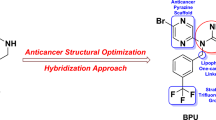
Abstract
A series of novel N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide derivatives 10(a–f), 12(a–c) and 14(a–c) were synthesized and characterized by FTIR, 1H-NMR, mass spectral and elemental analysis. The efficacy of these derivatives to inhibit in vivo angiogenesis was evaluated using chick chorioallantoic membrane (CAM) model and their DNA cleavage abilities were evaluated after incubating with calf thymus DNA followed by gel electrophoresis. These novel piperidine analogues efficiently blocked the formation of blood vessels in vivo in CAM model and exhibited differential migration and band intensities in DNA binding/cleavage assays. Among the tested compounds 10a, 10b, 10c, 12b, 14b and 14c showed significant anti-angiogenic and DNA cleavage activities compared to their respective controls and the other derivatives used in this study. These observations suggest that the presence of electron donating and withdrawing groups at positions 2, 3 and 4 of the phenyl ring of the side chain may determine their potency and as anticancer agents by exerting both anti-angiogenic and cytotoxic effects

.
Similar content being viewed by others
Introduction
There is growing evidence that tumor-initiated neovascularization, called tumor angiogenesis, is a central process involved in the aggressive growth of tumors and of their metastases. The requirement of angiogenesis for sustained tumor growth has led to the development of alternative strategies for treating cancer based on the selective interference with the growth of tumor micro vessels [1]. Cancer, the second largest cause of mortality in the world, is continuing to be a major health hazard in developing as well as in developing countries [2]. Design and development of anticancer drugs with fewer or no side effects are important for the treatment of cancer. The search for such potential anticancer drugs has led to the discovery of synthetic molecules with anticancer activity.
DNA is an important drug target and it regulates many biochemical processes that occur in the cellular system. The different alleles present in the DNA are involved in various processes such as gene activation, gene transcription, mutagenesis, carcinogenesis etc. [3]. Many small molecules exert their anticancer activities by binding with DNA, thereby altering DNA replication and inhibiting the growth of tumour cells. DNA cleavage reaction is also considered of prime importance as it proceeds by targeting various parts of DNA such as purine and pyrimidine bases, deoxyribose sugar and phosphodiester linkage.
Small molecules that hydrolyze the DNA are useful in genetic engineering, molecular biotechnology and robust anticancer drug design [4, 5]. Heterocyclic compounds have emerged as potential therapeutic agents because of conformational rigidity, improved physical properties, charge density, lipophilicity and pharmacological advantages such as metabolic stability and oral bioavailability [6].
Pyrimidines and their analogues represent an important class of biologically active nitrogen containing heterocyclic molecules, and many of which are either available as natural compounds or by designed synthetic routes [7,8,9]. The pyrimidine derivatives comprise a diverse and interesting group of drugs and have been discussed [10,11,12]. Pyrimidine, being an integral part of DNA and RNA, have imparts diverse biological activity viz. anticancer [13, 14], antiviral [15, 16] antiprotozoal [17], antihypertensive [18], antihistaminic [19], anti-inflammatory [20], central nervous activities [21], antibacterial [22, 23], antifungal [24, 25] and in particular antiangiogenic agents [26]. Specifically, disubstituted pyrimidines have shown potent anticancer activity as CDK inhibitors [27], TNF-α inhibitors [28], Abl tyrosine protein kinase inhibitors [29], PI-3 kinase inhibitors [30], Akt kinase inhibitors [31], and cytokines inhibitors [32].
Imatinib, an anti-cancer agent prepared by an intermediate N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidinamine, and it is currently marketed as Gleevec. Imatinib selectively inhibits Bcr–Abl kinase and was first approved to treat both adult and children with Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML) and later it has been approved to treat gastrointestinal stromal tumors (GISTs) [33] and other malignancies. Due to its high selectivity towards Bcr–Abl kinase, it has shown high efficacy and mild side effects in patients and has been listed as essential medicines by World Health Organization [34]. The use of combinatorial approaches toward the synthesis of drug-like scaffolds is a powerful tool in helping to speed up drug discovery. In the view of the facts mentioned above and as part of our initial efforts to discover potentially active new agents [35,36,37], we have synthesized some novel N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide derivatives as anticancer cancer agents, which have demonstrated efficient DNA binding and antiangiogenic activity.
Materials and methods
Chemistry
Melting points were determined using SELACO-650 hot stage melting point apparatus and were uncorrected. Infrared (IR) spectra were recorded using a Jasco FTIR-4100 series. Nuclear magnetic resonance (1H NMR) spectra were recorded on Shimadzu AMX 400-Bruker, 400 MHz spectrometer using DMSO-d6 as a solvent and TMS as internal standard (chemical shift in δ ppm). Spin multiplets are given as s (singlet), d (doublet), t (triplet) and m (multiplet). Mass and purity were recorded on a LCMSD-Trap-XCT. Silica gel column chromatography was performed using Merck 7734 silica gel (60–120 mesh) and Merck made TLC plates.
Synthesis of 3-dimethylamino-1-(pyridin-3-yl)prop-2-en-1-one (3)
A mixture of 3-acetylpyridine 1 (25 g, 20.63 mmol) and N,N-dimethylformamide dimethyl acetyl 2 (31.95 g, 26.82 mmol) was refluxed for 16 h under nitrogen. Upon completion of the reaction, the mixture was concentrated under reduced pressure. To the residue, cyclohexane was added and the mixture was cooled to 0 °C. The precipitate was collected by filtration to afford the product as yellow crystals (90%). MP: 78–80 °C. 1H-NMR (CDCl3) δ: 9.0 (d, 1H, Py-H), 8.62 (dd, 1H, Py-H), 8.25 (dt, 1H, Py-H), 7.81 (d, 1H, –COCH=CH), 7.35 (dd, 1H, Py-H), 5.75 (d, 1H, –COCH=CH), 3.25 (s, 3H, –CH3), 3.02 (s, 3H, –CH3). IR (KBr, cm−1): 3080, 1685, 1620, 1448, 1354, 748. MS (ESI) m/z: 177.09.
