Abstract
The reaction pathways of PCB-77 in the atmosphere with ·OH, O2, NO x , and 1O2 were inferred based on density functional theory calculations with the 6-31G* basis set. The structures the reactants, transition states, intermediates, and products were optimized. The energy barriers and reaction heats were obtained to determine the energetically favorable reaction pathways. To study the solvation effect, the energy barriers and reaction rates for PCB-77 with different polar and nonpolar solvents (cyclohexane, benzene, carbon tetrachloride, chloroform, acetone, dichloromethane, ethanol, methanol, acetonitrile, dimethylsulfoxide, and water) were calculated. The results showed that ·OH preferentially added to the C5 atom of PCB-77, which has no Cl atom substituent, to generate the intermediate IM5. This intermediate subsequently reacted with O2 via pathway A to generate IM5a, with an energy barrier of 7.27 kcal/mol and total reaction rate of 8.45 × 10−8 cm3/molecule s. Pathway B involved direct dehydrogenation of IM5 to produce the OH-PCBs intermediate IM5b, with an energy barrier of 28.49 kcal/mol and total reaction rate of 1.15 × 10−5 cm3/molecule s. The most likely degradation pathway of PCB-77 in the atmosphere is pathway A to produce IM5a. The solvation effect results showed that cyclohexane, carbon tetrachloride, and benzene could reduce the reaction energy barrier of pathway A. Among these solvents, the solvation effect of benzene was the largest, and could reduce the total reaction energy barrier by 25%. Cyclohexane, carbon tetrachloride, benzene, dichloromethane, acetone, and ethanol could increase the total reaction rate of pathway A. The increase in the reaction rate of pathway A with benzene was 8%. The effect of solvents on oxidative degradation of PCB-77 in the atmosphere is important.

The reaction pathways of PCB-77 in the atmosphere with •OH, O2, NOx, and 1O2 were inferred based on density functional theory calculations with the 6-31G* basis set. Different polar and nonpolar solvents: cyclohexane, benzene, carbon tetrachloride, chloroform, acetone, dichloromethane, ethanol, methanol, acetonitrile, dimethylsulfoxide, and water were selected to study the solvation effect on the favorable reaction pathways. The investigated results showed what kind of pathway was most likely to occur and the solvent effect on the reaction pathway
Similar content being viewed by others
Introduction
The pollution influence of PCBs
Polychlorinated biphenyls (PCBs) have been widely used as flame retardants, dielectric and heat transfer fluids, and plasticizers in industrial and household products [1]. As typical chlorinated organic pollutants, large quantities of PCB byproducts are released into the environment causing serious pollution and adverse impacts on ecosystems [2, 3]. Because of their volatility, persistence, bioaccumulation, and high toxicity, they have been listed by the United Nations Environment Programme as one of 12 kinds of persistent organic pollutants that need to be controlled [4]. The physical and chemical properties of PCBs are very stable. Once PCBs come into the environment, they remain in the environment for a long time and are very difficult to degrade (half-life is about 40 years) [5]. PCBs are not degraded by hydrolysis or similar reactions in the environment at a marked rate, and only a small fraction of PCBs are converted by photolysis in the soil and by photolysis of sunlight and ultraviolet light. Contaminated water and soil are also difficult to recover and have high residual in the environment. Under the stress of PCBs, the whole community structure of soil bacteria will change, which leads to the change of soil ecological environment quality [6]. Because of its lipophilic and hydrophobic characteristics, its long-term accumulation and the high bioaccumulation of some isoforms and congeners, PCBs have strong accumulation in the organism and are gradually enriched through the food chain. If the water contains 0.01 μg/L PCBs, its accumulation in the fish can reach 20 × 104 times of the concentration of water, and which in the body of birds and animals who eat fish will be higher. Some big fish in the sea and fierce birds in the air, such as sharks, seals and raptors, the concentration in their body can be 107–108 times higher than the surrounding environment [7]. The characteristics of the PCBs enable them to be migrated remotely and can be dispersed globally through the pathway of volatiles—atmospheric transport—sedimentation. From Antarctic penguins to the Arctic seals, they have been detected PCBs in their bodies. PCBs pollution has become a global problem. Related toxicological studies have shown that PCBs have an induced effect on lesions, or even concretization, of skin, liver, gastrointestinal system, nervous system, reproductive system, and immune system. Some homologues affect the reproduction of mammals and birds, and are potentially carcinogenic to human health. They are prone to accumulate in adipose tissue, causing brain, skin and visceral diseases and affect the nervous, reproductive and immune systems [8].
