Abstract
Background
Radix Glycyrrhizae is the rhizome of Glycyrrhiza inflata Bat., Glycyrrhiza uralensis Fisch. or Glycyrrhiza glabra L. The present paper describes the isolation and the structural elucidation of three new dihydroisocoumarins obtained from the 70% EtOH extract of Radix Glycyrrhizae. And the cytotoxic activities of these new compounds were also evaluated using four cell lines, subsequently.
Results
A pair of new dihydroisocoumarin epimers ((3R,4S)-4,8-dihydroxy-3-methyl-1-oxoisochroman-5-yl)methyl acetate (1) and ((3R,4R)-4,8-dihydroxy-3-methyl-1-oxoisochroman-5-yl)methyl acetate (2) along with a new dihydroisocoumarin (3R,4R)-4,8-dihydroxy-3,5-dimethylisochroman-1-one (3) were isolated from Radix Glycyrrhizae. Their structures were elucidated on the basis of chemical and spectral analysis, including 1D, 2D NMR analyses, HR–ESI–MSand ECD calculation comparing with those of experimental CD spectra. Cytotoxic activities of the three compounds were evaluated using the HepG2, A549, LoVo and Hela cell lines, respectively. IC50 values indicated compounds 1–3 exhibited moderate or less cytotoxic activity in vitro.
Conclusions
Dihydroisocoumarin is not the common components in Radix Glycyrrhizae, a series of dihydroisocoumarin were obtained in this plant could be a supplement to the chemical study of this plant.
Similar content being viewed by others
Background
Radix Glycyrrhizae is the rhizome of Glycyrrhiza inflata Bat., Glycyrrhiza uralensis Fisch. or Glycyrrhiza glabra L. They are widely distributed in the northwest and northeast of China [1]. The pharmacological activities of Radix Glycyrrhizae are mainly represented by the main triterpene saponins, glycyrrhizin, glycyrrhizic acid, glycyrrhizinic acid and its aglycone, glycyrrhetinic acid [2, 3]. Its root possesses wide broad pharmacological actions. According to literature reports, its pharmacological activities include the following aspects: effects on central nerve system; cardiovascular system and endocrine system; liver, renal and pancreas functions, anti-ulcer action, anticancer action, anti-allergic and anti-inflammatory effects, anti-virus and antibacteria activities, and effect on immune function and so on [4, 5]. In this paper, we describe the isolation and the structural elucidation of three new dihydroisocoumarins obtained from the 70% ethyl alcohol (EtOH) extract of Radix Glycyrrhizae. Their structures (Fig. 1) were established by extensive spectroscopic data analysis and comparison with those of literature values.
Results and discussion
Compound 1 was obtained as yellow crystal (CH3OH), with the molecular formula C13H14O6 as determined by high resolution electrospray ionization mass spectra (HR–ESI–MS) at m/z 289.0681 [M + Na]+, indicating the presence of seven degrees of unsaturation. The 1H-NMR spectrum of compound 1 (Table 1) displayed one hydroxyl proton signal at δH11.20 (1H, s), two methyl signals at δH1.45 (3H, d, J = 6.4 Hz) and 2.04 (3H, s), two aromatic proton signals at δH6.99 (1H, d, J = 8.8 Hz) and 7.64 (1H, d, J = 8.8 Hz), along with some other methylene and methine proton signals [δH5.70 (1H, d, J = 7.2 Hz), 5.09 (1H, d, J = 12.4 Hz), 5.17 (1H, d, J = 12.4 Hz), 4.66 (1H, dd, J = 6.8, 1.6 Hz) and 4.72 (1H, qd, J = 6.4, 1.6 Hz)]. The 13C-NMR spectrum of compound 1 (Table 1) showed 13 carbon signals, including eight sp2 carbons (δC169.8, 124.7, 138.3, 117.3, 161.1, 170.8, 141.3 and 108.2), three oxygenated sp3 carbons (δC78.5, 62.3 and 62.2), and two methyl carbons (δC16.6 and 21.2). All the NMR data and the degree of unsaturation revealed the presence of a 1,2,3,4-substituted benzyl group and a lactone ring. Signal assignments were specified by Heteronuclear Single Quantum Coherence (HSQC) experiment. The Heteronuclear Multiple-Bond Correlation (HMBC) spectrum (Fig. 2) showed the long-rang correlations between H-7 at δH6.99and C-8a at δC108.2, C-5 at δC124.7, C-8 at δC161.1; between H-6 at δH7.64 and C-10 at δC62.2, C-8a at δC108.2, C-4a at δC141.3; between H-10 at δH5.09/5.17 and C-5 at δC124.7, C-4a at δC 141.3, C-11 at δC 170.8, between H-9 at δH1.45 and C-4 at δC62.3, between H-4 at δH4.66 and C-3 at δC78.5, C-4a at δC 141.3, C-5 at δC 124.7, between H-4 at δH4.66 and C-5 at δC124.7, which led to a conclusion that the planar structure of compound 1 was similar to that of (3R, 4R)-4,8-dihydroxy-5-(hydroxymethyl)-3-methylisochroman-1-one [6], except for the acetylated of hydroxyl groups at C-10. Thus, the planar structure of 1 was determined as shown in Fig. 1.