Synthesis of N-(2-methyl-5-nitrophenyl)-4-pyridin-3-yl-pyrimidin-2-ylamine (5)
To a mixture of 3-dimethylamino-1-(pyridin-3-yl)propenone 3 (25 g, 11.34 mmol) and N-(2-methyl-5-nitrophenyl)guanidinium nitrate 4 (47.66 g, 14.74 mmol) in n-butanol (200 mL), sodium hydroxide (8.63 g, 216 mmol) was added. The mixture was refluxed for 16 h and then cooled to 0 °C. The precipitate was collected by filtration and washed with methanol and diethyl ether and dried to get the product (92%) as a yellow solid. MP: 196–197 °C. 1H-NMR δ: 8.93 (d, 1H, Py-H), 8.71 (dd, 1H, Py-H), 8.60 (s, 1H, –NH), 8.45 (d, 1H, pyrimidyl-H), 8.30 (d, 1H, Py-H), 7.45 (dd, 1H, Py-H), 7.30 (d, 1H, pyrimidyl-H), 6.75 (d, 1H, Ar–H), 6.70 (d, 1H, Ar–H), 6.38 (dd, 1H, Ar–H), 2.08 (s, 3H, –CH3). IR (KBr, cm−1): 3076, 1655, 1521, 1476, 870. MS (ESI) m/z: 308.11.
General procedure for the synthesis of 6-methyl-N 1-(4-(pyridin-3-yl)pyrimidin-2-yl)benzene-1,3-diamine (6)
To a solution of stannous chloride dihydrate in hydrochloric acid (30 mL) at 0 °C, N-(2-methyl-5-nitrophenyl)-4-pyridin-3-yl-pyrimidin-2-ylamine 5 was added in portions and stirred for 6 h. Progress of reaction was monitored by TLC. Upon completion, the mixture was poured into crushed ice, made alkaline with solid sodium hydroxide, and extracted with ethyl acetate. The combined organic layer was washed two to three times with water and dried over anhydrous sodium sulfate. The solvent was evaporated to get crude product, which was purified by recrystallization from methylene chloride to get the compound as a yellow solid.
Synthesis of 6-methyl-N 1-(4-(pyridin-3-yl)pyrimidin-2-yl)benzene-1,3-diamine (6)
The product obtained was yellow solid (75%) from N-(2-methyl-5-nitrophenyl)-4-pyridin-3-yl-pyrimidin-2-ylamine 5 (10 g, 3.254 mmol), and stannous chloride dihydrate (29 g, 12.974 mmol) in 35 mL hydrochloric acid. MP: 142–144 °C. 1H-NMR δ: 8.98 (d, 1H, Py-H), 8.65 (dd, 1H, Py-H), 8.58 (s, 1H, –NH), 8.42 (d, 1H, pyrimidyl-H), 8.34 (d, 1H, Py-H), 7.48 (dd, 1H, Py-H), 7.30 (d, 1H, pyrimidyl-H), 6.82 (d, 1H, Ar–H), 6.75 (d, 1H, Ar–H), 6.30 (dd, 1H, Ar–H), 4.80 (br, 2H, –NH2), 2.05 (s, 3H, –CH3). MS (ESI) m/z: 278.13.
General procedure for the synthesis of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8)
Piperidine-4-carboxylic acid 7 was taken in dry N,N-dimethyl formamide and cooled to 0–5 °C in ice bath. Then isobutyl chloroformate and N-methyl morpholine were added to the reaction mixture. The reaction mixture was allowed to stir for 10–15 min. After that 6-methyl-N1-(4-(pyridin-3-yl)pyrimidin-2-yl)benzene-1,3-diamine 6 was added, then reaction mixture was allowed to room temperature under stirring for 5–6 h. Progress of reaction was monitored by TLC. Upon completion, the solvent was removed under reduced pressure and residue was taken in water and extracted with ethyl acetate. The organic layer was dried with anhydrous sodium sulphate, the solvent was evaporated to get crude product which was purified by column chromatography over silica gel (60–120 mesh) using MDC and methanol (1:1) to get N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8).
Synthesis of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8)
The product obtained was pale yellow color from piperidine-4-carboxylic acid 7 (0.046 g, 0.36 mmol), 6-methyl-N1-(4-(pyridin-3-yl)pyrimidin-2-yl)benzene-1,3-diamine 6 (0.1 g, 0.36 mmol), isobutyl chloroformate (0.078 g, 0.772 mmol) and N-methyl morpholine (0.078 g, 0.772 mmol). MP: 118–120 °C. 1H-NMR δ: 9.20 (s, 1H, –CO–NH), 8.95 (d, 1H, Py-H), 8.70 (dd, 1H, Py-H), 8.61 (s, 1H, –NH), 8.46 (d, 1H, pyrimidyl-H), 8.32 (d, 1H, Py-H), 7.40 (dd, 1H, Py-H), 7.33 (d, 1H, pyrimidyl-H), 6.76 (d, 1H, Ar–H), 6.69 (d, 1H, Ar–H), 6.32 (dd, 1H, Ar–H), 3.53 (t, 2H, –CH2), 3.28 (s, 1H, –NH), 3.20 (t, 2H, –CH2), 2.79-2.89 (bs, 1H, –CH), 2.35 (t, 2H, –CH2), 2.10 (t, 2H, –CH2), 2.01 (s, 3H, –CH3). MS (ESI) m/z: 389.2 (100.0%). Anal. calcd. for C22H24N6O (in %): C-68.02, H-6.23, N-21.63. Found: C-67.96, H-6.17, N-21.65.
General procedure for the synthesis of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide derivatives 10(a–f)
The N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) was dissolved in dry dichloromethane. To this reaction mixture triethylamine was added and cooled to 0–5 °C in ice bath. Then different sulfonyl chlorides 9(a–f) are added. The reaction mixture was monitored by TLC. Upon completion, the solvent was removed under reduced pressure and residue was taken in water and extracted with ethyl acetate. The organic layer was dried with anhydrous sodium sulphate and the solvent was evaporated to get crude product which was purified by column chromatography over silica gel (60–120 mesh) using dichloromethane and methanol (1:1).
Synthesis of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-1-((4-nitrophenyl) sulfonyl)piperidine-4-carboxamide (10a)
The product obtained was pale yellow color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol), 4-nitrobenzene sulfonyl chloride (9a) (0.055 g, 0.257 mmol) and triethylamine (0.078 g, 0.772 mmol). 1H-NMR δ: 9.23 (s, 1H, –CO–NH), 8.92 (d, 1H, Py-H), 8.75 (dd, 1H, Py-H), 8.60 (s, 1H, -NH), 8.47 (d, 1H, pyrimidyl-H), 8.40 (d, 2H, Ar–H), 8.30 (d, 1H, Py-H), 8.15 (d, 2H, Ar–H), 7.43 (dd, 1H, Py-H), 7.30 (d, 1H, pyrimidyl-H), 6.71 (d, 1H, Ar–H), 6.69 (d, 1H, Ar–H), 6.35 (dd, 1H, Ar–H), 3.50 (t, 2H, –CH2), 3.25 (t, 2H, –CH2), 2.80–2.88 (bs, 1H, –CH), 2.38 (t, 2H, –CH2), 2.12 (t, 2H, –CH2), 2.03 (s, 3H, –CH3). 13C NMR (100.6 MHz, DMSO-d 6) δ: 17.5, 29.1, 38.1, 46.3, 103.3, 108.1, 111.7, 123.9, 124.2, 124.8, 128.3, 130.0, 133.1, 134.2, 136.3, 142.2, 145.8, 147.4, 148.0, 151.3, 154.5, 161.1, 168.7, 172.9. MS (ESI) m/z: 574.18 (100.0%). Anal. calcd. for C28H27N7O5S (in %): C-58.63, H-4.74, N-17.09. Found: C-58.66, H-4.71, N-17.05.