The industrial recycling options of PCBs
The PCBs are the platform upon which microelectronic components such as semiconductor chips and capacitors are mounted. PCBs provide the electrical interconnections between components and are found in virtually all electrical and electronic equipment. Every year, 20–50 million tons of waste electrical and electronic equipment (WEEE) are generated worldwide, which could bring large amount of waste PCBs. Recycling of waste PCBs is an important subject not only from the treatment for waste but also from the recovery of precious metal, The typical metals in PCBs consist of copper, iron, tin, nickel, lead, zinc, silver, gold, and palladium [9]. Due to its complex composition, PCBs recycling requires a multidisciplinary approach intended to separate fibers, metals and plastic fractions and reduce environmental pollution. Recycling process for waste PCBs includes three processes which is pretreatment, physical recycling, and chemical recycling. PCBs recycling generally start from the pretreatment stage, which include disassembly of the reusable and toxic parts and then PCBs are treated using physical recycling or chemical recycling process [10]. In general, the recycling methods of waste PCBs can be summarized as physical recycling methods and chemical recycling methods. Physical processing for the separating the metal fraction and non-metal fraction from waste PCBs includes shape separation, magnetic separation, electric conductivity-based separation, density-based separation and corona electrostatic separation. The chemical recycling methods include pyrolysis, gasification and combustion. Metal fraction can be treated by pyrometallurgical, hydrometallurgical or biotechnological process [11].
Industrial recovery of PCBs is indeed an environmentally friendly technology and 85% of the PCBs wastes produced by industry are to be recycled [12]. Furthermore, Large amount of the PCBs wastes were untreated wastes and exposed to wind and rain, easy to evaporate into the atmosphere or deposited into the soil, which can pose a serious threat to local water, air environment and human health, and lead to PCBs are not easy to be recycled.
The atmospheric degradation research status of PCBs
Pathways for transformation and removal of PCBs in the environment are a hot topic. Because PCBs degrade very slowly, they are now ubiquitous in air, water, soil, sediment, and biota. During transportation, PCBs can be removed and transformed through photolysis, wet and dry deposition, and chemical reactions with ·OH, NO3, Cl radicals, and O3. The radical ·OH is considered to be the main initiator for the removal of volatile organic compounds in the atmosphere [13,14,15]. The reaction with ·OH is considered the dominant removal pathway of PCBs from the atmosphere [16]. Rate constants for the gas phase reactions of 14 PCB congeners with ·OH have been measured from 323 to 363 K, the experimental data suggested that the more highly chlorinated PCBs would become progressively less reactive with OH radicals, which would increase their lifetimes compared to lower chlorinated PCBs [17]. Using the MPWB1K functional, Sun et al. [18] investigated the degradation process of PCB-47 with oxygen and nitrogen oxides, and found that the main degradation product of PCB-47 was glyoxal, the reaction rate with ·OH was 1.27 × 10−12 cm3/molecule s, and the half-life was 9.1 days. Lee et al. [19] studied the mechanisms for the formation of polychlorinated dibenzodioxin–OH adducts using density functional theory (DFT), and found that carbon atoms connected to oxygen atoms were the main sites of ·OH addition. Altarawneh et al. studied the atmospheric degradation of polychlorinated dibenzofurans initiated by ·OH addition and found a reaction rate of 2.70 × 10−11 cm3/molecule s. They suggested that the ·OH adduct immediately reacted with O2 to generate polychlorinated dibenzofuran–OH–O2 adducts [20]. Because of the reactivity of ·OH, most studies on the reactions of persistent organic pollutants with free radicals or reactive molecules in the atmosphere have focused on ·OH [21]. Because of the complexity of environmental conditions, it is difficult to use computational and theoretical chemistry to accurately evaluate environmental effects, and particularly the effect of solvents. More complete reaction mechanisms for oxidative degradation induced by ·OH, and studies of the effects of different solvents on the reaction mechanisms are required.