Compound 2 was also obtained as yellow crystal. The molecular formula was determined to be C13H14O6 by HR–ESI–MS at m/z 289.0670 [M + Na]+. The 1H and 13C NMR signals of 2 were almost identical to those of 1 with slight difference at C-1, C-3, C-4, C-5, and C-4a. The CD spectrum of 2 gave an exactly opposite absorption band at 250 nm compared with that of 1, and thus 2 was suggested to be the epimer of 1 at C-3. HMBC correlations of 2 shown in Fig. 3 verified the planar structure of 2, which was the same as that of 1. The relative configurations of 1 and 2 were established by NOESY analysis (Fig. 3). For compound 2, NOESY cross-peak between active proton of C-4 and H-3 was given while for compound 1, NOESY cross-peak between active proton of C-4 and 9-CH3 was observed, indicating the axial orientation of the active proton of C-4 as C-4 active proton could only give one NOESY cross-peak with either H-3 or 9-CH3.
The ECD (Electronic Circular Dichroism Spectroscopy) calculating study of 1 and 2 was performed based on the relative configuration of 1 and 2. Having two chiral centers, there are four possible stereo-isomers for 4,8-dihydroxy-3-methyl-1-oxoisochroman-5-yl)methyl acetate as shown in Fig. 4. The ECD results of each possible isomer and the experimental CD (Circular Dichroism Spectroscopy) curves of 1 and 2 were also expressed in Fig. 4a, b. The ECD results were represented in shot dashed line in Fig. 4a, d that both gave negative cotton effect at 250 nm, and so did Fig. 4b, c that both exhibited positive cotton effect at 250 nm, indicating that C-4 orientation dominated the cotton effect around 250 nm. Thus, via comparing the ECD results with those of the experimental CD curves of 1 and 2, the absolute configurations of C-3 and C-4 were determined to be (R), (S) and (R), (R) for 1 and 2, respectively.
Compound 3 was obtained as yellow crystal. The molecular formula was determined to be C11H12O4 by HR–ESI–MS at m/z 231.0637 [M + Na]+. The 1H and 13C NMR spectral data of 3 were similar to those of 2, expect for the disappearance of an acetoxy group at C-10. The absolute configuration of 3 was established by the analysis of its CD spectrum. A positive Cotton effect at 250 nm was shown in the CD spectrum (Fig. 5) of 3, indicating the (3R,4R)-configuration same as 2.
The cytotoxic activities of compounds 1-3 were evaluated using the HepG2, A549, LoVo and Hela cell lines, respectively. The IC50 values of these compounds were shown in Table 2. As a result, all the compounds exhibited moderate or less cytotoxic activity in vitro.
Methods
General experimental procedures
The UV spectrum was recorded on a Shimadzu UV-2201 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The IR spectrum was obtained from a Bruker IFS-55 spectrophotometer using a KBr pellet (Bruker Optik BmbH, Ettlingen, Germany). The HR-ESI–MS data were obtained on a microTOF-Q Bruker mass instrument (Bruker Daltonics, Billerica, MA, USA). CD spectra were recorded with a Biologic MOS-450 spectrometer using MeOH as solvent. 1D and 2D NMR spectra were run on a Bruker AVANCE 600 spectrometer (Bruker BioSpin, Rheinstetten, Germany). 1H chemical shifts (δH) were measured in ppm, relative to TMS, and 13C chemical shifts (δC) were measured relative to DMSO-d6 and converted to TMS scale. Column chromatography (CC) was performed on Silica gel (200–300 mesh; Qingdao Marine Chemical Co., Qingdao, China) and Sephadex LH-20 (Pharmacia, Uppsala, Sweden) columns. HPLC was performed on a Shimadzu LC-10AVP liquid chromatograph with a YMC-pack C18 (ODS) column (10 × 250 mm, 5 μm, apan) and a Shimadzu LC-8AVP liquid chromatograph with a Diamonsil C18 (ODS) column (4.6 × 250 mm, 5 μm, China). All reagents for isolation were HPLC or analytical grade and were purchased from Tianjin Damao Chemical Company (Tianjin, China). Fetal bovine serum and Dulbecco’s modified eagle medium (DMEM) were from Thermo Fisher Scientific, 96-well flat bottom plate were purchased from Corning Inc. (NY, USA), 3-[4,5-dimethyl-2-thiazolyl]-2,5 diphenyltetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Corporation (MA, USA).