Synthesis of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-1-(o-tolylsulfonyl)piperidine-4-carboxamide (10b)
The product obtained was pale yellow color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol), 2-methylbenzene sulfonyl chloride (9b) (0.049 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.23 (s, 1H, –CO–NH), 8.91 (d, 1H, Py-H), 8.73 (dd, 1H, Py-H), 8.60 (s, 1H, -NH), 8.49 (d, 1H, pyrimidyl-H), 8.40 (d, 1H, Py-H), 7.78 (d, 1H, Ar–H), 7.53 (d, 1H, Ar–H), 7.45 (t, 2H, Ar–H), 7.37 (dd, 1H, Py-H), 7.28 (d, 1H, pyrimidyl-H), 6.73 (d, 1H, Ar–H), 6.60 (d, 1H, Ar–H), 6.35 (dd, 1H, Ar–H), 3.50 (t, 2H, –CH2), 3.25 (t, 2H, –CH2), 2.78-2.85 (bs, 1H, –CH), 2.70 (s, 3H, –CH3), 2.32 (t, 2H, –CH2), 2.15 (t, 2H, –CH2), 2.05 (s, 3H, –CH3). MS (ESI) m/z: 543.21 (100.0%). Anal. calcd. for C29H30N6O3S (in %): C-64.19, H-5.57, N-15.49. Found: C-64.16, H-5.51, N-15.45.
Synthesis of 1-((4-methoxyphenyl)sulfonyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (10c)
The product obtained was pale yellow color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol), 4-methoxybenzene sulfonyl chloride (9c) (0.053 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.18 (s, 1H, –CO–NH), 8.90 (d, 1H, Py-H), 8.74 (dd, 1H, Py-H), 8.65 (s, 1H, –NH), 8.40 (d, 1H, pyrimidyl-H), 8.35 (d, 1H, Py-H), 7.63 (dd, 2H, Ar–H), 7.35 (dd, 1H, Py-H), 7.30 (d, 1H, pyrimidyl-H), 7.10 (d, 2H, Ar–H), 6.70 (d, 1H, Ar–H), 6.63 (d, 1H, Ar–H), 6.30 (dd, 1H, Ar–H), 3.84 (s, 3H, –OCH3), 3.50 (t, 2H, –CH2), 3.24 (t, 2H, –CH2), 2.80–2.88 (bs, 1H, –CH), 2.34 (t, 2H, –CH2), 2.11 (t, 2H, –CH2), 2.03 (s, 3H, –CH3). MS (ESI) m/z: 559.20 (100.0%), Anal. calcd. for C29H30N6O4S (in %): C-62.35, H-5.41, N-15.04. Found: C-62.29, H-5.37, N-15.05.
Synthesis of 1-((3-chlorophenyl)sulfonyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (10d)
The product obtained was pale yellow color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol) and 3-chlorobenzene sulfonyl chloride (9d) (0.054 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.21 (s, 1H, –CO–NH), 8.95 (d, 1H, Py-H), 8.74 (dd, 1H, Py-H), 8.60 (s, 1H, –NH), 8.51 (d, 1H, pyrimidyl-H), 8.36 (d, 1H, Py-H), 8.21 (s, 1H, Ar–H), 7.75 (d, 1H, Ar–H), 7.66 (t, 1H, Ar–H), 7.51 (d, 1H, Ar–H), 7.42 (dd, 1H, Py-H), 7.31 (d, 1H, pyrimidyl-H), 6.74 (d, 1H, Ar–H), 6.67 (d, 1H, Ar–H), 6.30 (dd, 1H, Ar–H), 3.55 (t, 2H, –CH2), 3.22 (t, 2H, –CH2), 2.79–2.89 (bs, 1H, –CH), 2.36 (t, 2H, –CH2), 2.12 (t, 2H, –CH2), 2.05 (s, 3H, –CH3). MS (ESI) m/z: 563.29 (100.0%). Anal. calcd. for C28H27ClN6O3S (in %): C-59.73, H-4.83, N-14.93. Found: C- C-59.70, H-4.81, N-14.90.
Synthesis of 1-((3,4-difluorophenyl)sulfonyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (10e)
The product obtained was dark brown color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol) and 3,4-difluorobenzene sulfonyl chloride (9e) (0.054 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.19 (s, 1H, –CO–NH), 8.92 (d, 1H, Py-H), 8.75 (dd, 1H, Py-H), 8.66 (s, 1H, –NH), 8.42 (d, 1H, pyrimidyl-H), 8.30 (d, 1H, Py-H), 7.89 (s, 1H, Ar–H), 7.73 (dd, 1H, Ar–H), 7.49 (dd, 1H, Ar–H), 7.41 (dd, 1H, Py-H), 7.30 (d, 1H, pyrimidyl-H), 6.78 (d, 1H, Ar–H), 6.62 (d, 1H, Ar–H), 6.36 (dd, 1H, Ar–H), 3.50 (t, 2H, –CH2), 3.20 (t, 2H, –CH2), 2.75-2.83 (bs, 1H, –CH), 2.30 (t, 2H, –CH2), 2.15 (t, 2H, –CH2), 2.00 (s, 3H, –CH3). MS (ESI) m/z: 565.17 (100.0%), Anal. calcd. for C28H26F2N6O3S (in %): C-59.56, H-4.64, N-14.88. Found: C-59.50, H-4.61, N-14.92.
