Abstract
Background
The overproduction of nitric oxide (NO) is known to involve in various inflammatory processes. A methanol extract of the tubers of Ophiopogon japonicus was found to strongly inhibit NO production. The present paper deals with the isolation, structural identification and NO inhibitory effect of five compounds isolated from the MeOH extract of O. japonicus tubers.
Results
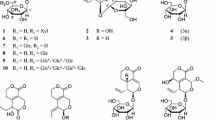
Three new compounds were elucidated to be (2R)-(4-methoxybenzyl)-5,7-dimethyl-6-hydroxyl-2,3-dihydrobenzofuran (1), 2-(2-hydroxyl-4-methoxy-benzyl)-5-methyl-6-methoxyl-2,3-dihydrobenzofuran (2), and 2-(4-hydroxy-benzyl)-5,6-dihydroxybenzofuran (3). In addition, two known compounds were isolated from a natural source for the first time including 2-(4-methoxy-benzyl)-6,7-dimethoxyl-2,3-dihydrobenzofuran (4), and 2-(4-methoxy-benzyl)-6,7-methylenedioxy-2,3-dihydrobenzofuran (5). The absolute configuration of compound 1 was determined by experimental and calculated circular dichroism spectra. The effects of the isolated compounds on LPS-induced NO production in RAW264.7 cells were evaluated. Compound 1 and 2 showed the inhibitory activity with IC50 values of 11.4 and 29.1 μM, respectively.
Conclusions
The class of 2-benzyl-2,3-dihydrobenzofuran is uncommon in nature. In this work, three such compounds were isolated from O. japonicus. Two of them showed promising anti-inflammatory activity by inhibition of NO production.
Similar content being viewed by others
Background
Ophiopogon japonicus (L.f) Ker-Gawl (Convallariaceae) occurs widely in Vietnam and it has been used in traditional medicine to treat cough, fever, epistaxis, inflammation, respiratory disease, constipation, and gastrointestinal disorders [1]. Steroidal saponins are among the main characteristic components of O. japonicus and have anticancer, anti-inflammatory, antioxidative, and neuritogenic effects [2–4]. Homoisoflavonoids are also characteristic of O. japonicus and possess anti-inflammatory, antioxidative, and cytotoxic activities [4–8]. O. japonicus is also rich in polysaccharides that have antidiabetic, antioxidative, anti-inflammatory, and immunomodulatory properties [4, 7]. In addition, phenolic acids, sesquiterpenes, fatty acids, and lignans have been identified from O. japonicus [4, 9, 10].
Nitric oxide (NO) is produced by inducible nitric oxide synthase (iNOS) in macrophages, hepatocytes, and renal cells. When produced in excess, NO directly damages normal tissues and triggers inflammation. Therefore, inhibitors of NO production have potential therapeutic value as anti-inflammatory agents [11]. In our search for anti-inflammatory compounds from natural sources, a methanol (MeOH) extract of the tubers of O. japonicus was found to strongly inhibit NO production. Phytochemical fractionation of the CHCl3-soluble fraction of the MeOH extract led to the isolation of five 2-benzyl benzofurans, including three new (1–3) and two known (4, 5) compounds (Fig. 1). Compound 1 strongly inhibited NO production in lipopolysaccharide (LPS)-induced RAW264.7 cells.
