Abstract
Cu2O supported on peanut shell (Cu2O@PS) was prepared by the reaction of copper acetate and peanut shell powder as a naturally available biopolymer support. The prepared catalyst was used as an efficient and reusable heterogeneous catalyst in the click reaction of benzyl halide or phenacyl bromides, acetylenes and sodium azide for the synthesis of potentially biologically active 1,2,3-triazoles under ultrasonic irradiation in EtOH-H2O as green solvent.

Similar content being viewed by others
Introduction
Green chemistry is one of the most important research activities for chemists, both in the laboratory and industry. Therefore, many efficient, eco-friendly and clean synthetic strategies have been developed for the synthesis of biologically and industrially active molecules [1,2,3,4,5]. Meanwhile, metal-catalyzed multi-component reaction is one of the significant areas of green chemistry research. Transition metal-catalyzed click synthesis of triazoles is a powerful method for the synthesis of diverse complex molecules. Triazoles derivatives have developing application in medicinal chemistry and biological activities [6,7,8,9,10]. They also have numerous industrial applications as florescent whiteners, dyestuffs, photo-stabilizers of polymers, and optical brightening agents [11, 12]. Forasmuch as copper-catalyzed click reaction is one of the best methods for the synthesis of 1,2,3-triazoles [13, 14], numerous homogeneous copper catalysts have been reported [15, 16]. Most of these successful methods suffer from non-reusability of the catalysts, and the usage of toxic and/or expensive ligands [17,18,19,20]. To overcome these problems, many researchers have focused their efforts on copper-based heterogeneous systems [17, 21, 22].
Natural biopolymers are the attractive subjects for the design of bio-supported catalysts due to their eco-friendly, low cost and non-toxic properties [23,24,25,26,27,28,29,30]. Peanut shell as an agro-industrial waste containing considerable fraction of the biodegradable lignocellulosic waste [31] is discarded in the environment or burned about 13.7 million tons per year [32, 33]. This promising natural and renewable raw material consists of a combination of lignin, cellulose, proteins and hemicellulose biopolymers (Fig. 1) [34, 35]. There are many polar functional groups such as hydroxyl, methoxy and carboxyl groups on the surface of peanut shell. Therefore, peanut shell is an attractive candidate as a natural, renewable, non-toxic and very low, or no cost environmentally friendly support for metal nanoparticles.
These days, the application of ultrasonic technology has reported for organic compounds synthesis, emulsification, extraction, nanoparticle formation, and degassing [36,37,38,39]. Sonication method has important advantages such as high efficiency, selectivity and yield, economic performance, short reaction time, and low environmental pollution [20, 40,41,42].
Herein, in continuation of our research toward the development of nano bio-based catalytic systems [43,44,45], the synthesis of copper oxide supported on peanut shell (Cu2O@PS) as a heterogeneous nano-biocatalyst and its catalytic activity for the click synthesis of triazoles in EtOH-H2O as green solvent under ultrasonic irradiation is reported.
Results and discussion
Synthesis and characterizations of the catalyst
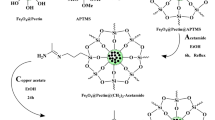
The preparation of the Cu2O@PS nanocomposite is described schematically in Scheme 1. The Cu2O@PS nanocomposite obtained by the reaction of peanut shell powder with copper acetate in water at 70 °C for 5 h. The catalyst was centrifuged and washed with water, ethanol, and acetone then dried in the oven at 70 °C.

(The source of this diagram is taken from “http://nolinsteel.com/peanuts/” and the used softwares are Chemdraw and Paint. The Scheme was designed by authors)
Schematic diagram of catalyst preparation
The Cu2O@PS nanocomposite was characterized by FT-IR, thermogravimetric analysis (TGA), atomic absorption spectroscopy (AAS), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDS) analysis and X-ray diffraction (XRD) measurements. The FT-IR spectrum of peanut shell and Cu2O@PS are shown in Fig. 2. The band around 3400 cm−1 was ascribed to the mixed stretching vibration absorption band of amino and hydroxyl groups. The bands at 2950 cm−1 were assigned to aliphatic C–H, mainly CH2 stretching. The small band obtained at 1738 cm−1 was assigned to the carbonyl groups stretching vibration [29, 46]. As can be seen in the FT-IR spectrum of Cu2O@PS, the presence of characteristic bands of PS in the 1738 and 3500 cm−1 regions clearly confirms the existence of PS in the final catalyst. Also, the band shift from 1738 cm−1 (in PS) to 1727 cm−1 (in Cu2O@PS) reveals the coordination of copper to peanut shell [46].