Synthesis of 1-((2,6-difluorophenyl)sulfonyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (10f)
The product obtained was dark brown color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol) and 2,6-difluorobenzene sulfonyl chloride (9f) (0.054 g, 0.257 mmol) and triethylamine (0.078 g, 0.772 mmol). 1H-NMR δ: 9.22 (s, 1H, –CO–NH), 8.98 (d, 1H, Py-H), 8.68 (dd, 1H, Py-H), 8.59 (s, 1H, –NH), 8.47 (d, 1H, pyrimidyl-H), 8.35 (d, 1H, Py-H), 7.45 (dd, 1H, Py-H), 7.36 (d, 1H, pyrimidyl-H), 7.25 (dd, 2H, Ar–H), 7.19 (t, 1H, Ar–H), 6.80 (d, 1H, Ar–H), 6.71 (d, 1H, Ar–H), 6.36 (dd, 1H, Ar–H), 3.57 (t, 2H, –CH2), 3.22 (t, 2H, –CH2), 2.78–2.88 (bs, 1H, –CH), 2.32 (t, 2H, –CH2), 2.15 (t, 2H, –CH2), 2.04 (s, 3H, –CH3). MS (ESI) m/z: 565.17 (100.0%). Anal. calcd. for C28H26F2N6O3S (in %): C-59.56, H-4.64, N-14.88. Found: C-59.52, H-4.60, N-14.90.
Synthesis of 1-(4-chlorobenzoyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (12a)
The product obtained was dark brown color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol) and 4-chlorobenzoyl chloride (11a) (0.045 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.18 (s, 1H, –CO–NH), 8.90 (d, 1H, Py-H), 8.75 (dd, 1H, Py-H), 8.66 (s, 1H, –NH), 8.50 (d, 1H, pyrimidyl-H), 8.34 (d, 1H, Py-H), 7.82 (dd, 2H, Ar–H), 7.65 (dd, 2H, Ar–H), 7.40 (dd, 1H, Py-H), 7.33 (d, 1H, pyrimidyl-H), 6.72 (d, 1H, Ar–H), 6.65 (d, 1H, Ar–H), 6.38 (dd, 1H, Ar–H), 3.50 (t, 2H, –CH2), 3.23 (t, 2H, –CH2), 2.78–2.87 (bs, 1H, –CH), 2.37 (t, 2H, –CH2), 2.11 (t, 2H, –CH2), 2.02 (s, 3H, –CH3). 13C NMR (100.6 MHz, DMSO-d 6) δ: 17.6, 29.7, 38.3, 44.7, 103.5, 108.1, 111.5, 123.8, 124.7, 128.7, 129.6, 133.0, 134.1, 135.3, 136.3, 142.2, 147.5, 148.0, 154.5, 161.1, 168.7, 170.0, 172.9. MS (ESI) m/z: 527.018 (100.0%), Anal. calcd. for C29H27ClN6O2 (in %): C-66.09, H-5.16, N-15.95. Found: C-66.05, H-5.13, N-15.92.
Synthesis of 1-(4-fluorobenzoyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (12b)
The product obtained was dark brown color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol) and 4-fluorobenzoyl chloride (11b) (0.040 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.20 (s, 1H, –CO–NH), 8.93 (d, 1H, Py-H), 8.79 (dd, 1H, Py-H), 8.61 (s, 1H, -NH), 8.55 (d, 1H, pyrimidyl-H), 8.30 (d, 1H, Py-H), 7.80 (dd, 2H, Ar–H), 7.65 (dd, 2H, Ar–H), 7.44 (dd, 1H, Py-H), 7.30 (d, 1H, pyrimidyl-H), 6.74 (d, 1H, Ar–H), 6.60 (d, 1H, Ar–H), 6.42 (dd, 1H, Ar–H), 3.55 (t, 2H, –CH2), 3.28 (t, 2H, –CH2), 2.76–2.87 (bs, 1H, –CH), 2.35 (t, 2H, –CH2), 2.14 (t, 2H, –CH2), 2.01 (s, 3H, –CH3). MS (ESI) m/z: 511.21 (100.0%), Anal. calcd. for C29H27FN6O2 (in %): C-68.22, H-5.33, N-16.46. Found: C-68.20, H-5.29, N-16.41.
Synthesis of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)-1-(4-(trifluoromethyl)benzoyl)piperidine-4-carboxamide (12c)
The product obtained was dark brown color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol) and 4-(trifluoromethyl)benzoyl chloride (11c) (0.053 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.20 (s, 1H, –CO–NH), 8.93 (d, 1H, Py-H), 8.81 (dd, 1H, Py-H), 8.65 (s, 1H, –NH), 8.52 (d, 1H, pyrimidyl-H), 8.34 (d, 1H, Py-H), 7.98 (dd, 2H, Ar–H), 7.86 (dd, 2H, Ar–H), 7.50 (dd, 1H, Py-H), 7.36 (d, 1H, pyrimidyl-H), 6.74 (d, 1H, Ar–H), 6.63 (d, 1H, Ar–H), 6.40 (dd, 1H, Ar–H), 3.53 (t, 2H, –CH2), 3.25 (t, 2H, –CH2), 2.74-2.85 (bs, 1H, –CH), 2.32 (t, 2H, –CH2), 2.14 (t, 2H, –CH2), 2.03 (s, 3H, –CH3). MS (ESI) m/z: 561.21 (100.0%), Anal. calcd. for C30H27F3N6O2 (in %): C-64.28, H-4.85, N-14.99. Found: C-64.22, H-4.80, N-14.93.
Synthesis of 1-((4-chlorophenyl)carbamothioyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (14a)
The product obtained was dark brown color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol) and 4-chlorophenyl isothiocyanate (13a) (0.043 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.22 (s, 1H, –CO–NH), 9.16 (s, 1H, –CS–NH), 8.93 (d, 1H, Py-H), 8.74 (dd, 1H, Py-H), 8.59 (s, 1H, –NH), 8.42 (d, 1H, pyrimidyl-H), 8.30 (d, 1H, Py-H), 7.46 (dd, 1H, Py-H), 7.35 (d, 1H, pyrimidyl-H), 7.29 (dd, 2H, Ar–H), 6.60 (dd, 2H, Ar–H), 6.71 (d, 1H, Ar–H), 6.62 (d, 1H, Ar–H), 6.35 (dd, 1H, Ar–H), 3.50 (t, 2H, –CH2), 3.23 (t, 2H, –CH2), 2.79–2.89 (bs, 1H, –CH), 2.33 (t, 2H, –CH2), 2.11 (t, 2H, –CH2), 2.03 (s, 3H, –CH3). 13C NMR (100.6 MHz, DMSO-d 6) δ: 17.6, 29.7, 38.3, 51.0, 103.5, 108.1, 111.5, 123.8, 124.7, 128.7, 129.6, 131.7, 133.0, 133.7, 134.1, 136.3, 142.2, 147.7, 148.0, 154.5, 161.1, 168.5, 172.9, 186.7. MS (ESI) m/z: 557.17 (100.0%), Anal. calcd. for C29H28ClN7OS (in %): C-62.41, H-5.06, N-17.57. Found: C-62.37, H-5.01, N-17.53.