Research content
Quantitative structure activity relationship studies have shown that PCBs with meta- and para-chlorine substituents are the most similar to dioxins among the PCBs. Toxicological studies have shown that PCBs that are not coplanar or ortho-substituted, such as the 14 dioxin like PCBs (congener numbers 77, 81, 105, 114, 118, 123, 126, 156, 157, 169, 170, 180, and 189) have high reactivity and toxicity [22]. In the present study, PCB-77 (3,3′,4,4′-Tetrachlorobiphenyl), which has two meta- and two para-chlorine substituents, was selected for study by quantum chemistry calculations using DFT. The results were used to predict the degradation reactions of PCBs with the common oxidants ·OH, O2, NO x , and 1O2. The intermediates and transition states in the reactions were predicted, and the rate constants were calculated by transition state theory (TST). The effects of different solvents on the reaction pathways were studied based on the solvation effect.
Computational methods
The Gaussian 09 program [23] was used to perform all calculations. The degradation reactions of PCB-77 were optimized for all reactants, transition states, intermediates, and products. The optimal structure was obtained using DFT at the B3LYP/6-31G*level. This method has yielded satisfying results in previous research [24, 25]. The vibrational frequencies were calculated at the same level, and showed that the transition state has only one imaginary frequency, and the intermediate for each transition state has no imaginary frequency. The intrinsic reaction coordinate was calculated to identify the connections between reactants, transition states, and products. To obtain more accurate Gibbs free energies, the basis set of 6-311G(2df, p) was used with the same method to calculate the single point energies based on the optimal material configuration, taking into consideration the zero point energy correction. The reaction rates at 298 K and standard atmospheric pressure were calculated using transition state theory (TST), taking into consideration the tunneling effect, with KiSTheIP software [26]. The polarizable continuum model (PCM) was used to optimize the reaction parameters of the intermediates and transition states of PCB-77 degradation, and analyze the effect of solvation. PCM is widely used in studies of the solvation effect as the classical quantum chemical calculation method [27]. Many studies show that the results of PCM can be directly compared with the outcome of the experimental measurements,and the results are in good agreement with the experimental values [28,29,30]. To eliminate systematic error between the theoretical and experimental values, the calculations were multiplied by a calibration factor of 0.960 [31].
Results and discussion
The reaction of PCB-77 with ·OH
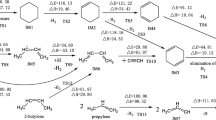
Because of the large proportion of oxygen in the atmosphere, ·OH is considered to be the most important atmospheric oxidant. This ·OH can react with environmental pollutants via either ·OH addition or H abstraction. The possible pathways for the reactions of PCB-77 with ·OH radicals are shown in Figs. 2 and 3. PCB-77 (Fig. 1) shows C1 symmetry, and contains six different carbon atoms that could be sites for ·OH addition. Among the six transition states for these carbon atoms (TS1–TS6, Fig. 2a), TS3 and TS4 present the highest energy barriers to ·OH addition. This can be attributed to the steric hindrance of the chlorine atom, which can block ·OH addition to C3 and C4. The energy barrier for TS1 is much higher than those for TS2, TS5, and TS6, mainly because of the position of C1 and steric hindrance from the benzene ring. The energy barriers for ·OH addition to C2, C5, and C6 are relatively low, and ·OH addition is likely to occur at these sites. Among the possible pathways, addition of ·OH to the C5 atom to form the intermediate IM5 is the most favorable pathway with the lowest energy barrier (0.439 kcal/mol), and is exothermic (21.52 kcal/mol). Three direct H abstraction pathways were identified (Fig. 2b). These were H atom abstraction from the C2–H, C5–H, and C6–H bonds. Formation of IM8 is the most favorable pathway with the lowest energy barrier (4.68 kcal/mol), and is exothermic (5.76 kcal/mol). These results confirm that the C5 atom is more reactive than the other carbon atoms. Consequently, IM5 and IM8 were selected for further investigation. When the ·OH addition and H abstraction were compared, the H abstraction reaction had a higher energy barrier than the addition reaction, and was less exothermic. PCB-77 preferentially occurred addiction reaction with OH, which is consistent with the results of Chen et al [32,33,34]. The Optimization structure of transition state (TS1–TS9) as shown in Fig. 3.