Thermogravimetric analysis was further used to study the composition of the catalyst (Fig. 3). The TGA curve of the catalyst shows a weight loss at ~ 100 °C that is associated with the release of physically adsorbed water. The weight loss above ~ 230 °C (and continued to ~ 600 °C) is related to the decomposition of PS and organic groups on the Cu2O@PS. Thermal analysis showed that the catalyst has good thermal stability up to 230 °C. Also, the copper content on the Cu2O@PS nanocomposite was measured 0.28 mmol g−1 by atomic absorption spectroscopy.
Morphologies of fresh peanut shell and the Cu2O@PS nanocomposite were determined by SEM. The fresh PS is basically smooth (Fig. 4a). The SEM images of Cu2O@PS show the formation of spherical particles in size around 30–40 nm on the surface of the peanut shell (Fig. 4b).
In addition, comparing the EDS analysis of Ps and Cu2O@PS clearly shows the presence of Cu, C, O, and N elements in this composite and demonstrate that copper was anchored to the PS (Fig. 5a and b). In the XRD pattern of the Cu2O@PS nanocomposite, the diffractions at 2θ = 36.4°, 42.5°, 61.4°, and 73.4° can be assigned to the (111), (200), (220) and (311) lattice planes of Cu2O, in accordance with Cu2O standard data (JCPDS card NO. 05–0667) (Fig. 6).
Catalytic studies
The catalytic activity of Cu2O@PS nanocomposite was investigated in the click synthesis of 1,2,3-triazoles. The reaction of phenyl acetylene 1, 4-nitrobenzyl bromide 2 and sodium azide (NaN3) was chosen as a model reaction under different conditions. As the first experiment, K2CO3 was used as a base in the presence of 1 mol% of catalyst, and this reaction was tested by employing various solvents such as toluene, MeCN, MeOH, EtOH, H2O, EtOH-H2O, and MeOH-H2O at 50 °C under ultrasonic irradiation for 45 min (Table 1, entries 1–7). A superior yield was obtained when EtOH-H2O (1:1) was used as the solvent (Table 1, entry 6). Then, different base were screened in the model reaction. A moderate yields were obtained with Cs2CO3 and KOt-Bu (entries 8 and 9) and the reaction proceed in fairly good yields in the presence of NaOH and KOH (entries 10 and 11). Also, some experiments were carried out at different temperatures, and finally 50 °C was chose as optimum reaction temperature (entries 6, 12–14). Effect of the catalyst loading was also investigated under the optimum reaction conditions. It was found that, when the amount of catalyst increased from 0.5 and 1 to 2 mol%, the yield of product changed from 65 and 91% to 92%, respectively. So, 1 mol% of catalyst is sufficient to promote this reaction (entries 6, 15–16). When this reaction was carried out without catalyst, the yield of the product was trace (entry 17). To delineate the role of ultrasound, the reaction was investigated without ultrasonic irradiation at 50 °C in various solvents. In all reactions, the result obtained by the use of ultrasound irradiation leads to a higher yield (entries 18–20). Finally, when this reaction was carried out with PS or Cu2O as catalyst, the yield of the product was trace and 78% yield, respectively (entries 21 and 22).

To explore the scope of the click reaction various benzyl bromides and aryl acetylenes, containing both electron donating and electron withdrawing functionalities were screened in optimized reaction conditions and high isolated yields were obtained (Table 2). Under the same reaction conditions benzyl chlorides provided target products in good yields (Table 2, entries 12–14).

Observation of great potential activity of Cu2O@PS nano-biocatalyst in the Click reaction of benzyl bromides and aryl acetylenes encouraged us to investigate the Click reaction of aryl acetylenes 1 with phenacyl bromides 4 and sodium azide in the same reaction conditions. As can be seen from Table 3, the Click reaction of aryl acetylenes and phenacyl bromides contain electron withdrawing or donation groups provide 1H-1, 2, 3-triazol-ethan-1-one derivatives 5 in good isolated yields in the presence of 1 mol% of catalyst in EtOH-H2O under ultrasound irradiation at 50 °C.

