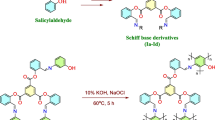
Abstract
Sevelamer (polyallyamine resin)-supported sulfonic acid (S-SO3H) has been prepared from the reaction of sevelamer with chlorosulfonic acid and characterized using FT-IR spectroscopy, scanning electronmicroscopy (SEM) and thermogravimetric analysis (TGA). The catalytic activity of S-SO3H was investigated in the synthesis of 1,8-dioxo-octahydroxanthene derivatives. All of the reactions were fast and gave excellent yields. The catalyst was easily recovered and reused for 5 runs without significant loss of its catalytic activity.

Similar content being viewed by others
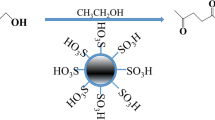
Introduction
Heterogeneous catalysis involving functional polymers has been very important over the past few decades [1,2,3]. Catalysts immobilized on a functional polymer support shows efficiency strategy for the isolation and catalyst recycling, leading to economic and environmental advantages [4, 5].
The most commonly used polymer-supports involves polystyrene cross-linked with divinylbenzene (PS/DVB) [6,7,8]. However, there are some drawbacks to PS/DVB. The number of chemical modification reactions, such as chloromethylation, sulfonation that can be carried out to introduce functional groups onto the polystyrene (PS)-based beads are limited and the reactions themselves may be difficult to control. On the other hand, the induced functional groups are limited and reduce the number of catalytic sites. It’s useful that functional polymers contain reactive groups that can be used directly for the further introduction of functional groups. The amount of induced functional groups can be easily controlled. Sevelamer is a co-polymer of epichlorohydrin and polyallylamine [9]. Sevelamer is an environmentally friendly solid material with excellent mechanical strength and stability. This compound contains abundant amine groups, which can be easily transformed into various functionality.
The use of solid acids in organic synthesis and the industrial manufacture of materials is of increasing importance [10]. Heterogeneous acid catalysts such as solid acid zeoilte, solid superacid sulfated zirconia and Nafion have been explored in both organic synthesis and large scale industry. Polymer supported acids, such as polystyrene resin and perfluorinated ion exchange polymers, have also been prepared and widely used in organic synthesis [11,12,13]. These catalysts are suitable for a variety of synthetic conditions. However, they are associated with one or more disadvantages such as lower reactivity, the use large quantities of the solid support and not being suitable for reactions conducted in aqueous media.
In the past decade, xanthene and its derivatives has been widely used in pharmaceuticals, drug discovery, dyes, fluorescent materials and biochemistry. Xanthenes, especially 1,8-dioxo-octahydroxanthenes, are prepared using a range of different methods, due to their wide range of synthetic, industrial and pharmacological applications [14,15,16,17,18]. Various catalysts have been used for the preparation of these xanthenes. A wide range of catalysts such as MCM-41-SO3H [19], SbCl3/SiO2 [20], p-dodecylbenzene sulfonic acid [21], triethylbenzylammonium chloride [22], β-cyclodextrin SO3H [23], Amberlyst-15 [24], diammonium hydrogen phosphate [25] and nano-TiO2 [26] have been used for the preparation of 1,8-dioxo-octahydroxanthenes. On the other hand, there have been several reports describing the use of SO3H immobilized on polymers towards the synthesis of 1,8-dioxo-octahydroxanthenes. However, many of these methods suffer from disadvantages such as low yields, long reaction times, the use of harmful volatile organic solvents and tedious work-ups.
Recently, we have focused our attention on the use of polymeric catalysts as recyclable catalysts in organic synthesis in environmentally sustainable chemistry [27]. Functional polymers used as the solid support are very important for the definition of efficient synthetic procedures especially when safer reaction media, ecofriendly alternatives to organic solvents or solvent-free conditions (SolFC) are used.
Experimental
Instruments
TGA was carried out on a Mettler-Toledo 851E instrument. 1H NMR spectra were recorded on a 400 MHz spectrometer (Varian, Palo Alto, USA) in DMSO solution. Chemical shift values are given in parts per million. IR spectra were recorded on a Perkin Elmer (model: spectrum BX) FT-IR Spectrometer using KBr pellets.
Preparation of polyallylamine hydrochloride
Allylamine (15.0 g) was slowly added to concentrated HCl (26.8 g) maintaining the temperature below 10 °C. Nitrogen gas was introduced into the flask after 30 min and the solution was heated at 50 °C. Afterwards, 2,2′-diamidinyl-2,2′-azo-propane dihydrochloride (1.0 g) was added to the solution. The reaction mixture was stirred at 50 °C for 24 h. A second portion of 2,2′-diamidinyl-2,2′-azo-propane dihydrochloride (1.0 g) was then added to the solution. The reaction mixture was maintained for another 24 h at 50 °C. The pale yellow, transparent and viscous solution obtained in this process was added into a large amount of methanol and a white polymer precipitate was subsequently produced. This precipitate was filtered and extracted with methanol using a Soxhlet extractor for 10 h. After being dried at 50 °C, polyallylamine hydrochloride was obtained (15.6 g).