Materials
Radix Glycyrrhizae was purchased from Anhui Yishengyuan Traditional Chinese Medicine Pellets Co., Ltd., P. R. China, and all the materials were identified by Dr. Xiao Fu, Department of Traditional Chinese Medicine, First Affiliated Hospital of Jinzhou Medical University. The voucher specimen (20150610) has been deposited at First Affiliated Hospital of Jinzhou Medical University.
Extraction and isolation
Radix Glycyrrhizae (25 kg) was cut and extracted with 70% EtOH for two times. The combined extracts were concentrated in vacuo to yield a residue, and the residue was then suspended in H2O and successively partitioned with petroleum ether, dichloromethane (CH2Cl2), ethyl acetate (EtOAc). The EtOAc crude extracts (2.3 kg) were applied on a silica gel column and eluted with petroleum ether-acetone gradient (from 500:0 to 0:100) to afford nine fractions. Fr. 6 was subjected to Sephadex LH-20, semi-preparative HPLC to yield compound 1 (12.0 mg) and 2 (9.2 mg). Fr. 7 was subjected to Sephadex LH-20, semi-preparative HPLC to yield compound 3 (15 mg).
((3R,4S)-4,8-Dihydroxy-3-methyl-1-oxoisochroman-5-yl)methyl acetate (1)
Yellow needle crystal (CH3OH); UV (MeOH) λmax(log ε) 214, 315 nm; IR (KBr) νmax 3408.9, 2920.2, 2850.0, 1674.3, 1446.1,1384.2, 1207.2, 1138.8 cm−1; CD (mdeg): Δε212 nm + 25.0,Δε250 nm − 14.6, Δε290 nm + 5.6; 1H and 13C-NMR spectral data, see Table 1. HR-ESI–MS: m/z 289.0681 [M + Na]+ (calcd. for C13H14O6Na, 289.0683).
((3R,4R)-4,8-Dihydroxy-3-methyl-1-oxoisochroman-5-yl)methyl acetate (2)
Yellow needle crystal (CH3OH); UV (MeOH) λmax(log ε) 218, 316 nm; IR (KBr) νmax 3418.5, 2920.1, 2850.8, 1675.2, 1477.9,1383.7, 1208.1, 1171.9 cm−1; CD (mdeg): Δε198 nm − 12.5,Δε212 nm + 5.0,Δε250 nm + 25.0, Δε316 nm − 3.7;1H and 13C-NMR spectral data, see Table 1. HR–ESI–MS: m/z 289.0670 [M + Na]+ (calcd. for C13H14O6Na, 289.0683).
(3R,4R)-4,8-Dihydroxy-3,5-dimethylisochroman-1-one (3)
Yellow needle crystal (CH3OH); CD (mdeg): Δε198 nm − 6.5,Δε212 nm + 7.0,Δε250 nm + 13.8, Δε316 nm − 1.1;1H and 13C-NMR spectral data, see Table 1. HR-ESI-MS: m/z 231.0637 [M + Na]+ (calcd. for C11H12O4Na, 231.0628).
Cytotoxic activity assay
Four cell lines including HepG2, A549, LoVo and Hela cell lines were purchased from the American Type Culture Collection. All the cell lines were used to evaluate the cytotoxic activities of compounds 1-3 in vitro by the method of MTT. Briefly, HepG2, A549, LoVo and Hela cells were seeded in 96-well flat bottom plates at a density of about 1 × 104 cells/well, respectively. After incubating 12–18 h, 20 μL of compounds 1–3 were added into each well at a final concentration of 1, 5, 10, 25, 50, 100 and 200 μΜ. All the cells in the plates were incubated for another 48 h respectively. Subsequently, cell lines were incubated with MTT at the concentration of 0.5 mg/mL for 4 h, and then the cells were re-suspended in 150 μL of Dimethyl sulfoxide (DMSO). Inhibitory concentrations of compounds were calculated and half maximal inhibitory concentrations (IC50) values were confirmed. 5-Fluorouracil and dimethyl sulfoxide (DMSO, 0.1%, v/v) were used as positive control and negative control, respectively.