Results and discussion
Compound 1 was obtained as a brown solid. Its molecular formula was determined to be C18H20O3 from high-resolution electrospray ionisation mass spectrometry (HRESIMS) (m/z 283.1365 [M − H]−). Its 1H NMR spectrum showed the characteristic resonance of an AA′BB′ aromatic ring [δH 7.19 (2H, d, J = 8.5 Hz, H-2′, 6′), and 6.86 (2H, d, J = 8.5 Hz, H-3′, 5′)], one aromatic singlet [δH 6.67 (1H, s, H-4)], one oxygenated methine proton [δH 4.86 (partially overlapped with HDO signal, H-2)], one methoxyl group [δH 3.78 (3H, s, 4′-OMe)], two methylene groups [δH 3.07 (1H, dd, J = 15.0, 8.5 Hz, Ha-3), 2.82 (1H, dd, J = 15.0, 7.5 Hz, Hb-3), 3.02 (1H, dd, J = 14.0, 7.0 Hz, Ha-7′), 2.84 (1H, dd, J = 14.0, 6.5 Hz, Ha-7′)] and two aromatic methyl groups [δH 2.12 (3H, s, Me-5) and 2.05 (3H, s, Me-7)] (Table 1). The 13C NMR and DEPT spectra revealed the presence of two methyl carbons at δC 9.2 (7-Me) and 16.5 (5-Me), two methylene carbons at δC 35.9 (C-3) and 42.0 (C-7′), one methoxy carbon at δC 55.7 (4′-OMe), one oxygenated methine carbon at δC 85.1 (C-2), five methine carbons at δC 123.9 (C-4), 131.4 (C-2′, 6′), and 114.7 (C-3′, 5′), and seven quaternary carbons at δC 153.8 (C-6, observed from HMBC spectrum), 158.1 (C-7a), and 159.8 (C-4′), 118.0 (C-3a), 117.3 (C-5), 108.1 (C-7), and 131.2 (C-1′) (Table 2). These data suggested the 2-benzyl-2,3-dihydrobenzofuran skeleton of 1 [12, 13]. The HMBC correlations from aromatic singlet H-4 to C-3, C-3a, C-5, C6, C-7a, and from Me-5 to C-4, C-5, C-6, as well as from Me-7 to C-6, C-7, C-7a indicated the presence of a dihydrobenzofuran skeleton with a hydroxyl group located at C-6 and two methyl groups located at C-5 and C-7. The methoxyl group was placed on C-4′ based on the HMBC correlation of the proton of this group with C-4′ (Fig. 2). From these data, 1 was identified as 2-(4-methoxybenzyl)-5,7-dimethyl-6-hydroxyl-2,3-dihydrobenzofuran. The quantum chemical electronic circular dichroism (ECD) calculation method, based on time-dependent density functional theory (TDDFT), was used to determine of the absolute configuration at C-2 [14]. The predicted ECD patterns for 2R were consistent with the experimentally measured ECD of 1 (Fig. 3). Thus, compound 1 was assigned as (2R)-(4-methoxybenzyl)-5,7-dimethyl-6-hydroxyl-2,3-dihydrobenzofuran.
Compound 2 was obtained as a brown solid. Analysis of the HRESIMS spectrum indicated that compound 2 has molecular formula C18H20O4 (m/z 301.1436 [M + H]+). The 1H NMR spectrum of 2 showed the presence of two aromatic singlets (δH 6.87 and 6.39), an aromatic ABX spin system [δH 6.48 (1H, d, J = 2.5 Hz, H-3′), 6.43 (1H, dd, J = 8.0, 2.5 Hz, H-5′), and 6.98 (1H, d, J = 8.0 Hz, H-6′)], one aromatic methyl (δH 2.10) and two methoxy groups (δH 3.75 and 3.76). The 13C NMR and DEPT spectra of 2 showed the characteristics of 2-benzyl-2,3-dihydrobenzofuran, which are similar to those of 1 with one oxygenated methine carbon at δC 85.8 (C-2) and two methylenes at 34.0 (C-3) and 36.9 (C-7) (Table 2). The HMBC correlations confirmed the positions of the methyl and methoxy groups (Fig. 2). In a test of the optical rotation, this compound was optically inactive. Therefore, the structure of compound 2 was determined to be 2-(2-hydroxyl-4-methoxy-benzyl)-5-methyl-6-methoxyl-2,3-dihydrobenzofuran.
Compound 3 was obtained as a brown solid. Its HRESIMS revealed a molecular ion peak at m/z 255.0632 [M − H]− corresponding to a molecular formula of C15H12O4. The 1H NMR spectrum showed signals due to an olefinic proton [δH 6.16 (1H, s)], two aromatic protons [δH 6.82 (1H, s), and 6.83 (1H, s)], a 1,4-disubstituted benzene ring with two apparent doublets [δH 7.10 (2H, d, J = 8.5 Hz), 6.74 (2H, d, J = 8.5 Hz)], and a methylene group [δH 3.91 (2H, s)]. The 13C NMR and DEPT experiments showed one methylene, seven methines, five oxygenated aromatic carbons, and two quaternary carbons. In the HMBC spectrum, the correlations between the olefinic proton (δH 6.16, H-3) and C-3a (δC 122.0), C-4 (δC 106.0), and C-7a (δC 150.7), H-4/C-3 (δC 103.5), C-3a, C-5 (δC 143.1), C-6 (δC 144.3), and C-7a, as well as H-7/C-3a, C-5, C-6, and C-7a indicated the presence of a benzofuran skeleton with two oxygen substituents on ring A (Fig. 2). Similarly, the correlations between the 1,4-disubstituted protons (δH 7.10, H-2′,6′) and the oxygenated aromatic carbon (δC 157.0) and the methylene carbon (δC 34.8) indicated the presence of a 4-hydroxybenzyl group. Moreover, the HMBC correlations of the methylene protons (δH 3.19, H-7′) with C-2, C-3, and C-4 suggested the location of the 4-hydroxy-benzyl group at C-2 (Additional file 1). This compound was also optically inactive. Therefore, the structure of 3 was assigned as 2-(4-hydroxy-benzyl)-5,6-dihydroxybenzofuran.