Then, we examined the heterogeneous nature of the catalyst. Firstly, to assess the copper leaching of the catalyst, we performed hot filtration test for the click reaction of 4-nitro benzyl bromide 2, phenylacetylene 1 and NaN3. The reaction was stopped after ~ 50% of the reaction time. Hot filtrate was transferred to another flask containing base and H2O-EtOH at 50 °C. Upon further heating of the catalyst-free solution for 1.5 h, no considerable progress was observed by GC analysis (Fig. 7a). Moreover, atomic absorption spectroscopy (AAS) of the same reaction solution at the midpoint of completion indicated that no significant quantities of copper were lost to the reaction medium during the process. Furthermore, the reusability of catalyst was investigated in the reaction of 4-nitro benzyl bromide, phenylacetylene, and NaN3. The catalyst could be reused successively five times without significant loss of activity (Fig. 7b). Moreover, atomic absorption spectroscopy revealed that the loading of copper was 0.27 mmol g−1 after five runs and there was no significant change in the copper content of the recovered catalyst. All results confirm the reaction occurs mainly via a heterogeneous pathway. The SEM micrographs of reused catalyst after five times reveal that the reused catalyst has a similar texture with fresh catalyst (see Additional file 1).
Based on literature reports [61], a possible mechanism for click catalytic synthesis of triazole is proposed in Scheme 2. Synthesis of triazole proceeds through the formation of copper acetylide (A). The coordination of organic azide (B) (formed in situ by the reaction of organic bromide with NaN3) to the copper acetylide, followed by the Huisgen 1, 3-dipolar cycloaddition reaction of (A) and (B) give the complex (C). Subsequently, the desired triazole was obtained by copper-acidic hydrogen exchange followed by regeneration of the catalyst for the next use in the catalytic cycle. It is notable; functional groups such as hydroxyl, amine, methoxy and carboxyl groups on the surface of peanut shell have good potential to coordinate with copper nanoparticles.

(The sources of Graphical abstract are internet “https://pngtree.com/freepng/vector-arrow-earth_520723.html”, “http://aisphysicalscience.pbworks.com/w/page/1623001/29%20Cu%20-%20Copper” and “https://pngtree.com/so/peanut-shells” and the used softwares are Chemdraw and Paint. The Scheme was designed by authors)
The proposed mechanism of the reaction. The Scheme was designed by authors
Reports in Table 4, compares the efficiency of Cu2O@PS nanocomposite with some other heterogeneous copper catalysts in literature in the Click reaction of phenylacetylene, phenacyl bromides and NaN3. Table 4 shows that although all of methods have good efficiency, the present catalyst affords some advantages such as biodegradability using green nano bio-support for immobilization of copper, reasonable reaction time and low temperature which are all energy and time-consuming processes.
Experimental
Material and measurements
All chemicals were purchased from Merck, Aldrich or Fluka were used without further purification. IR spectra were recorded on a Shimadzu FT-IR-470 FT-IR spectrophotometer. EDS characterization was performed using an electron microscopy Oxford Instrument Company, Germany. Field emission scanning electron microscopy (FESEM) was performed using a ZEISS instrument, SIGMA VP model, Germany. The NMR spectra were recorded on a Brukerdrx-300Avance spectrometer. The concentration of Cu was estimated using a Shimadzu AA-680 flame atomic absorption spectrophotometer. Diffraction data were collected on a STOE STADI P with scintillation detector, secondary monochromator and Cu-Ka1 radiation (λ = 1. 5406 Å). Gas chromatography was performed on a Trace GC ultra from the Thermo Company equipped with FID detector and Rtx®-1 capillary column. Melting points of products were measured with an Electrothermal 9100 apparatus and are uncorrected. Thermogravimetric analysis (TGA) was done by D-32609 Hullhorst. The peanut shell was obtained from Astaneh Ashrafiyeh Township located in 37° 16′ latitude and 49° 56′ longitude in north of Iran.
Preparation of Cu2O@Peanut shell
Crushed peanut shells were ground in a ball mill to a fine powder. A mixture of peanut shell powder (1 g) and copper acetate (0.1 g) was stirred in de-ionized water (30 ml) at 70 °C for 5 h. The catalyst was then centrifuged and washed with water, ethanol, and acetone and dried in the oven at 70 °C to obtain Cu2O@Peanut shell.