Synthesis of 1-((2-methoxyphenyl)carbamothioyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (14b)
The product obtained was dark brown color color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol) and 2-methoxyphenyl isothiocyanate (13b) (0.042 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.20 (s, 1H, –CO–NH), 9.14 (s, 1H, –CS–NH), 8.92 (d, 1H, Py-H), 8.75 (dd, 1H, Py-H), 8.63 (s, 1H, –NH), 8.40 (d, 1H, pyrimidyl-H), 8.35 (d, 1H, Py-H), 7.42 (dd, 1H, Py-H), 7.31 (d, 1H, pyrimidyl-H), 6.86 (d, 1H, Ar–H), 6.79 (d, 1H, Ar–H), 6.70 (dd, 2H, Ar–H), 6.68 (d, 1H, Ar–H), 6.59 (d, 1H, Ar–H), 6.34 (dd, 1H, Ar–H), 3.85 (s, 3H, –OCH3), 3.56 (t, 2H, –CH2), 3.24 (t, 2H, –CH2), 2.75–2.86 (bs, 1H, –CH), 2.37 (t, 2H, –CH2), 2.14 (t, 2H, –CH2), 2.05 (s, 3H, –CH3). MS (ESI) m/z: 554.22 (100.0%), Anal. calcd. for C30H31N7O2S (in %): C-65.08, H-5.64, N-17.71. Found: C-65.02, H-5.60, N-17.66.
Synthesis of 1-((3-methoxyphenyl)carbamothioyl)-N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (14c)
The product obtained was dark brown color from N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) (0.1 g, 0.257 mmol) and 3-methoxyphenyl isothiocyanate (13c) (0.042 g, 0.257 mmol) and triethylamine(0.078 g, 0.772 mmol). 1H-NMR δ: 9.21 (s, 1H, –CO–NH), 9.15 (s, 1H, –CS–NH), 8.90 (d, 1H, Py-H), 8.75 (dd, 1H, Py-H), 8.64 (s, 1H, –NH), 8.42 (d, 1H, pyrimidyl-H), 8.36 (d, 1H, Py-H), 7.48 (dd, 1H, Py-H), 7.34 (d, 1H, pyrimidyl-H), 7.10 (t, 1H, Ar–H), 6.78 (d, 1H, Ar–H), 6.62 (d, 1H, Ar–H), 6.38 (d, 1H, Ar–H), 6.30 (dd, 1H, Ar–H), 6.25 (bs, 1H, Ar–H), 6.08 (d, 1H, Ar–H), 3.83 (s, 3H, –OCH3), 3.54 (t, 2H, –CH2), 3.25 (t, 2H, –CH2), 2.79–2.89 (bs, 1H, –CH), 2.38 (t, 2H, –CH2), 2.15 (t, 2H, –CH2), 2.01 (s, 3H, –CH3). MS (ESI) m/z: 554.226 (100.0%), Anal. calcd. for C30H31N7O2S (in %): C-65.08, H-5.64, N-17.71. Found: C-65.03, H-5.60, N-17.73.
Biology
Fertilized eggs were obtained from IVRI, Bangalore, India. CT DNA was purchased from Sigma. All chemicals and solvents were reagent grade purchased from Merck. DNA stock solution was prepared by dilution of CT DNA to buffer (containing 150 mM NaCl and 15 mM trisodium citrate at pH 7.0) followed by exhaustive stirring at 4 °C for 3 days, and kept at 4 °C for no longer than a week. The stock solution of CT DNA gave a ratio of UV absorbance at 260 and 280 nm (A260/A280) of 1.89, indicating that the DNA was sufficiently free of protein contamination. The DNA concentration was determined by the UV absorbance at 260 nm after 1:20 dilution using ε = 6600 M−1 cm−1.
Shell less chorioallantoic membrane (CAM) assay
Antiangiogenic effect of the novel N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide derivatives 10(a–f), 12(a–c) and 14(a–c) was evaluated according to the method of Auerbach et al. [38]. Fertilized hens eggs were surface sterilized using 70% alcohol. The eggs were incubated in fan assisted humidified incubator at 37 °C. On the 4th day, the eggs were cracked out into thin films of the hammock within a laminar flow cabinet and were further incubated. On the day 5th when blood vessels were seen proliferating from the center of the eggs within the hammock, filter paper discs loaded with 100 µg of 10(a–f), 12(a–c) and 14(a–c) were placed over the proliferating blood vessels and the eggs were returned to the incubator. Results for antiangiogenic effect of the each compound were observed after 24 h comparing to untreated controls (paper discs with solvent only).
DNA cleavage experiments
DNA cleavage experiments were carried out according to the previously described procedure [39]. Briefly, the solution of compounds in DMF (1 mg/mL) was prepared and these test samples (1 µg) were added to the 500 ng of Calf thymus-DNA (CT-DNA) in TE buffer and incubated for 2 h at 37 °C. Agarose gel electrophoresis was performed after loading the samples on to the gel in TAE buffer system at 50 V for 2 h. At the end of electrophoresis, the gel was carefully stained with EtBr (Ethedium bromide) solution (10 µg/mL) for 10–15 min and visualized under UV light using a Bio-Rad Trans illuminator and the images were captured.
Results and discussions
Chemistry
Synthesis of the key intermediate N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) is outlined in Scheme 1. To prepare the pyrimidine ring system, the general method was used [40], which involved reacting 3-acetylpyridine (1) with N,N-dimethylformamide dimethyl acetyl (2) to give the 3-dimethylamino-1-(pyridin-3-yl)prop-2-en-1-one (3) in 90% yield. The enaminone (3) reacts with 1-(2-methyl-5-nitrophenyl)guanidine (4) in presence of base to give N-(2-methyl-5-nitrophenyl)-4-(pyridin-3-yl)pyrimidin-2-amine (5). Reduction of compound (5) with SnCl2·2H2O afforded 6-methyl-N1-(4-pyridin-3-yl-pyrimidin-2-yl)benzene-1,3-diamine (6) in 75% yield. 6-Methyl-N1-(4-(pyridin-3-yl)pyrimidin-2-yl)benzene-1,3-diamine (6) (1.0 eq) and piperidine-4-carboxylic acid (7) (1.0 eq) in N,N-dimethyl formamide in the presence of base N-methyl morpholine, isobutyl chloroformate, and reaction mixture was stirred for 5–6 h at room temperature, which gave target key intermediate (8). The absence of –COOH proton peak and presence of –NH proton peak confirmed the formation of compound (8) with a good yield of 88%. The nucleophilic substitution reaction of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide (8) with different substituted aromatic sulfonyl chlorides 9(a–f) (R–SO2Cl)/aromatic acid chlorides 11(a–c) (R–CO–Cl)/aromatic isothiocyanates 13(a–c) (R–N=C=S) was carried out in the presence of triethylamine and dichloromethane as solvent with a good yield of 81–88%. The absence of –NH and presence of –CS–NH proton peak in synthesized derivatives 10(a–f), 12(a–c) and 14(a–c) in 1H NMR spectra confirmed the identity of the products. It is also confirmed by IR data, for sulfonamide series 10(a–f) which showed asymmetric stretching frequency of O=S=O in the range 1350–1370 cm−1 and symmetric stretching frequency at 1270–1290 cm−1. For carboxamide series 12(a–c), IR data showed stretching frequency of –C=O at 1630–1670 cm−1 and similarly for 14(a–c), stretching frequency at 3350–3360 cm−1 for –NH and 1640–1660 cm−1 for –C=O group. The chemical structures of all the synthesized compounds are given in Table 1.