Compounds 4 and 5 were identified as 2-(4-methoxy-benzyl)-6,7-dimethoxyl-2,3-dihydrobenzofuran, and 2-(4-methoxy-benzyl)-6,7-methylenedioxy-2,3-dihydrobenzofuran, respectively [12]. Both compounds were isolated for the first time from the nature. It is noted that the class of 2-benzyl-2,3-dihydrobenzofuran is uncommon and only a few compounds of this type have been isolated so far from natural source [15].
The isolated compounds were tested for their ability to inhibit NO production in LPS-stimulated RAW264.7 cells. NO is an important signalling molecule in various physiological and pathophysiological responses, including the circulation, blood pressure, platelet function, and host defence. The overproduction of NO is also important in inflammation and related processes [16]. High NO levels are used as a marker for the treatment of inflammatory disorders. According to its specific functions and characteristics, inhibition of NO production by immune cells, typically macrophages, is suggested as one strategy for the development of anti-inflammatory agents. Our test revealed that compounds 1 and 2 reduced NO levels in LPS-stimulated RAW264.7 cells. Compound 1 was the most active compound with an IC50 of 11.4 μM, while compound 2 had a moderate effect (IC50 = 29.1 μM). Compounds 3–5 were inactive up to the highest concentration tested (30 μM). The cell viability test showed that neither compound had significant toxicity at its effective dose for NO inhibition (data not shown). 2,3-Dihydrobenzofurans have been known as potent anti-inflammatory compounds. Closse et al. [17] demonstrated that the synthetic analogues of 2,3-dihydrobenzofuran-2-one had powerful anti-inflammatory activity in vivo, and 5-chloro-6-cyclohexyl-2,3-dihydrobenzofuran-2-one was significantly more potent than the reference compound, diclofenac, in all testing models. More recently, a series of dihydrobenzofurans was isolated from the seeds of Prunus tomentosa, some of which strongly inhibited NO production in LPS-stimulated BV-2 cells [18]. Consistently, our results suggest that O. japonicus is a potential natural source of anti-inflammatory dihydrobenzofurans.
Methods
General experimental procedures
Optical rotation values were recorded on a JASCO P-2000 digital polarimeter (JASCO, Tokyo, Japan). The IR spectra were obtained from a Tensor 37 FT-IR spectrometer (Bruker, Ettlingen, Germany). CD spectra were obtained with a JASCO J-1100 spectropolarimeter. NMR experiments were carried out on a Bruker AM500 FT-NMR spectrometer (Bruker, Rheinstetten, Germany) using residual solvent peak as a reference or tetramethylsilane (TMS) as internal standard. The HR-ESI-MS were recorded on a Waters Q-TOF micromass spectrometer Waters Q-TOF micromass spectrometer and an LTQ Orbitrap XL™ Mass spectrometer. Absorbance of bioassay solutions was read on an xMark microplate spectrophotometer.
Plant materials
The tubers of O. japonicus were collected in Feb. 2014 at Me Linh, Hanoi and identified by Prof. Tran Huy Thai, Institute of Ecology and Biological Resources, Vietnam Academy of Science and Technology. The voucher specimens were deposited at the Department of Bioactive Products, Institute of Marine Biochemistry, Vietnam Academy of Science and Technology.
Extraction and isolation
The air-dried and powdered tubers of O. japonicus (2.4 kg) were extracted with methanol (4 L × 3 times) in a sonic bath for 30 min at 40 °C. The combined extracts were concentrated under a vacuum to obtain a crude residue (360 g), which was then resuspended in water (2 L), and extracted by chloroform (1 L × 3 times) to obtain chloroform (8 g) and water residues. The chloroform residue was chromatographed on a silica gel column eluted with a gradient of 1–100% ethyl acetate in hexane to afford nine fractions F1–F9. Fraction F1 was fractionated on a silica gel column eluted with hexane–ethyl acetate (20:1 v/v) to give nine subfractions F1.1–F1.9. Compound 5 (69.5 mg) was purified from F1.4 by using a reverse phase C18 column eluted with acetone–water (2:1 v/v). Compound 1 (70.0 mg) and 4 (18.2 mg) were isolated from F1.7 by using a reverse phase C18 column eluted with acetone–water (3:2 v/v). The F1.9 was chromatographed on a silica gel column (hexane–acetone 8:1, v/v) to afford 2 (20.8 mg). Compound 3 (20.0 mg) was obtained from F9 by repeated C18 column (methanol–water 1:1 v/v) and silica gel column (dichloromethane-acetone 6:1, v/v).