Continuous addition reaction pathway of IM5 and O2
The addition reaction of PCB-77 and ·OH to produce the reactive intermediate IM5 can be followed by two pathways (Fig. 4a). Path A involves direct addition of O2 to IM5 to generate IM5a. Path B involves H abstraction of IM5 to generate OH-PCBs (IM5b). The energy barrier for O2 addition was 7.27 kcal/mol, and the reaction was exothermic (29.5 kcal/mol). The energy barrier for direct H abstraction was 28.49 kcal/mol, and the reaction was exothermic (33.30 kcal/mol). Therefore, under these conditions, intermediate IM5 easily reacts with O2 to form IM5a. IM5a contains peroxy radicals, is strongly reactive, and will react with other substances in the air. Because NO is a free radical with a single electron, it can participate in many free radical reactions [35]. The reaction of IM5a and NO was evaluated (Fig. 4b). The intermediate IM5a1 was obtained via direct NO addition to the peroxy radical, and this reaction was barrierless and exothermic (18.19 kcal/mol). Single molecule decomposition of IM5a1 generates IM5a2 and NO2, with an energy barrier of 23.20 kcal/mol in an exothermic reaction (2.60 kcal/mol). IM5a2 can generate products P5a1 and P5a2 by bimolecular reaction and self-decomposition. IM5a2 can generate diol product P5a1 and ·OH, with an energy barrier of 7.71 kcal/mol in an exothermic reaction (10.60 kcal/mol). The newly produced ·OH initiates a new round of PCB degradation. This phenomenon has been found in the atmospheric oxidation of polychlorinated dibenzodioxins/furans in the presence of water vapor [36]. The ring-opening reaction of IM5a2 to produce P5a2 occurs by cleavage of the C4–C5 bond, and the oxygen free radicals are converted to double bonds. The energy barrier for this reaction is 1.13 kcal/mol, and it is exothermic (25.60 kcal/mol). Comparison of the two pathways indicated that IM5a2 was more prone to self-decomposition. The Optimization structure of transition state (TS5a–TS5a5) as shown in Fig. 4c.
Addition reaction pathway of IM8
IM8 is strongly reactive with a single electron, and can react with larger molecules or radicals in the atmosphere. A possible reaction pathway is shown in Fig. 5a. The addition reactions of IM8 with ·OH, O2, and NO2 are barrierless and exothermic (43.23, 21.09, and 23.10 kcal/mol, respectively). The addition reaction of IM8 with ·OH also produces IM5b. The reaction of IM8 with O2 generates IM8a, which contains the dioxygen radical. The reaction pathway is similar to that for IM5a in the atmosphere, and finally generates IM5b. Addition of NO2 produces P8b. The Optimization structure of transition state (TS8a1, TS8a2) as shown in Fig. 5b.
Addition pathway of intermediate IM5b with singlet oxygen (1O2)
Reactions of IM5 and IM8 in the atmosphere can generate IM5b, which is hydroxylated PCBs. OH-PCBs have been widely detected in the abiotic environment in air, rain, and snow. These compounds have potential estrogenic activity, thyroid effects in vitro and in vivo, and are toxic [37, 38]. 1O2 is an important reactive oxygen species. The electron configuration of its molecular orbital is different from ground state oxygen. 1O2 is a form of excited state molecular oxygen, and can easily react with unsaturated compounds because of its high activity. Studies have found that chlorophenol and 1O2 reactions are the main contributors to decomposition [39]. We deduced an addition reaction pathway of IM5b with 1O2. Generally, the following four principal types of oxygen addition reactions that produce aromatic and unsaturated compounds are recognized [40]: (1) 1,3-addition to a double bond connected to a hydrogen-carrying group to form allylic hydroperoxides; (2) 1,4-cycloaddition to a system containing at least two conjugated double bonds to form 1,4-peroxides; (3) [p2 + p2] 1,2-cycloaddition to an isolated double bond to form 1,2-peroxides; and (4) 1,4-addition to phenols and naphthols to form hydroperoxide ketones.