Conclusion
A mount of chemical constituents have been isolated and identified from Radix Glycyrrhizae. Triterpenoids including glycyrrhizic acid, glycyrrhetinic acid and flavonoids including isoliquiritigenin, liquiritigenin, isoliquiritin, Licochalcone A, B and E are considered as the main characteristic constituents of the herb. And the anticancer bioactivities of these characteristic compounds were assayed frequently. Compared to triterpenoids, flavonoids possessed stronger anticancer bioactivities. In this study, three dihydroisocoumarins (1–3) showed the less toxicities on A549 and HepG2 cell lines than that of flavonoids constituents reported previously. IC50 value of isoliquiritigenin, licochalcone A and E on A549 cell lines were 18.5, 14.3 and 17.3 μM, respectively. Licochalcone A possessed almost the same toxicity on HepG2 cell lines (IC50, 10 μM). But the dihydroisocoumarins (1–3) showed more toxicity on A549 cell lines than that of Glycyrrhizic acid [7]. Besides anticancer activity, dihydroisocoumarin and its derivatives also exhibited anti-inflammatory and anti-bacterial effects [8, 9]. And these bioactivities assay were also the aim of our research in the future project.
References
Liu YY, Liu CS, Zeng BF, Fan BD, Li PS, Xu TH et al (2013) Research progress on germplasm resources of Glycyrrhizae Radix et Rhizoma. Zhongcaoyao 44:3593–3598
Rauchensteiner F, Matsumura Y, Ya MY, Yamaji S, Tani T (2005) Analysis and comparison of Radix Glycyrrhizae(licorice) from Europe and China by capillary-zoneelectrophoresis (CZE). J Pharm Biomed Anal 38:594–600
Lin ZJ, Qiu SX, Wufuer A, Shum L (2005) Simultaneous determination of glycyrrhizin, a marker component in Radix Glycyrrhizae, and its major metabolite glycyrrhetic acid in human plasma by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 814:201–207
Shon YH, Lee HS, Kim CH, Lim JK, Jeon BH, Nam KS (2004) Effect of herbal medicines on cytokine-induced cytotoxicity and major histo compatibility complex (MHC) class II antigen expression in rat thyroid cells. Bio Pharm Bull 27:371–374
Kim SC, Byun SH, Yang CH, Kim CY, Kim JW, Kim SG (2004) Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage). Toxicology 197:239–251
Zheng W, Ji YB, Li WL, Dong J, Chen N, Yu XF et al (2017) A pair of new isocoumarin epimersfrom soil fungus Hypoxylon sp. J Asian Nat Prod Res 19:993–999
Tang ZH, Li T, Tong YG, Chen XJ, Chen XP, Wang YT et al (2015) A systematic review of the anticancer properties of compounds isolated from Licorice (Gancao). Planta Med 81:1670–1687
Li YF, Plitzko I, Zaugg J, Hering S, Hamburger M (2010) HPLC-based activity profiling for GABAA receptor modulators: a new dihydroisocoumarin from haloxylon scoparium. J Nat Prod 73:768–770
Kim DC, Quang TH, Ngan NTT, Yoon CS, Sohn JH, Yim JH et al (2015) Dihydroisocoumarin derivatives from marine-derived fungal isolates and their anti-inflammatory effects in lipopolysaccharide-induced BV2 microglia. J Nat Prod 78:2948–2955
Authors’ contributions
ZY and LHW conceived and designed the experiments; ZSS and WJ performed the experiments; ZSS, YXJ and ZZZ analyzed the data; ZSS and YXJ wrote the paper; ZY and LHWmodified the paper. All authors read and approved the final manuscript.
Acknowledgements
This work was financially supported by Young innovative talent training plan of College in Heilongjiang Province (UNPYSCT-2016182) and Beijing CSCO clinical cancer research foundation (201510784).
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional file
Additional file 1.
NMR and MS spectrum of compound 1–3.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, S., Yan, X., Zhao, Y. et al. Dihydroisocoumarins from Radix Glycyrrhizae. Chemistry Central Journal 12, 58 (2018). https://doi.org/10.1186/s13065-018-0427-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-018-0427-0


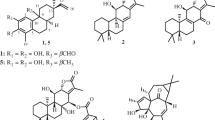



 ) correlations of compound 1–3
) correlations of compound 1–3