General procedure for Click reactions
A mixture of Cu2O@PS (1 mol% of Cu, 40 mg), K2CO3 (2 mmol), aryl bromide (1.0 mmol), phenyl acetylene (1.2 mmol), and NaN3 (1.2 mmol) in H2O-EtOH (3 ml, 1:1) was sonicated at 50 °C for an appropriate time. After completion of the reaction monitored by TLC (EtOAc:n-hexane (1:3), the catalyst was separated and the filtrate was extracted with Chloroform (2 × 2 ml). The organic solvents were removed under vacuum and the pure product was obtained by recrystallization with CHCl3:n-hexane (1:3). All of the Click products are known compound and were reported previously.
Conclusions
In summary, Cu2O@Peanut shell nano-biocomposite was synthesized and used as an effective heterogeneous catalyst in a one-pot Huisgen 1,3-dipolar cycloaddition reaction under ultrasonic irradiation in EtOH-H2O as a green solvent for the synthesis of 1,2,3-triazole derivatives. The reusability of the catalyst is high and the catalyst can be reused five times without a significant decrease in its catalytic activity. Notable features of this catalytic reaction are bio-degradable and bio-renewable polymeric support, compatibility with a wide range of substrate, mild reaction conditions, high atom economy, good-yields of the products, ligand-free, leaching-free and eco-friendliness characteristics of the catalyst.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its Additional file 1].
References
Bai L, Wang J-X, Zhang Y (2003) Rapid microwave-promoted Suzuki cross coupling reaction in water. Green Chem 5(5):615–617
Pironti V, Colonna S (2005) Microwave-promoted synthesis of [small beta]-hydroxy sulfides and [small beta]-hydroxy sulfoxides in water. Green Chem 7(1):43–45
Leadbeater NE (2005) Fast, easy, clean chemistry by using water as a solvent and microwave heating: the Suzuki coupling as an illustration. Chem Commun 23:2881–2902
Al-Amin M, Akimoto M, Tameno T, Ohki Y, Takahashi N, Hoshiya N et al (2013) Suzuki-Miyaura cross-coupling reactions using a low-leaching and highly recyclable gold-supported palladium material and two types of microwave equipments. Green Chem 15(5):1142–1145
Frost CG, Mutton L (2010) Heterogeneous catalytic synthesis using microreactor technology. Green Chem 12(10):1687–1703
Klich K, Pyta K, Kubicka MM, Ruszkowski P, Celewicz L, Gajecka M et al (2016) Synthesis, antibacterial, and anticancer evaluation of novel spiramycin-like conjugates containing C(5) triazole arm. J Med Chem 59(17):7963–7973
Velázquez S, Alvarez R, Pérez C, Gago F, Clercq ED, Balzarini J et al (1998) Regiospecific synthesis and anti-human immunodeficiency virus activity of novel 5-substituted N-alkylcarbamoyl and N, N-dialkyl carbamoyl 1,2,3-triazole-TSAO analogues. Antivir Chem Chemother 9(6):481–489
Pereira D, Fernandes P (2011) Synthesis and antibacterial activity of novel 4-aryl-[1,2,3]-triazole containing macrolides. Bioorg Med Chem Lett 21(1):510–513
Buckle DR, Outred DJ, Rockell CJM, Smith H, Spicer BA (1983) Studies on v-triazoles. 7. Antiallergic 9-oxo-1H,9H-benzopyrano[2,3-d]-v-triazoles. J Med Chem 26(2):251–254
Fung-Tomc JC, Huczko E, Minassian B, Bonner DP (1998) In vitro activity of a new oral triazole, BMS-207147 (ER-30346). Antimicrob Agents Chemother 42(2):313–318
Wamhoff H (1984) Comprehensive heterocyclic chemistry. Pergamon, Oxford
Fan W-Q, Katritzsky AR (1996) Comprehensive heterocyclic chemistry II. Elsevier, Oxford
Aucagne V, Leigh DA (2006) Chemoselective formation of successive triazole linkages in One Pot: “Click–Click” chemistry. Org Lett 8(20):4505–4507
Siemsen P, Livingston RC, Diederich F (2000) Acetylenic coupling: a powerful tool in molecular construction. Angew Chem Int Ed 39(15):2632–2657