(2R)-(4-methoxybenzyl)-5,7-dimethyl-6-hydroxyl-2,3-dihydrobenzofuran (1)
Brown solid. [α] 24D = + 86.5 (c 0.05, MeOH). IR (KBr): 3446, 1615, 1513, 1472, 1247, 1097. CD (MeOH): 220 (+17.8), 288 (+3.2). 1H-(CD3OD, 500 MHz) and 13C-NMR (CD3OD, 125 MHz): see the Tables 1 and 2. HR-ESI-MS (neg.): 283.1365 [M − H]− (calc. for C18H19O3, 283.1334).
2-(2-Hydroxyl-4-methoxy-benzyl)-5-methyl-6-methoxyl-2,3-dihydrobenzofuran (2)
Brown solid. IR (KBr): 3440, 1610, 1482, 1245, 1120. 1H-(CDCl3, 500 MHz) and 13C-NMR (CDCl3, 125 MHz): see the Tables 1 and 2. HR-ESI-MS (pos.): 301.1436 [M + H]+ (calc. for C18H21O4, 301.1440).
2-(4-Hydroxy-benzyl)-5,6-dihydroxybenzofuran (3)
Brown solid. IR (KBr): 3450, 1613, 1514, 1484, 1242, 1142. 1H-(CD3OD, 500 MHz) and 13C-NMR (CD3OD, 125 MHz): see the Tables 1 and 2. HR-ESI-MS (neg.): 255.0632 [M − H]− (calc. for C15H11O4, 255.0657).
Assay for inhibition of NO production
The effects of compounds on the NO production in LPS-stimulated RAW264.7 cells were evaluated as previously described [19]. The cells were seeded in 96-well plate at 2 × 105 cells/well and incubated for 12 h. The plate were pretreated with compounds in various concentrations (from 1 to 30 µM) for 30 min and then incubated for another 24 h with or without 1 μg/ml LPS. 100 μl of the culture supernatant were transferred to other 96-well plate and 100 μl of Griess reagent were added. The absorbance of the reaction solution was read at 570 nm with a microplate reader (XMark microplate reader, Biorad, USA). The remaining cell solutions in cultured 96-well plate were used to evaluate cell viability by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Cardamonin was used as a positive control (IC50 = 2.80 μM).
Conclusion
In summary, three 2-benzyl-2,3-dihydrobenzofurans and two 2-benzyl-benzofurans were isolated from the CHCl3-soluble fraction of the MeOH extract of O. japonicus tubers. Their structures were found to be (2R)-(4-methoxybenzyl)-5,7-dimethyl-6-hydroxyl-2,3-dihydrobenzofuran (1), 2-(2-hydroxyl-4-methoxy-benzyl)-5-methyl-6-methoxyl-2,3-dihydrobenzofuran (2), 2-(4-hydroxy-benzyl)-5,6-dihydroxybenzofuran (3), 2-(4-methoxy-benzyl)-6,7-dimethoxyl-2,3-dihydrobenzofuran (4), and 2-(4-methoxy-benzyl)-6,7-methylenedioxy-2,3-dihydrobenzofuran (5). Compounds 1 and 2 inhibited NO production in LPS-stimulated RAW264.7 cells.