Typical procedure for the preparation of support s
Polyallylamine hydrochloride (5.0 g) was dissolved in water (75.0 mL) to obtain a clear solution at 30 °C. The solution was further cooled to 10 °C. Then, NaOH (1.38 g) was added to the solution at 10 °C and stirred for 30 min. Then, 40 mL of toluene and SPAN-85 (0.2 g) were added to the mixture at 10 °C. The reaction mixture was maintained for another 30 min at 10 °C. The temperature was then increased to 55 °C and maintained at this temperature for 15 min. Afterwards, epichlorohydrin (0.45 g) was added to the reaction mixture at 55 °C and maintained at this temperature for 3 h. The reaction mixture was cooled to room temperature. Sevelamer hydrochloride was then filtered, washed with water and dried in vacuo. The compound was then soaked in NaOH solution (10.0 g, 10% w/v) at room temperature for 24 h. The product was filtered and washed with hot water until it became pH neutral. Finally, the product was dried at 60 °C for 12 h under vacuum. S (3.22 g, 94%) was obtained as a pale-yellow solid.
Preparation of S-SO3H
A suspension of sevelamer (4.0 g) and Na2CO3 (7.35 g) in dry CH2Cl2 (20 mL) was added to a suction flask. Then, chlorosulfonic acid (3.0 mL, 30 mmol) was added dropwise over a period of 15 min at room temperature while the mixture was stirred slowly at room temperature. After the addition was complete, the mixture was stirred for 12 h. Then, the CH2Cl2 was removed by filtration under reduced pressure and the solid powder dissolved in water (200.0 mL). The mixture was filtered again. The solid powder was washed with water, then soaked in H2SO4 solution (10.0 g, 10% v/v) at room temperature for 24 h. The product was filtered and washed with water until it became pH neutral. Finally, the product was dried at 70 °C for 12 h under vacuum. A yellow solid of polyallylamine-supported sulfonic acid was obtained (6.1 g).
Results and discussion
Preparation of S-SO3H
The synthetic strategy used to prepare S-SO3H is shown in Scheme 1. At first, sevelamer was synthesized according to our previous work. Then, sevelamer was used to support SO3H.
Catalyst characterization
Scanning electron microscopy
Scanning electron micrographs which shows the preparation of sevelamer support are reported in Fig. 1. Resin sevelamer (Fig. 1a) shows a fine and uniform surface texture as compared to (Fig. 1b). Resin S-SO3H (Fig. 1b) show rough contamination with fragments on its surface.
FT-IR spectroscopy
The FT-IR spectra of sevelamer and S-SO3H are shown in Fig. 2. The absorbance bands ranging between 3200–3500 cm−1 were shows acid adsorption (Fig. 2a) and amine (Fig. 2b). Strong peak at 1144 cm−1 represents the S–N stretching (the weak band observed at the same frequency in the sevelamer spectrum) was notable. In Fig. 2a, the absorption range at 1176–1284 and 1006–1088 cm−1 shows the possibility of O–S–O asymmetric and symmetric stretching modes, respectively and the S\O stretching mode lies at 675–852 cm−1 showing the presence of the sulfonic acid functional group, which was consistent with the reported IR spectra for SO3H.
Thermogravimetric analysis
The thermogravimetric analysis (TGA) of S-SO3H in comparison with sevelamer is shown in Fig. 3. Weight loss of (5 wt%) below 100 °C for sevelamer is displayed by the TGA curves in (Fig. 3a), which corresponds to the loss of physically adsorbed water. The ash content was only 1.6%. In the TGA curve for S-SO3H (Fig. 3b), three regions corresponding to different mass lose ranges exist. The first mass loss region was below 150 °C and was attributed to the loss of trapped water from the catalyst. A mass loss of approximately 10 wt% occurred between 100 and 200 °C, which was related to the slow mass loss of SO3H groups. Finally, a mass loss of approximately 50 wt% occurred between 240 and 300 °C, which was related to the sudden mass loss of the resin. The ash content was up to 20%. This result shows the S-SO3H easily turns into carbon or sulfate slats under the acidic atmosphere and high temperature. Also, from the TGA results, it was found that sevelamer has a greater thermal stability than S-SO3H. However, S-SO3H can still be safely used in organic reactions at temperatures in the range of 0–120 °C.