Astruc D, Liang L, Rapakousiou A, Ruiz J (2012) Click dendrimers and triazole-related aspects: catalysts, mechanism, synthesis, and functions. A bridge between dendritic architectures and nanomaterials. Acc Chem Res 45(4):630–640
Gawande MB, Bonifacio VDB, Luque R, Branco PS, Varma RS (2013) Benign by design: catalyst-free in-water, on-water green chemical methodologies in organic synthesis. Chem Soc Rev 42(12):5522–5551
Lal K, Rani P. Recent developments in copper nanoparticle-catalyzed synthesis of 1,4-disubstituted 1,2,3-triazoles in water. Arkivoc. 2016;i:307–341
Huisgen R (1989) Kinetics and reaction mechanisms: selected examples from the experience of forty years. Pure Appl Chem 61(4):613
Pressly ED, Amir RJ, Hawker CJ (2011) Rapid synthesis of block and cyclic copolymers via click chemistry in the presence of copper nanoparticles. J Polym Sci Part A Polym Chem 49(3):814–819
Cintas P, Barge A, Tagliapietra S, Boffa L, Cravotto G (2010) Alkyne–azide click reaction catalyzed by metallic copper under ultrasound. Nat Protoc 5(3):607–616
Ghosh S, Saha S, Sengupta D, Chattopadhyay S, De G, Basu B (2017) Stabilized Cu2O nanoparticles on macroporous polystyrene resins [Cu2O@ARF]: improved and reusable heterogeneous catalyst for on-water synthesis of triazoles via click reaction. Ind Eng Chem Res 56(41):11726–11733
Khodaei MM, Bahrami K, Meibodi FS (2017) Ferromagnetic nanoparticle-supported copper complex: a highly efficient and reusable catalyst for three-component syntheses of 1, 4-disubstituted 1, 2, 3-triazoles and C-S coupling of aryl halides. Appl Organomet Chem 31(10):e3714
Zhou P, Wang H, Yang J, Tang J, Sun D, Tang W (2012) Bacteria cellulose nanofibers supported palladium (0) nanocomposite and its catalysis evaluation in heck reaction. Ind Eng Chem Res 51(16):5743–5748
Rezaei R, Sheikhi MR (2015) Starch–sulfuric acid as a bio-supported and recyclable solid acid catalyst for rapid synthesis of α, α′-benzylidene bis(4-hydroxycoumarin) derivatives. Res Chem Intermed 41(3):1283–1292
Sin E, Yi S-S, Lee Y-S (2010) Chitosan-g-mPEG-supported palladium (0) catalyst for Suzuki cross-coupling reaction in water. J Mol Catal A Chem 315(1):99–104
Mohammad Zaheri H, Javanshir S, Hemmati B, Dolatkhah Z, Fardpour M (2018) Magnetic core–shell Carrageenan moss/Fe3O4: a polysaccharide-based metallic nanoparticles for synthesis of pyrimidinone derivatives via Biginelli reaction. Chem Cent J 12(1):108
Punnadiyil RK, Sreejith MP, Purushothaman E (2016) Isolation of microcrystalline and nano cellulose from peanut shells. J Chem Pharm Sci 1:12–16
Bao C, Ma J, Zhou L, Shao Y, Wu Q, Wang F (2015) Self-template synthesis of hierarchical magnetic porous carbon fibers derived from Fe(BTC)-coated bamboo fibers for fast removal of methylene blue. RSC Adv 5(106):87616–87625
Zhu C-S, Wang L-P, Chen W-B (2009) Removal of Cu(II) from aqueous solution by agricultural by-product: peanut hull. J Hazard Mater 168(2–3):739–746
Johnson P, Watson M, Brown J, Jefcoat I (2002) Peanut hull pellets as a single use sorbent for the capture of Cu(II) from wastewater. Waste Manag 22(5):471–480
Kumar M, Revathi K, Khanna S (2015) Biodegradation of cellulosic and lignocellulosic waste by Pseudoxanthomonas sp R-28. Carbohydr Polym 134:761–766
Zhao X, Chen J, Du F (2012) Potential use of peanut by-products in food processing: a review. J Food Sci Technol 49(5):521–529
Ding J, Wang H, Li Z, Cui K, Karpuzov D, Tan X et al (2015) Peanut shell hybrid sodium ion capacitor with extreme energy-power rivals lithium ion capacitors. Energy Environ Sci 8(3):941–955
Anike FN, Yusuf M, Isikhuemhen OS (2016) Co-substrating of peanut shells with cornstalks enhances biodegradation by Pleurotus ostreatus. J Bioremediat Biodegrad 7:327–334
Tanyildizi MŞ (2011) Modeling of adsorption isotherms and kinetics of reactive dye from aqueous solution by peanut hull. Chem Eng J 168(3):1234–1240