References
Do TL (2004) Vietnamese medicinal herbs and remedies. Medicinal Publisher, Hanoi
Qi J, Hu ZF, Zhou YF, Hu YJ, Yu BY (2015) Steroidal sapogenins and glycosides from the fibrous roots of Ophiopogon japonicus and Liriope spicata var. prolifera with anti-inflammatory activity. Chem Pharm Bull 63:187–194
Ye Y, Qu Y, Tang R, Cao S, Yang W, Xiang L, Qi J (2013) Three new neuritogenic steroidal saponins from Ophiopogon japonicus (Thunb.) Ker-Gawl. Steroids 78:1171–1716
Chen MH, Chen XJ, Wang M, Lin LG, Wang YT (2016) Ophiopogon japonicus—a phytochemical, ethnomedicinal and pharmacological review. J Ethnopharmacol 181:193–213
Duan CL, Kang ZY, Lin CR, Jiang Y, Liu JX, Tu PF (2009) Two new homoisoflavonoids from the fibrous roots of Ophiopogon japonicus (Thunb.) Ker-Gawl. J Asian Nat Prod Res 11:876–879
Hung TM, Thu CV, Dat NT, Ryoo SW, Lee JH, Kim JC, Na M, Jung HJ, Bae K, Min BS (2010) Homoisoflavonoid derivatives from the roots of Ophiopogon japonicus and their in vitro anti-inflammation activity. Bioorg Med Chem Lett 20:2412–2416
Fan Y, Ma X, Zhang J, Ma L, Gao Y, Zhang W, Song X, Hou W, Guo C, Tong D (2015) Ophiopogon polysaccharide liposome can enhance the non-specific and specific immune response in chickens. Carbohydr Polym 119:219–227
Lin Y, Zhu D, Qi J, Qin M, Yu B (2010) Characterization of homoisoflavonoids in different cultivation regions of Ophiopogon japonicus and related antioxidant activity. J Pharm Biomed Anal 52:757–762
Cheng ZH, Wu T, Bligh SW, Bashall A, Yu BY (2004) cis-Eudesmane sesquiterpene glycosides from Liriope muscari and Ophiopogon japonicus. J Nat Prod 67:1761–1763
Liu C, Ju A, Zhou D, Li D, Kou J, Yu B, Qi J (2016) Simultaneous qualitative and quantitative analysis of multiple chemical constituents in YiQiFuMai injection by ultra-fast liquid chromatography coupled with ion trap time-of-flight mass spectrometry. Molecules 21:E640
Luiking YC, Engelen MP, Deutz NE (2010) Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care 13:97–104
Qi P, Jiang J, Qi H, Jin Y, Shen Q, Wu Y, Song H, Zhang W (2013) Synthesis and antiproliferative activity of new polyoxo 2-benzyl-2,3-dihydrobenzofurans and their related compounds. Lett Drug Des Discov 10:886–894
Ortega N, Urban S, Beiring B, Glorius F (2012) Ruthenium NHC catalyzed highly asymmetric hydrogenation of benzofurans. Angew Chem Int Ed 51:1710–1713
Kwon J, Hiep NT, Kim DW, Hong S, Guo Y, Hwang BY, Lee HJ, Mar W, Lee D (2016) Chemical constituents isolated from the root bark of Cudrania tricuspidata and their potential neuroprotective effects. J Nat Prod 79:1938–1951
Tsai YC, Hsu CC, El-Shazly M, Chiang SY, Wu CC, Wu CC, Lai WC, Yen MH, Wu YC, Chang FR (2015) Phytochemicals and estrogen-receptor agonists from the aerial parts of Liriope platyphylla. Molecules 20:6844–6855
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75:639–653
Closse A, Haefliger W, Hausser D, Gubler HU, Dewald B, Baggiolini M (1981) 2,3-Dihydrobenzofuran-2-ones: a new class of highly potent antiinflammatory agents. J Med Chem 24:1465–1471
Liu QB, Huang XX, Bai M, Chang XB, Yan XJ, Zhu T, Zhao W, Peng Y, Song SJ (2014) Antioxidant and anti-inflammatory active dihydrobenzofuran neolignans from the seeds of Prunus tomentosa. J Agri Food Chem 62:7796–7803
Dat NT, Binh PT, Quynh LTP, Huong HT, Minh CV (2012) Sanggenon C and O inhibit NO production, iNOS expression and NF-κB activation in LPS-induced RAW264.7 cells. Immunopharmacol Immunotoxicol 34:84–88
Authors’ contributions
NHD and NTD designed research and wrote the paper; NDC, HMT and NVT performed research; NTH and DL analyzed CD data. All authors read and approved the final manuscript.
Acknowledgements
This work is supported by the National Foundation for Science and Technology Development (NAFOSTED 104.01-2014.05). We thank the Institute of Chemistry (VAST) for the NMR experiments.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Additional file
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Dang, N.H., Chung, N.D., Tuan, H.M. et al. 2-Benzyl-benzofurans from the tubers of Ophiopogon japonicus . Chemistry Central Journal 11, 15 (2017). https://doi.org/10.1186/s13065-017-0242-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-017-0242-z