BET surface area analysis
The surface area of the catalysts is an important factor influencing the catalytic activity. The surface area of polymer resin and catalyst was determined using the nitrogen gas adsorption method. BET surface area of sevelamer (2.668 m2 g−1) is almost same as the surface area of S-SO3H (3.010 m2 g−1).
Application of S-SO3H as an efficient catalyst in organic reactions
The studies demonstrated for the use inexpensive and environmentally friendly S-supported sulfonic acid (S-SO3H) as a heterogeneous solid acid catalyst shows very satisfactory results. In the present work, S-SO3H was easily prepared and used as a highly efficient, heterogeneous, reusable and inexpensive solid acid catalyst for synthesis of 1,8-dioxo-octahydroxanthene derivatives.
The 1,8-dioxo-octahydroxanthenes are an important structural unit in many natural compounds with biological and pharmaceutical activities. Water is considered to be an environmentally benign solvent. Therefore, the reaction of benzylaldehyde and 1,3-cyclohexanedione was selected as a model reaction to screen the experimental conditions in aqueous media. Reaction progress was monitored by thin-layer chromatography. To examine the temperature effects, the model reaction was carried out with catalyst loadings of 0.010 g S-SO3H in aqueous media. The reaction proceeded smoothly at room temperature, but the product was obtained in 73% in 120 min (Table 1, entry 1). With an increase in temperature, the reaction time was significantly decreased. This reaction was completed in 30 min at 90 °C (Table 1, entry 4). To justify the efficiency of the S-SO3H, the model reaction was carried out in the presence of different amounts of S-SO3H in aqueous media. It was found that the product 3a was formed in high yields with catalyst loadings of 0.0100 g (Table 1, entry 8). A higher amount of catalyst did not accelerate the reaction time or promote the reaction yield (Table 1, entry 9). A lower yield of product 3a was obtained with a lower amount of catalyst and in a reaction time of 8 h (Table 1, entry 5).
After optimizing the conditions, the scope of the method was successfully studied using a variety of aldehydes in water. Both electron-rich and electron-deficient aromatic aldehydes worked well, giving high yields of the desired products. Electron-deficient aldehydes needed shorter reaction times and gave somewhat higher yields than their electron-rich counterparts (Table 2, entries 1–6). In almost all cases, the reactions proceeded smoothly within 30-50 min.
Five consecutive syntheses of 1,8-dioxo-octahydroxanthene was done to demonstrate the easy recyclability using the recovered S-SO3H catalyst. Once product 3a was filtered from the reaction mixture, the S-SO3H catalyst was used directly in another cycle without drying. The catalyst showed no appreciable loss in activity. All reactions were completed in 30 min and the desired product was obtained in 91–94% yield.
Conclusion
Sevelamer (polyallyamine resin)-supported sulfonic acid (S-SO3H) was used as an efficient heterogeneous catalyst for the synthesis of 1,8-dioxo-octahydroxanthene derivatives in summary. This catalyst could be easily separated from the reaction mixture and directly reused after a simple extraction step. The catalyst was recycled for seven consecutive cycles without any obvious loss in its catalytic activity. As a good heterogeneous solid acid, S-SO3H is stable in air, easy to prepare and can be recovered easily. The scope and synthetic applications of the S-SO3H catalyst are currently under investigation in our laboratory.
Availability of data and materials
All data are fully available without restriction.