Serra S, Fuganti C, Brenna E (2005) Biocatalytic preparation of natural flavours and fragrances. Trends Biotechnol 23(4):193–198
Azizi N, Gholibeglo E, Maryami M, Nayeri SD, Bolourtchian SM (2013) Ultrasound mediated efficient ring opening of epoxides by in situ generated dithiocarbamates in green reaction media. C R Chim 16(5):412–418
Chen G-F, Jia H-M, Zhang L-Y, Chen B-H, Li J-T (2013) An efficient synthesis of 2-substituted benzothiazoles in the presence of FeCl3/Montmorillonite K-10 under ultrasound irradiation. Ultrason Sonochem 20(2):627–632
Zhang Z, Zha Z, Gan C, Pan C, Zhou Y, Wang Z et al (2006) Catalysis and regioselectivity of the aqueous Heck reaction by Pd(0) nanoparticles under ultrasonic irradiation. J Org Chem 71(11):4339–4342
Kaur G, Sharma A, Banerjee B (2018) Ultrasound and ionic liquid: an ideal combination for organic transformations. ChemistrySelect 3(19):5283–5295
Banerjee B (2017) Recent developments on ultrasound assisted catalyst-free organic synthesis. Ultrason Sonochem 35:1–14
Tasdelen MA, Kiskan B, Yagci Y (2016) Externally stimulated click reactions for macromolecular syntheses. Prog Polym Sci 52:19–78
Hemmati B, Javanshir S, Dolatkhah Z (2016) Hybrid magnetic Irish moss/Fe3O4 as a nano-biocatalyst for synthesis of imidazopyrimidine derivatives. RSC Adv 6(56):50431–50436
Javanshir S, Saghiran Pourshiri N, Dolatkhah Z, Farhadnia M (2017) Caspian Isinglass, a versatile and sustainable biocatalyst for domino synthesis of spirooxindoles and spiroacenaphthylenes in water. Monatsh Chem 148(4):703–710
Pourian E, Javanshir S, Dolatkhah Z, Molaei S, Maleki A (2018) Ultrasonic-assisted preparation, characterization, and use of novel biocompatible core/shell Fe3O4@GA@ isinglass in the synthesis of 1, 4-dihydropyridine and 4H-pyran derivatives. ACS Omega 3(5):5012–5020
Liu S, Xu W-H, Liu Y-G, Tan X-F, Zeng G-M, Li X et al (2017) Facile synthesis of Cu(II) impregnated biochar with enhanced adsorption activity for the removal of doxycycline hydrochloride from water. Sci Total Environ 592:546–553
Reddy VH, Reddy YVR, Sridhar B, Reddy BVS (2016) Green catalytic process for click synthesis promoted by copper oxide nanocomposite supported on graphene oxide. Adv Synth Catal 358(7):1088–1092
Naeimi H, Shaabani R (2017) Ultrasound promoted facile one pot synthesis of triazole derivatives catalyzed by functionalized graphene oxide Cu(I) complex under mild conditions. Ultrason Sonochem 34:246–254
Asano K, Matsubara S (2010) Effects of a flexible alkyl chain on a ligand for CuAAC reaction. Org Lett 12(21):4988–4991
Szadkowska A, Staszko S, Zaorska E, Pawlowski R (2016) A theophylline based copper N-heterocyclic carbene complex: synthesis and activity studies in green media. RSC Adv 6(50):44248–44253
Chavan PV, Pandit KS, Desai UV, Kulkarni MA, Wadgaonkar PP (2014) Cellulose supported cuprous iodide nanoparticles (Cell-CuI NPs): a new heterogeneous and recyclable catalyst for the one pot synthesis of 1,4-disubstituted-1,2,3-triazoles in water. RSC Adv 4(79):42137–42146
Shaabani A, Afshari R, Hooshmand SE, Tabatabaei AT, Hajishaabanha F (2016) Copper supported on MWCNT-guanidine acetic acid@Fe3O4: synthesis, characterization and application as a novel multi-task nanocatalyst for preparation of triazoles and bis(indolyl)methanes in water. RSC Adv 6(22):18113–18125
Kalhor-Monfared S, Beauvineau C, Scherman D, Girard C (2016) Synthesis and cytotoxicity evaluation of aryl triazolic derivatives and their hydroxymethine homologues against B16 melanoma cell line. Eur J Med Chem 122:436–441
Yamada YMA, Sarkar SM, Uozumi Y (2012) Amphiphilic self-assembled polymeric copper catalyst to parts per million levels: click chemistry. J Am Chem Soc 134(22):9285–9290