Between IM5b and singlet oxygen, these four types of reactions would, in principle, occur in a similar way (Fig. 6). The optimal structures and thermodynamic properties of reactants, transition states, and products of the 12 reaction paths of IM5b with 1O2 were calculated. For each reaction pathway, the Gibbs free energy change (∆G) was calculated (Table 1). Reactions a1, a2, c1, c2, c3, c4, and d1 all had negative ∆G, and could be spontaneous. The ∆G of reactions b1, b2, b3, c5, and c6 were positive. For the reaction of 2, 4-chlorophenesic acid with 1O2, the ∆G of reaction pathways a and d were negative and those of reaction pathways b and c were positive [41]. In contrast to the results of Song et al. in this paper, the ∆G of pathways c1–c4 were negative. This could be because IM5b has meta- and para-chlorine substituents, whereas the 2,4-dichlorophenol studied by Song et al. has ortho- and para-chlorine substituents. The meta-chlorine atoms could change the benzene ring electron distribution and reduce the stability of the benzene ring. This could mean the addition reaction could occur, and 1O2 could add to the double bond of the benzene ring so that the reaction pathway of c1–c4 could be spontaneous. The reaction pathway of c5 and c6 cannot be spontaneous, because the C1 atom is attached to two benzene rings, and it is not easy for the carbon in this position to react because of conjugation. However, the thermodynamic study only suggests this is reaction possible, the reaction mechanism still needs to be confirmed by dynamics calculations. Next, we calculated the reaction energy barriers (Table 2) and determined the optimal pathway.
The energy barrier of reaction pathway a2 was the smallest (0.88 kcal/mol), and when the energy barriers of reaction pathways a, c, and d were compared, the energy barrier for pathway a was the smallest. Therefore, IM5b and 1O2 are more likely to react in a substitution reaction. 1O2 is preferentially added to the ortho carbon atom, which does not have chlorine substituents. From ·OH, the hydrogen atom migrates to the peroxide group, and the addition of oxygen is carried out at the same time as hydrogen extraction. The pathway for a2 in the atmosphere is shown in Fig. 7a. Because of the lack of stability of ·OOH, the C2–C3 bond was broken into intermediate IMa2 by an open loop reaction with an energy barrier of 1.65 kcal/mol. This reaction is exothermic (20.14 kcal/mol). IMa2 can produce ·OH and aldehyde product Pa2 by monomolecular decomposition with an energy barrier of 2.57 kcal/mol in an exothermic reaction (18.95 kcal/mol). The Optimization structure of transition states as shown in Fig. 7b.
After the addition of ·OH, PCB-77 could degrade via pathway A or B. The energy barrier of pathway A is smaller than that of pathway B (Fig. 8). Therefore, PCB-77 preferentially degrades via pathway A in the atmosphere.
Solvation effect of degradation reaction pathway A
The effect of solvation on the reaction energy barrier
From the above study, we can know that the pathway A is the most likely pathway for degradation of PCB-77 in the atmosphere. The solvent has some influence on the reaction and the parameters of the solute, that is, the reaction pathway can be affected by the solvent. We investigated four polar solvents (cyclohexane, benzene, carbon tetrachloride, and chloroform) and seven non-polar solvents (acetone, dichloromethane, ethanol, methanol, acetonitrile, dimethyl sulfoxide, and water). The PCM was used to study the effects of these different solvents on pathway A. Comparison of the reaction energy barriers with the different solvents (Table 3) showed that if the dielectric constant of the solvent was less than five, such as with cyclohexane, carbon tetrachloride, benzene, and chloroform, the energy barrier of was reduced compared to that in the atmosphere. Therefore, PCB-77 is more likely to react with these four solvents than under standard atmospheric conditions, and these solvents could be used to promote this degradation reaction. Among these solvents, benzene could reduce the total reaction energy barrier by 25% compared to that under standard atmospheric conditions. For the solvents with dielectric constants greater than five (dichloromethane, acetone, ethanol, methanol, acetonitrile, dimethyl sulfoxide, and water), the energy barriers were higher than those under standard atmospheric conditions. Therefore, the presence of these solvents would inhibit the reaction. The increase in the dielectric constant would increase interactions between the molecules and change bond lengths [42]. As the dielectric constant increases, it becomes more difficult for PCB-77 to react with the free radicals, and the energy barrier increases because the energy required for bond breaking or formation increases.
