitol. 1926;13:146–53.
Zhu XQ, Jacobs D, Chilton N, Sani R, Cheng N, Gasser RB. Molecular characterization of a Toxocara variant from cats in Kuala Lumpur, Malaysia. Parasitology. 1998;117:155–64.
Hu M, Chilton NB, Gasser RB. The mitochondrial genomics of parasitic nematodes of socio-economic importance: recent progress, and implications for population genetics and systematics. Adv Parasitol. 2003;56:133–212.
Zhan B, Li T, Zhang F, Hawdon JM. Species-specific identification of human hookworms by PCR of the mitochondrial cytochrome oxidase I gene. J Parasitol. 2001;87:1227–9.
Blouin MS, Yowell CA, Courtney CH, Dame JB. Substitution bias, rapid saturation, and the use of mtDNA for nematode systematics. Mol Biol Evol. 1998;15:1719–27.
Wolfgang RW. Dochmoides yukonensis sp. nov. from the brown bear (Ursus americanus) in the Yukon. Can J Zool. 1956;34:21–7.
Olsen OW. Uncinaria rauschi (Strongyloidea: Nematoda), a new species of hookworms from Alaskan bears. Can J Zool. 1968;46:1113–7.
Zhao S, Zheng P, Dong S, Zhan X, Wu Q, Guo X, et al. Whole-genome sequencing of giant pandas provides insights into demographic history and local adaptation. Nat Genet. 2013;45:67–71.
Brooks DR, Hoberg EP, Gardner SL, Boeger W, Galbreath KE, Herczeg D, et al. Finding them before they find us: informatics, parasites and environments in accelerating climate change. Comp Parasitol. 2014;81:155–64.
Hoberg EP, Brooks DR. A macroevolutionary mosaic: Episodic host-switching, geographic colonization, and diversification in complex host-parasite systems. J Biogeogr. 2008;35:1533–50.
Hoberg EP, Brooks DR. Beyond vicariance: integrating taxon pulses, ecological fitting and oscillation in historical biogeography and evolution. In: Morand S, Krasnov BR, editors. The geography of host-parasite interactions. Oxford: Oxford University Press; 2010. p. 7–20.
Hoberg EP, Brooks DR. Evolution in action: climate change, biodiversity dynamics and emerging infectious disease. Philos Trans R Soc Lond B Bio Sci. 2015;5:370.
Araujo SBL, Braga MP, Brooks DR, Agosta S, Hoberg EP, von Hathental F, Boeger WA. Understanding host-switching by ecological fitting. PLoS One. 2015;10:e0139225.
Acknowledgements
We acknowledge the staff of the Fengtongzai Natural Nature Reserve (Sichuan, China) and Conglong Lai, Guorui Sha and Rongxue Peng from the Sichuan Agricultural University (Ya’an, China) for their assistance during specimen sampling; Min Yan from the Sichuan Agricultural University of China for technical assistance in preliminary examinations, photo-micrographic imaging and morphometrics of specimens.
Funding
This work was supported by the grant from the Research Fund for the Chengdu Research of Giant Panda Breeding, China (no. CPF2012-13) and the fellowship from the China Scholarship Council (CSC 201406910034). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. Nucleotide sequences generated in the present study have been deposited in the GenBank database under accession numbers KP842921–KP842924. The type-material has been deposited in Sichuan Agricultural University under accession numbers GYY-XY1301–13017.
Authors’ contributions
YGY and XY led the study and drafted the initial manuscript. XY and YGY collected samples. XY performed the laboratory work and carried out the molecular genetic study. YGY, JFU and EPH oversaw the study. YGY, EPH, XY and YZJ prepared the morphological description of the specimens. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval
This study was performed in strict accordance with the Wild Animal Protection Law of the People’s Republic of China (released in 1989), and all procedures were reviewed and approved by the Animal Ethics Committee of Sichuan Agricultural University (Sichuan, China; approval no. 2011–028). The worm specimens were collected from a recently deceased giant panda with permission of the Sichuan Fengtongzai Natural Nature Reserve.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xie, Y., Hoberg, E.P., Yang, Z. et al. Ancylostoma ailuropodae n. sp. (Nematoda: Ancylostomatidae), a new hookworm parasite isolated from wild giant pandas in Southwest China. Parasites Vectors 10, 277 (2017). https://doi.org/10.1186/s13071-017-2209-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-017-2209-2