Pourjavadi A, Tajbakhsh M, Farhang M, Hosseini SH (2015) Copper-loaded polymeric magnetic nanocatalysts as retrievable and robust heterogeneous catalysts for click reactions. New J Chem 39(6):4591–4600
Dige NC, Patil JD, Pore DM (2017) Dicationic 1, 3-Bis (1-methyl-1H-imidazol-3-ium) propane copper(I) dibromate: novel heterogeneous catalyst for 1, 3-dipolar cycloaddition. Catal Lett 147(2):301–309
Movassagh B, Rezaei N (2014) Polystyrene resin-supported CuI-cryptand 22 complex: a highly efficient and reusable catalyst for three-component synthesis of 1, 4-disubstituted 1, 2, 3-triazoles under aerobic conditions in water. Tetrahedron 70(46):8885–8892
Cha H, Lee K, Chi DY (2017) Synthesis of N-unsubstituted 1,2,3-triazoles via aerobic oxidative N-dealkylation using copper(II) acetate. Tetrahedron 73(20):2878–2885
Ahmady AZ, Heidarizadeh F, Keshavarz M (2013) Ionic liquid containing copper(I): a new, green, homogeneous, and reusable catalyst for click cyclization. Synth Commun 43(15):2100–2109
Gaikwad S, Goswami A, De S, Schmittel M (2016) A metalloregulated four-state nanoswitch controls two-step sequential catalysis in an eleven-component system. Angew Chem Int Ed 55(35):10512–10517
Tajbakhsh M, Farhang M, Baghbanian SM, Hosseinzadeh R, Tajbakhsh M (2015) Nano magnetite supported metal ions as robust, efficient and recyclable catalysts for green synthesis of propargylamines and 1,4-disubstituted 1,2,3-triazoles in water. New J Chem 39(3):1827–1839
Pourjavadi A, Safaie N, Hosseini SH, Bennett C (2015) Graphene oxide/poly (vinyl imidazole) nanocomposite: an effective support for preparation of highly loaded heterogeneous copper catalyst. Appl Organomet Chem 29(9):601–607
Pourjavadi A, Hosseini SH, Moghaddam FM, Ayati SE (2015) Copper loaded cross-linked poly (ionic liquid): robust heterogeneous catalyst in ppm amount. RSC Adv 5(38):29609–29617
Banan A, Bayat A, Valizadeh H (2017) Copper immobilized onto polymer-coated magnetic nanoparticles as recoverable catalyst for ‘click’reaction. Appl Organomet Chem 31(5):e3604
Saadat S, Nazari S, Afshari M, Shahabi M, Keshavarz M (2015) Copper (I) iodide nanoparticles on polyaniline as a green, recoverable and reusable catalyst for multicomponent click synthesis of 1, 4-disubstituted-1H-1, 2, 3-triazoles. Orient J Chem 31(2):1005–1012
Jafari AA, Mahmoudi H, Firouzabadi H (2015) A copper acetate/2-aminobenzenthiol complex supported on magnetite/silica nanoparticles as a highly active and recyclable catalyst for 1, 2, 3-triazole synthesis. RSC Adv 5(130):107474–107481
Singh G, Kumar M, Bhalla V (2018) Supramolecular ensemble of perylene bisimide derivative and Cu2O-Ag nanoparticles: nano/“Dip Strip” catalytic system for one-pot, three-component click reaction at room temperature. ACS Sustain Chem Eng 6(9):11466–11472
Acknowledgements
We gratefully acknowledge financial support from the Shahid Beheshti University and the Iran University of Science & Technology.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ZD and AM performed the experiments, collected the data and drafted the manuscript; AB and SJ designed the study and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1.
Supporting information including the FESEM images of PS, Cu2O@PS, and reused Cu2O@PS after 5 times, characterization of triazole products, and HNMR spectrum of products.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Dolatkhah, Z., Mohammadkhani, A., Javanshir, S. et al. Peanut shell as a green biomolecule support for anchoring Cu2O: a biocatalyst for green synthesis of 1,2,3-triazoles under ultrasonic irradiation. BMC Chemistry 13, 97 (2019). https://doi.org/10.1186/s13065-019-0612-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-019-0612-9