Synthesis of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide and its analogs. Reagents and conditions: (i) 100 °C; (ii) NaOH, n-butanol, 110 °C; (iii) SnCl2·2H2O, hydrochloric acid, 0 °C-r.t.; (iv) IBCF, NMP, DMF; (v) MDC, TEA, 0 °C-r.t. 9a = 4-nitrobenzene sulfonyl chloride; 9b = 2-methylbenzene sulfonyl chloride; 9c = 4-methoxybenzene sulfonyl chloride; 9d = 3-chlorobenzene sulfonyl chloride; 9e = 3,4-difluorobenzene sulfonyl chloride; 9f = 2,6-difluorobenzene sulfonyl chloride; (vi) MDC, TEA, 0 0C-r.t. 11a = 4-chlorobenzoyl chloride; 11b = 4-fluorobenzoyl chloride; 11c = 4-(trifluoromethyl)benzoyl chloride; (vii) MDC, TEA, 0 0C-r.t; 13a = 4-chlorophenyl isothiocyanate; 13b = 2-methoxyphenyl isothiocyanate; 13c = 3-methoxyphenyl isothiocyanate
Biology
Choriallanotoic membrane (CAM) assay
The CAM assay is a simple, reliable, and inexpensive method of studying angiogenesis. In the present investigation anti-angiogenic activity of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide derivatives showed reduced proliferation of blood vessels in the shell less CAM assay model of developing embryos.
Pyrimidine is an important scaffold known to be associated with several biological activities. Some of the derivatives of pyrimidines potently inhibit angiogenesis [41, 42]. Some representatives of pyrimidine have been investigated as non-ATP competitive KDR inhibitors (type II) [43]. Donnini et al. demonstrated that inhibition of pyrazolo-pyrimidine-derived c-Src kinase activity reduces VEGF induced-angiogenesis both in tumor and endothelial cells [44].
In view of the above findings, the anti-angiogenic activity was assessed by carrying out the reactions of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide with different sulfonyl chlorides containing substituted aromatic rings. The proliferation of micro vessels were regressed around the zone of compounds treated (Fig. 1). Our data demonstrates that compounds 10a, 10b, 10c, 12b, 14b and 14c possess potential antiangiogenic activity.
DNA cleavage studies by gel electrophoresis
The pyrimidine entity is one of the most prominent structures found in nucleic acid chemistry. Some of the derivatives of 4-(4-(6-phenyl-pyrimidin-4-yl)-phenoxymethyl]-chromen-2-ones were tested for DNA cleavage activity by agarose gel electrophoresis method [45]. Shamsuzzaman et al. synthesized some steroidal pyrimidines for interaction with DNA and indicated higher binding affinity of compounds towards DNA [46]. In view of the above findings, the compounds synthesized in this study were evaluated for their DNA cleavage activity. After binding to DNA, synthetic molecule can induce several changes in DNA conformation and deformations, such as bending, local denaturation, (over winding and under winding), intercalation, micro loop formation and subsequent DNA shortening lead to alteration in molecular weight of DNA. Gel electrophoresis is an extensively used technique for the study of binding of compounds with nucleic acids: in this method segregation of the molecules will be on the basis of their relative rate of movement through a gel under the influence of an electric field. Gel electrophoresis images shown in Figs. 2, 3 and 4 shows differences in band width and ethidium-bromide staining intensities compared to the control. The difference observed in the band width and intensity is the criterion for the evaluation of binding/cleavage ability of synthetic molecule with calf thymus DNA. Figure 2 shows the bands with different band width and brightness compared to control. There is significant binding/cleavage of DNA in the lane 2, 3, 4 and 6 when compared to the control, where the intensity of the DNA is more. Figure 3 shows lane 2, 3 and 4 (treated with synthetic molecule: 12a, 12b, and 8) showed less intense DNA indicating degradation when compared with control. In the Fig. 4 lane 2, 3, 4 revealing less intense DNA compared to the control. The molecule 10f has completely degraded the DNA indicating better cleavage activity.
From the obtained results, it indicates that the substitution at N-terminal of the piperidine ring play a key role in its DNA binding activity. Thus, 10b, 10c, 14b and 14c having electron donating groups enhances their DNA binding/cleavage activity. Interestingly, compounds 10a and 12b having electron withdrawing nitro (para) and fluoro (ortho) groups, respectively also showed good activity. This could be attributed to the increased electron withdrawing effect of nitro and fluoro groups when compared to chloro group present in 10(d–f), 12a, 12c and 14a. On the other hand, as the electron donating efficiency increases, the activity also increases. We believe that introducing electron donating methoxy and methyl groups (5e, 5f) on the N-terminal of the piperidine ring at 2nd 3rd and 4th position resulted in increase in the activity. However, further studies are required to understand the exact mechanism of its action.
Conclusion
Among the tested compounds, compounds 10a, 10b, 10c, 12b, 14b and 14c showed a significant antiangiogenic and DNA cleavage activity. N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide and its derivatives which showed combined antiangiogenic and DNA cleavage activities may be used for the design of more potent anticancer drugs. In conclusion, the antitumor activity of N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide and its analogs still has to be established, and detailed studies are needed to investigate whether these compounds are able to induce apoptosis in activated endothelial cells and in tumor vasculature.