The effect of solvation on the degradation rate
In this study, the reaction rate constants (k) of pathways A and B at 298 K and standard atmospheric pressure were calculated using TST and taking into consideration the tunneling effect. The total reaction rates of pathways A and B were 8.45 × 10−8 and 1.15 × 10−5 cm3/molecule s, respectively. Pathway A is the most likely reaction path, so we then calculated the rate constants for each branch of this pathway, and obtained the Arrhenius equation in the temperature range 238–357 K (Table 4). The reaction rate of PCB-77 with ·OH was 6.67 × 10−12 cm3/molecule s. Our results cannot be compared with experimental values because of a lack of experimental data. Instead, we compared the rate constants with those of some congeners. A previous study found that the rate constants of PCB-29 (2,4,5-trichlorobiphenyl), PCB-31 (2,4′,5-trichlorobiphenyl), PCB-41 (2,2′,3,5′-tetrachlorobiphenyl) and PCB-47 (2,2′,4,4′-tetrachlorobiphenyl) with ·OH in the atmosphere were 1.3 × 10−12, 1.2 × 10−12, 0.8 × 10−12, and 1.0 × 10−12 cm3/molecule s at 298 K, respectively [17]. Considering the effect of the degree of chlorine substitution, we think our results agree with the results for these congeners. According to the deduced Arrhenius equation, the reaction rate constant of each pathway increases with increasing temperature. Table 4 shows that the reaction rate for the conversion of IM5a2 to IM5a4 is the fastest among the reactions in this pathway, and this step plays a key role in determining the overall reaction rate. Therefore, if you want to enhance the overall reaction rate, the reaction conditions that affect the pathway should be changed first.
Different solvents can change the degree of difficulty of a reaction (Table 3). To explore whether the solvent could affect the reaction process and the reaction rates were calculated using different solvents (Table 5). The results suggested the dielectric constants of different solvents would affect the rates of all steps of the reaction path. The reaction rate of the conversion of IM5a2 to IM5a4 increased in cyclohexane, carbon tetrachloride, benzene, dichloromethane, acetone, and ethanol, and this increased the overall reaction rate. Benzene could increase the overall reaction rate by 8% compared to that under standard atmospheric conditions. At the same time, cyclohexane, carbon tetrachloride, and benzene could reduce the energy barrier to improve the reaction rate. Therefore, the reaction conditions can be adjusted to enhance the reaction.
Conclusions
In this paper, the degradation processes of PCB-77 with ·OH, NO x , O2, and 1O2 in the atmosphere were evaluated using theoretical calculations. By comparing the energy barriers, the optimal degradation reaction pathway with the minimum energy barrier was selected. The reaction rate of each pathway was calculated by TST, and the effects of different solvents on the reaction pathway were studied based on the solvation effect. We reached the following conclusions:
-
1.
PCB-77 can react with ·OH in ·OH addition and H abstraction reactions in the atmosphere, and ·OH addition is dominant. The ·OH is added to the carbon atoms without substituents. After the addition reaction with ·OH, the reaction can diverge into pathway A or B. The energy barrier of pathway A is lower than that of pathway B, and it is more likely to occur. Most of the pathways in the reaction will produce OH-PCBs, and the OH-PCBs can easily react with singlet oxygen (1O2). This reaction occurs via one of the three types (a, c, and d) of the four principal types of oxygen addition reactions to aromatic and unsaturated compounds.
-
2.
In the atmosphere, the reaction rates of each step of pathway A increase with increasing temperature, and the final step of the whole reaction process is conversion of IM5a2 to IM5a4.
-
3.
The reaction energy barrier and reaction rate of optimal pathway A are affected by changes in the solvent. Among the investigated solvents, cyclohexane, carbon tetrachloride, and benzene can reduce the reaction energy barrier, and cyclohexane, carbon tetrachloride, benzene, dichloromethane, acetone, and ethanol can improve the reaction rate. The largest reduction in the energy barrier and increase in the reaction rate were obtained with benzene.