References
Lu J, Toy PH (2009) Organic polymer supports for synthesis and for reagent and catalyst immobilization. Chem Rev 109:815–838
Zaheri HM, Javanshir S, Hemmati B, Dolatkhah Z, Fardpour M (2018) Magnetic core–shell Carrageenan moss/Fe3O4: a polysaccharide-based metallic nanoparticles for synthesis of pyrimidinone derivatives via Biginelli reaction. Chem Cent J 12:108–118
Aravind A, George S, Kumar S (2012) Remarkable influence of microwave heating on Morita-baylis-Hillman reaction in PEG-200. Chem Cent J 6:30–35
Akelah A, Sherrington DC (1981) Application of functionalized polymers in organic synthesis. Chem Rev 81:557–587
Sherrington DC, Hodge P (1988) Synthesis and separation using functional polymers. Wiley, Chichester
Regen SL (1975) Triphase catalysis. J Am Chem Soc 97:5956
Regen SL (1979) Triphase catalysis. Angew Chem Int Ed 18:421–429
Sherrington C (1998) Preparation, structure and morphology of polymer supports. Chem Commun 30:2275–2286
Kioussis D, Smith D, Kofinas P (2001) Ammonium perchlorate-binding poly(allylamine hydrochloride) hydrogels for wastewater remediation. J Appl Polym Sci 80:2073–2083
Hattori H, Ono Y (2015) Solid acid catalysis: from fundamentals to applications. Pan Stanford, San Antonio
Okayasu T, Saito K, Nishide H, Hearn MTW (2009) Preparation of a novel poly(vinylsulfonic acid)-grafted solid phase acid catalyst and its use in esterification reactions. Chem Commun 31:4708–4710
Heitner-Wirguin C (1996) Recent advances in perfluorinated ionomer membranes: structure, properties and applications. J Membr Sci 120:1
Mauritz KA, Moore RB (2004) State of understanding of nafion. Chem Rev 104:4535
Feringa BL (2007) The art of building small: from molecular switches to molecular motors. J Org Chem 72:6635–6652
Kitahara Y, Tanaka K (2002) Synthesis, crystal structure and properties of thiaheterohelicenes containing phenolic hydroxy functions. Chem Commun 9:932–933
Ahmad M, King TA, Ko DK, Cha BH, Lee J (2002) Performance and photostability of xanthene and pyrromethene laser dyes in sol-gel phases. J Phys D Appl Phys 35:1473–1476
Knight CG, Stephens T (1989) Xanthene-dye-labelled phosphatidylethanolamines as probes of interfacial pH. Studies in phospholipid vesicles. J Biochem. 258(258):683–687
Shaheen F, Ahmad M, Khan SN, Hussain SS, Anjum S, Tashkhodjaev B (2006) New α-glucosidase inhibitors and antibacterial compounds from Myrtus communis L. Eur J Org Chem. 10:2371–2377
Rostamizadeh S, Amani AM, Mahdavinia GH, Amiri G, Sepehrian H (2010) Ultrasound promoted rapid and green synthesis of 1,8-dioxo-octahydroxanthenes derivatives using nanosized MCM-41-SO3H as a nanoreactor, nanocatalyst in aqueous media. Ultrason Sonochem 17:306–309
Zhang ZH, Liu YH (2008) Efficient photocatalytic H2 production under visible light irradiation over Ni doped Cd1–xZnxS microsphere photocatalysts. Catal Commun 9:1715–1719
Jin TS, Zhang JS, Xiao JC, Wang AQ, Lix TS (2004) A clean synthesis of 1,8-dioxo-octahydroxanthene derivatives catalyzed by p-dodecylbenezenesulfonic acid in aqueous media. Synlett 5:866–870
Wang XS, Shi DQ, Li YL, Chen H, Wei XY, Zong ZM (2005) Improved and highly versatile synthesis of 5-aryltropones. Synth Commun 35:97–104
Kokkirala S, Sabbavarapu NM, Yadavalli VDN (2011) β-Cyclodextrin mediated synthesis of 1,8-dioxooctahydroxanthenes in water. Eur J Chem 2:272–275
Das B, Thirupathi P, Mahender I, Reddy VS, Rao YK, Mol J (2006) Amberlyst-15: an efficient reusable heterogeneous catalyst for the synthesis of 1,8-dioxo-octahydroxanthenes and 1,8-dioxo-decahydroacridines. Catal A-Chem 247:233–239
Darvish F, Balalaei S, Chadegani F, Salehi P (2007) Diammonium hydrogen phosphate as a neutral and efficient catalyst for synthesis of 1,8-dioxo-octahydroxanthene derivatives in aqueous media. Synth Commun 37:1059–1067
Khazaei A, Moosavi-Zare AR, Mohammadi Z, Zare A, Khakyzadeh V, Darvishi G (2013) Efficient preparation of 9-aryl-1,8-dioxo-octahydroxanth, enes catalyzed by nano-TiO2 with high recyclability. RSC Adv. 3:1323–1326
Zhao XL, Yang KF, Zhang YP, Xu LW (2014) Sevelamer as an efficient and reusable heterogeneous catalyst for the Knoevenagel reaction in water. Chin Chem Lett 25(25):1141–1144
Acknowledgements
We gratefully acknowledge financial support by the National Natural Science Foundation of China (Nos. 21302168, 51203037).
Funding
This study was financially supported by the National Natural Science Foundation of China (Nos. 21302168, 51203037).
Author information
Authors and Affiliations
Contributions
ZXL, YKF conceived and designed the experiments; ZXL and MS performed the experiments; YKF analyzed the data; ZXL wrote the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, XL., Shelton, M. & Yang, KF. Sulfonic acid-functionalized polyallylamine (sevelamer) as an efficient reusable strong solid acid catalyst for the synthesis of xanthenes derivatives. BMC Chemistry 13, 98 (2019). https://doi.org/10.1186/s13065-019-0609-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-019-0609-4