References
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other diseases. Nat Med 1:27–31
Eckhardt S (2002) Recent progress in the development of anticancer agents. Cur Med Chem Anticancer Agents 3(2):419–439
Sreelatha S, Padma PR, Umadevi M (2009) Protective effect of corandrum sativum extracts on carbon tetrachloride-induced heptotoxity in rats. Food Chem Toxicol 47(4):702–708
Komiyama M, Sumaoka J (1998) Progress towards synthetic enzymes for phosphoester hydrolysis. Cur Opin Chem Biol 2(6):751–757
Hong J, Jiao Y, Yan J, He W, Guo Z, Zhu L, Zhang J (2010) DNA cleavage promoted by trigonal-bipyrimidal zinc(II) and copper(II) complexes formed by asymmetric tripodal tetradentate 2-[bis(2-aminoethyl)amino]ethanol. Inorg Chemica Acta 363(4):793–798
Marcaccino S, Pepino R, Pozo MC, Basurto S, Garia-valverde M, Torroba T (2004) One- pot synthesis of quinolin-2-(1H)-ones via tandem Ugi-Knoevenagel condensations. Tet Lett 45(21):3999–4001
Looper RE, Runnegar MTC, Williams RM (2005) Synthesis of the putative structure of 7-deoxycylindrospermopsin: C7-oxygenation is not required for the inhibition of protein synthesis. Angew Chemie Int Ed 44:3879–3881
Kobayashi J, Kanda F, Ishibashi M, Shigemori H, Manzacidins AC, Manzacidins AC (1991) Novel tetrahydropyrimidine alkaloids from the Okinawan marine sponge hymeniacidon sp. J Org Chem 56(14):4574–4576
Skinner GS, Wunz PR (1951) 2,5,5-Trialkyl-1,4,5,6-tetrahydropyrimidines. J Am Chem Soc 73(8):3814–3815
Chabner BA, Wilson W, Supko J (2001) Pharmocology and toxicity of antineoplastic drugs. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, Seligsohn U (eds) William Hematology, 6th edn. McGraw-Hill, New York
Hardman JG, Limbird LE, Molinof PB, Ruddon RW (2001) Gilman AG. In: Goodman G (ed) The pharmacological basics of therapeutics, 10th edn. McGraw-Hill, New York
Brown DJ. Pyrimidines and their benzo derivatives. In: Katritzky AR, Rees CW, editors. Comprehensive heterocyclic chemistry The structure, reactions, synthesis and uses of heterocyclic compounds. Pergamon Press: Oxford; 1984. Vol. 3, pp-57
Ghoshal K, Jacob ST (1997) An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem Pharmacol 53(11):1569–1575
Al-Safarjalani ON, Zhou XJ, Rais RH, Shi J, Schinazi RF, Naguib FNM, El Kouni MH (2005) 5-(Phenylthio)acyclouridine, a powerful enhancer of oral uridine bioavailability: relevance to chemotherapy with 5-fluorouracil and other uridine rescue regimens. Cancer Chemother Pharmacol 55(6):541–551
Lu X, Chen Y, Guo Y, Liu Z, Shi Y, Xu Y, Wang X, Zhang Z, Liu J (2007) The design and synthesis of N-1-alkylated-5-aminoarylalkylsubstituted-6-methyluracils as potential non-nucleoside HIV-1 RT inhibitors. Bioorg Med Chem 15(23):7399–7407
Wang X, Lou Q, Guo Y, Xu Y, Zhanga Z, Liu J (2006) The design and synthesis of 9-phenylcyclohepta[d]pyrimidine-2,4-dione derivatives as potent non-nucleoside inhibitors of HIV reverse transcriptase. Org Biomol Chem 4(17):3252–3258
McCarthy O, Musso-Buendia A, Kaiser M, Brun R, Ruiz-Perez LM, Johansson NG, Pacanowska DG, Gilbert IH (2009) Design, synthesis and evaluation of novel uracil acetamide derivatives as potential inhibitors of Plasmodium falciparum dUTP nucleotidohydrolase. Eur J Med Chem 44(2):678–688
Chang RSL, Chen T, O’Malley SS, Pettibone DJ, DiSalvo J, Francis B, Bock MG, Freidinger R, Nagarathnam D, Miao SW, Shen Q, Lagu B, Dhar TGM, Tyagarajan S, Marzabadi MR, Wong WC, Gluchowski C, Forray C (2000) In vitro studies on L-771,688 (SNAP 6383), a new potent and selective alpha1A-adrenoceptor antagonist. Eur J Pharmacol 409(3):301–312
Ikeda M, Maruyama K, Nobuhara Y, Yamada Y, Okabe S (1996) Synthesis and cytoprotective antiulcer activity of 2- or 4-(1H-pyrazol-1-yl)pyrimidine derivatives related to mepirizole and dulcerozine. Chem Pharm Bull 44(9):1700–1706
Falcao EPS, deMelo SJ, Srivastava RM, Catanho MTJA, Nascimento SC (2006) Synthesis and antiinflammatory activity of 4-amino-2-aryl-5-cyano-6-{3- and 4- (N-phthalimidophenyl)} pyrimidines. Eur J Med Chem 41(2):276–282
Goldberg HL, Finnerty RJ (1976) The comparative efficacy of buspirone and diazepam in the treatment of anxiety. Am J Psych 136(9):1184–1187
Kohlhoff SA, Sharma RI (2007) Expert Opin Invest Drugs 16(9):1441–1448
Locher HH, Schlunegger H, Hartman PG, Anghern P, Then RL (1996) Antibacterial activities of epiroprim, a new dihydrofolate reductase inhibitor, alone and in combination with dapsone. Antimicrob Agents Chemother 40:1376–1381
Morgan A, Cofer C, Stevens DL (2009) Iclaprim: a novel dihydrofolate reductase inhibitor for skin and soft tissue infections. Fut Microbiol 4(2):131–144
Mai A, Rotili D, Massa S, Brosch G, Simonetti G, Passariello C, Palamar AT (2007) Discovery of uracil-based histone deacetylase inhibitors able to reduce acquired antifungal resistance and trailing growth in Candida albicans. Bioorg Med Chem Lett 17(5):1221–1225
Aleem G, Sonali K, Michael AI, Jessica ET, Satyendra SS (2010) Synthesis and biological activity of N4-phenylsubstituted-6-(2,4-dichloro phenylmethyl)-7H-pyrrolo[2,3-d]pyrimidine-2,4-diamines as vascular endothelial growth factor receptor-2 inhibitors and antiangiogenic and antitumor agents. Bioorg Med Chem 18(10):3575–3587