References
Bedard DL (2004) Polychlorinated biphenyls in aquatic sediments: environmental fate and outlook for biological treatment. Springer, New York
Guvenius DM, Hassanzadeh P, Bergman A (2002) Metabolites of polychlorinated biphenyls in human liver and adipose tissue. Environ Toxicol Chem 21:2264–2269
Hites RA (2006) Persistent organic pollutants in the Great Lakes. Springer, Berlin
Zhang P, Song JM, Liu ZG, Zheng GX, Zhang NX, He ZP (2007) PCBs and its coupling with eco-environments in Southern Yellow Sea surface sediments. Mar Pollut Bull 54:1105–1115
Lammel G, Ghim YS, Grados A, Gao H, Hühnerfuss H (2007) Levels of persistent organic pollutants in air in China and over the Yellow Sea. Atmos Environ 41:452–464
Cogliano VJ (1998) Assessing the cancer risk from environmental PCBs. Environ Health Perspect 106:317–323
Fiandanese N, Borromeo V, Berrini A, Fischer B, Schaedlich K (2016) Maternal exposure to a mixture of di(2-ethylhexyl) phthalate (DEHP) and polychlorinated biphenyls (PCBs) causes reproductive dysfunction in adult male mouse offspring. Reprod Toxicol 65:123–132
Shiraishi F, Okumura T, Nomachi M, Serizawa S, Nishikawa J (2003) Estrogenic and thyroid hormone activity of a series of hydroxy-polychlorinated biphenyls. Chemosphere 52:33–42
He W, Li G, Ma X, Wang H, Huang J, Xu M, Huang C (2006) WEEE recovery strategies and the WEEE treatment status in China. J Hazard Mater 136:502–512
Sohaili J, Muniyandi SK, Mohamad SS (2011) A review on potential reuse of recovered nonmetallic printed circuit board waste. J Emerg Trends Eng Appl Sci 2:946–951
Cui J, Zhang L (2008) Metallurgical recovery of metals from electronic waste: a review. J Hazard Mater 158:228–256
Goosey M, Kellner R (2003) Recycling technologies for the treatment of end of life printed circuit boards (PCBs). Circuit World 29:33–37
Xing Y, Lu Y, Dawson RW, Shi Y, Zhang H (2005) A spatial temporal assessment of pollution from PCBs in China. Chemosphere 60:731–739
And DE, Pilling MJ (2003) Measurement of OH and HO2 in the Troposphere. Chem Rev 103:5163–5198
Archibald AT, Cooke MC, Utembe SR (2010) Impacts of mechanistic changes on HOx formation and recycling in the oxidation of isoprene. Atmos Chem Phys 10:8097–8118
Totten LA, Eisenreich SJ, Brunciak PA (2002) Evidence for destruction of PCBs by the OH radical in urban atmospheres. Chemosphere 47:735–746
Anderson PN, Hites RA (1996) OH radical reactions: the major removal pathway for polychlorinated biphenyls from the atmosphere. Environ Sci Technol 30:1756–1763
Sun Y, Zhang Q, Wang H (2015) Quantum chemical investigation on the mechanism and kinetics of OH radical-initiated atmospheric oxidation of PCB-47. Chemosphere 133:53–60
Lee JE, Choi W, Mhin BJ (2011) Theoretical study on the reaction of oh radicals with polychlorinated dibenzo-p-dioxins. J Phys Chem A 108:607–614
Altarawneh M, Kennedy EM, Dlugogorski BZ (2008) Computational study of the oxidation and decomposition of dibenzofuran under atmospheric conditions. J Phys Chem A 112:6960–6967
Wang YJ, Huang XS (2012) Mapping the compositions of zinc tantalate for optimum photocatalytic performance in degradation of organic pollutants. Chin J Struct Chem 31:1271–1280
Ross C (2004) The public healthy implications of polychlorinated biphenyls (PCBs) in the environment. Ecotoxicol Environ Safe 59:275–291
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE Jr, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision E.01. Gaussian Inc., Wallingford
Han XY, Wang ZY, Zhai ZC (2006) Estimation of n-octanol/water partition coefficients (Kow) of all PCB congeners by Ab initio and a Cl substitution position method. QSAR Comb Sci 25:333–341
Xie YJ, Liu HX, Wang ZY (2008) Prediction of aqueous solubility for 209 polychlorinated diphenyl ethers from molecular structural parameters by DFT method. Chin J Struct Chem 27:481–490
Canneaux S, Bohr F, Henon E (2014) KiSThelP: a program to predict thermodynamic properties and rate constants from quantum chemistry results. J Comput Chem 35:82–93
Mennucci B, Tomasi JR, Cammi R (2002) Polarizable continuum model (PCM) calculations of solvent effects on optical rotations of chiral molecules. J Phys Chem A 106:6102–6113
Zhang XH, Wang KT, Niu T (2015) Theoretical study on reaction mechanism of an N-heterocyclic carbene boryl azide with electron-deficient alkynes and nitriles. Struct Chem 26:599–606
Benchouk W, Mekelleche SM, Silvi B et al (2011) Understanding the kinetic solvent effects on the 1,3-dipolar cycloaddition of benzonitrile N-oxide: a DFT study. J Phys Org Chem 24:611–618
Ray PC (2004) Remarkable solvent effects on first hyperpolarizabilities of zwitterionic merocyanine dyes: ab initio TD-DFT/PCM approach. Chem Phys Lett 395:269–273
Computational chemistry comparison and benchmark dataBase (CCCBDB). http://cccbdb.nist.gov/vibscalejust.asp
Zhou J, Chen JW, Liang CH, Xie Q, Wang YN, Zhang S et al (2011) Quantum chemical investigation on the mechanismand kinetics of PBDE photooxidation by ·OH: a case study for BDE-15. Environ Sci Technol 45:4839–4845
Cao HJ, He MX, Han DD, Sun YH, Zhao SF, Ma HJ et al (2012) Mechanistic and kinetic study on the reaction of 2,4-dibrominated diphenyl ether (BDE-7) with OH radicals. Comput Theor Chem. 983:31–37
Wang S, Hao C, Gao ZX, Chen JW, Qiu JS (2012) Effects of excited-state structures and properties on photochemical degradation of polybrominated diphenyl ethers: a TDDFT study. Chemosphere 88:33–38
Cheng XL, Zhao YY, Li F, Wu RT (2008) Theoretical study of reaction mechanism of 1-propenyl radical with NO. Chin J Chem Phys 21:239–244
Sun X, Zhang C, Zhao Y (2012) Atmospheric chemical reactions of 2, 3, 7, 8-tetrachlorinated dibenzofuran initiated by an OH radical: mechanism and kinetics study. Environ Sci Technol 46:8148–8155
Jansen HT, Cooke PS, Porcelli J (1993) Estrogenic and antiestrogenic actions of PCBs in the femalrat: in vitro and in vivo studies. Reprod Toxicol 7:237–248
Ueno D, Darling C, Alaee M (2007) Detection of hydroxylated polychlorinated biphenyls (OH-PCBs) in the abiotic environment: surface water and precipitation from Ontario, Cananda. Environ Sci Technol 41:1841–1848
Cui C, Song Q, Wang H (2010) Theoretical study of the reaction of chlorophenols with 1O2 in gas phase and aqueous solutions. Comput Appl Chem 27:319–323
Matxain JM, Ristilä M, Strid A, Eriksson LA (2007) Theoretical study of the reaction of vitamin B6 with 1O2. Chemistry 13:4636–4642
Song Q, Niu T, Wang H (2008) Theoretical study of the reaction of 2,4-dichlorophenol with 1O2. J Mol Struc Thochem 61:27–32
Chen Y, Li Y (2016) QSAR model-assisted prediction of largest ultraviolet absorption wavelength of PCBs based on solvent effects. Chem Lett 45:1453–1456
Authors’ contributions
MLX responsible for the calculation of data and the preparation of the article. JWY is responsible for auxiliary calculation. YL is responsible for modifying the article. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Funding
Fundamental Research Funds for the Central Universities in 2013 (JB2013146) and the Key Projects in the National Science & Technology Pillar Program in the Eleventh five-Year Plan Period (2008BAC43B01).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xin, ML., Yang, JW. & Li, Y. The mechanism for enhanced oxidation degradation of dioxin-like PCBs (PCB-77) in the atmosphere by the solvation effect. Chemistry Central Journal 11, 61 (2017). https://doi.org/10.1186/s13065-017-0291-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-017-0291-3