Wang S, Meades C, Wood G, Osnowski A, Anderson S, Yuill R, Thomas M, Mezna M, Jackson W, Midgley C, Griffiths G, Fleming I, Green S, McNae I, Wu S, McInnes C, Zheleva D, Walkinshaw MD, Fischer PM (2004) 2-Anilino-4-(thiazol-5-yl)pyrimidine CDK inhibitors: synthesis, sar analysis, X-ray crystallography, and biological activity. J Med Chem 47(7):1662–1675
Brugel TA, Maier JA, Clark MP, Sabat M, Golebiowski A, Bookland RG, Laufersweiler MJ, Laughlin SK, VanRens JC, De B, Hsieh LC, Mekel MJ, Janusz MJ (2006) Development of N-2,4-pyrimidine-N-phenyl-N’-phenyl ureas as inhibitors of tumor necrosis factor alpha (TNF-alpha) synthesis Part 1. Bioorg Med Chem Lett. 16(13):3510–3513
Zimmermann J, Buchdunger E, Mett H, Meyer T, Lydon NB (1997) Potent and selective inhibitors of the abl-kinase: phenylamino-pyrimidine (PAP) derivatives. Bioorg Med Chem Lett 7(2):187–192
Bailey JP, Giles MB, Pass M. 2,4,6-trisubstituted pyrimidines as phosphotidylinositol (pi) 3-kinase inhibitors and their use in the treatment of cancer. WO 2006005914 A1, 2006
Bilodeau MT, Chua PC, Cosford NDP, Hoffman JM, Nagasawa J. WO 2005100344 A1, 2005
Srinivas ASSV, Tadiparthi R, Sharma GVR, Thirunavukkarasu S, Bhakiaraj DP, Kachhadia V, Narsimhan K, Thara SN, Rajagopal S, Reddy G, Narayanan S, Parameswaran V, Janarthanam V. US 20070167413 A1, 2007
Demetri GD, von Mehren M, Blanke CD (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. New Eng J Med 347(7):472–480
Peggs K, Mackinnon S (2003) Imatinib mesylate-the new gold standard for treatment of chronic myeloid leukemia. New Eng J Med 348(11):1048–1050
Vinaya K, Kavitha CV, Chandrappa S, Prasanna DS, Sateesh CR, Rangappa KS (2012) Synthesis and Antileukemic Activity of Novel 2-(4-(2,4-dimethoxybenzoyl)phenoxy)-1-(4-(3-(piperidin-4-yl)propyl)piperidin-1-yl) ethanone Derivatives. Chem Biol Drug Des 79(3):360–367
Vinaya K, Kavitha CV, Prasanna DS, Chandrappa S, Raghavan SC, Rangappa KS (2011) Synthesis and antileukemic activity of novel 4-(3-(piperidin-4-yl)propyl) piperidine derivatives. Chem Biol Drug Des 78(4):622–630
Vinaya K, Raja N, Ananda Kumar CS, Benaka Prasad SB, Chandrappa S, Ranganatha SR, Krishna V, Rangappa KS (2009) Evaluation of in vivo wound-healing potential of 2-[4-(2,4-dimethoxy-benzoyl)-phenoxy]-1-[4-(3-piperidin-4-yl-propyl)-piperidin-1-yl]-ethanone derivatives. Eur J Med Chem 44(8):3158–3165
Auerbach R, Kubai L, Knighton D, Folkman J (1974) A simple procedure for the long- term cultivation of chicken embryos. Dev Biol 41(2):391–394
Michael RG, Sambrook J (2012) Molecular cloning, a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, New York
Chang S, Yin SL, Wang J, Jing YK, Dong JH (2009) Design and synthesis of novel 2-phenylaminopyrimidine (PAP) derivatives and their antiproliferative effects in human chronic myeloid leukemia cells. Molecules 14(10):4166–4179
Weitensteiner SB, Liebl J, Krystof V, Havlicek L, Gucky T, Strnad M, Furst R, Vollmar AM, Zahler S (2013) Trisubstituted pyrazolopyrimidines as novel angiogenesis inhibitors. PLoS ONE 8(1):e54607
Miyazaki Y, Tang J, Maeda Y, Nakano M, Wang L, Nolte RT, Sato H, Sugai M, Okamoto Y, Truesdale AT, Hassler DF, Nartey EN, Patrick DR, Hoc ML, Ozawa K (2007) Orally active 4-amino-5-diarylurea-furo[2,3-d]pyrimidine derivatives as anti-angiogenic agent inhibiting VEGFR2 and Tie-2. Bioorg Med Chem Lett 17(6):1773–1778
Xiao-Yun W, Wen-Hua C, Shu-Guang W, Yuan-Xin T, Jia-Jie Z (2012) Pyrrolo[3,2-d]pyrimidine derivatives as type II kinase insert domain receptor (KDR) inhibitors: CoMFA and CoMSIA studies. Int J Mol Sci 13(2):2387–2404
Donnini S, Mont M, Castagnini C, Solito R, Botta M, Schenone S, Giachetti A, Ziche M (2006) Pyrazolo–pyrimidine-derived c-Src inhibitor reduces angiogenesis and survival of squamous carcinoma cells by suppressing vascular endothelial growth factor production and signaling. Int J Cancer 120(5):995–1004
Keri RS, Hosamani KM, Shingalapur RV, Hugar MH (2010) Analgesic, anti-pyretic and DNA cleavage studies of novel pyrimidine derivatives of coumarin moiety. Eur J Med Chem 45(6):2597–2605
Shamsuzzaman DAM, Yaseen Z, Alam K, Hussain A, Gatoo MA (2013) Steroidal pyrimidines: synthesis, characterization, molecular docking studies with DNA and in vitro cytotoxicity. J Mol Struct 1045(6):62–71
Authors’ contributions
VK, GKC and NDR performed experiments and VK, PDS and KP analyzed the results and prepared manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors are grateful to UGC, Govt. of India for financial support to V.K. under the UGC vide No. F. 39-810/2010 (SR) and the Principal, Government First Grade College, Kadur for the laboratory facilities to carry out this work successfully. V.K. acknowledges University Grants Commission, Government of India for a Raman Postdoctoral Fellowship for the year 2015–2016 (F No. 5-119/2016(IC)). PDS sincerely acknowleges Science and Engineering Research Board, Department of Science nd Technology, Government of India (No. YSS/2015/001930) for financial support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
All the authors consent to the publication.
Ethics approval and consent to participate
Not applicable.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kambappa, V., Chandrashekara, G.K., Rekha, N.D. et al. Synthesis, anti-angiogenic and DNA cleavage studies of novel N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)piperidine-4-carboxamide derivatives. Chemistry Central Journal 11, 122 (2017). https://doi.org/10.1186/s13065-017-0354-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-017-0354-5