Abstract
Background
Postoperative pulmonary complications (PPCs) are the most common perioperative complications following surgical site infection (SSI). They prolong the hospital stay and increase health care costs. A lung-protective ventilation strategy is considered better practice in abdominal surgery to prevent PPCs. However, the role of the inspiratory oxygen fraction (FiO2) in the strategy remains disputed. Previous trials have focused on reducing SSI by increasing the inhaled oxygen concentration but higher FiO2 (80%) was found to be associated with a greater incidence of atelectasis and mortality in recent research. The trial aims at evaluating the effect of different FiO2 added to the lung-protective ventilation strategy on the incidence of PPCs during general anesthesia for abdominal surgery.
Methods and design
PROtective Ventilation with a low versus high Inspiratory Oxygen fraction trial (PROVIO) is a single-center, prospective, randomized controlled trial planning to recruit 252 patients undergoing abdominal surgery lasting for at least 2 h. The patients will be randomly assigned to (1) a low-FiO2 (30% FiO2) group and (2) a high-FiO2 (80% FiO2) group in the lung-protective ventilation strategy. The primary outcome of the study is the occurrence of PPCs within the postoperative 7 days. Secondary outcomes include the severity grade of PPCs, the occurrence of postoperative extrapulmonary complications and all-cause mortality within the postoperative 7 and 30 days.
Discussion
The PROVIO trial assesses the effect of low versus high FiO2 added to a lung-protective ventilation strategy on PPCs for abdominal surgery patients and the results should provide practical approaches to intraoperative oxygen management.
Trial registration
www.ChiCTR.org.cn, identifier: ChiCTR18 00014901. Registered on 13 February 2018.
Similar content being viewed by others
Background
About 2.0 to 5.6% of more than 234 million patients undergoing surgery develop postoperative pulmonary complications (PPCs), especially after general and vascular surgeries (approximately 40%), which makes PPCs the most common perioperative complications following surgical site infection (SSI) [1,2,3,4,5,6]. PPCs, especially respiratory failure, add to the morbidity and mortality risk in hospitalized patients [1, 4, 5]. Moreover, PPCs prolong hospital stay and increase medical expense and resource utilization [2, 5]. A reduction of PPCs is a very important evaluation index of medical quality management. A possible explanation for increasing morbidity in patients who develop PPCs is that mechanical ventilation under general anesthesia results in gas-exchange impairment, a local inflammatory response and circulatory disorder [7, 8]. Thus, decreased lung volumes, ventilator-induced lung injury and atelectasis are strongly associated with the incidence of PPCs [9].
Prior studies noted that so-called lung-protective ventilation, referring to low-tidal-volume (VT), appropriate positive end-expiratory pressure (PEEP) level and recruitment maneuvers, seems to be the optimum option for the surgical and intensive care unit (ICU) populations [10,11,12,13]. The decrease in PPCs, mortality and health care costs have been observed in the protective-ventilated population. On the basis of the robust evidence available, a combination of low VT (6–8 ml/kg of predicted body weight) [11, 14], a level of PEEP at 5–8 cmH2O [15] and repeated recruitment maneuvers [16] are now widely adopted.
Setting the inspiratory oxygen fraction (FiO2) intraoperatively is a significant task of anesthetists, but has not been based on evidence-based guidelines. Obtaining comprehensive knowledge about hyperoxia caused by high FiO2 has been stressed as important by clinicians over the past few decades, including its potentially deleterious effects on lung. Even mildly elevated FiO2 levels have been reported to exacerbate lung injury by up-regulating pro-inflammatory cytokines and inducing neutrophil infiltration in the alveolar spaces [17,18,19].
Even if there is no significant difference in pulse oximetry and the oxygenation index for several time-points with 30 or 80% FiO2 intraoperatively, hyperoxia and substantial oxygen exposure are common in clinical practice [20, 21]. Questions have been raised about the use of oxygen in ventilated patients undergoing elective surgery. A recent systematic review revealed that the trials of this decade about the effects of FiO2 on SSI have been inconclusive, and we should also focus on clinically relevant pulmonary side-effects and other adverse events (AEs) [22,23,24,25]. In addition, exposure to oxygen is related to adverse effects in critically ill patients [26, 27]. The ideal FiO2 level in the lung-protective ventilation strategy to protect against PPCs and improve clinical outcomes has not been addressed in the perioperative period.
The relationship between FiO2 and PPCs in surgical patients is mainly affected by a hyperoxia-induced change in the respiratory mechanism. Higher FiO2 seems to be associated with pulmonary complications and adverse clinical outcomes, but the existing evidence is insufficient to warrant its effect to promote PPCs [28,29,30]. We hypothesize that a low level of FiO2 (30%) compared with high FiO2 (80%) could decrease the incidence of PPCs in patients undergoing abdominal surgery when lung-protective ventilation strategy is administered.
Methods and design
Study design
The PROVIO trial is a single-center, prospective, randomized controlled and two-arm study and is conducted in accordance with the Declaration of Helsinki. The trial will be conducted in West China Hospital of Sichuan University, China. We aim to assess the effect of FiO2 in a lung-protective ventilation strategy, in an abdominal surgical population of patients, on PPCs, extrapulmonary complications (e.g., SSI, sepsis, etc.), hospital stay and mortality.
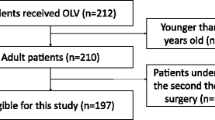
The protocol follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 Statement. The SPIRIT checklist can be found inAdditional file 1. The diagram of the Consolidated Standards of Reporting Trials (CONSORT), which are also followed, is presented in Fig. 1.
Study population
The inclusion criteria of the study are: American Society of Anesthesiologists (ASA) physical status I–III patients aged 18 years or older, scheduled for elective abdominal surgery with an expected duration of at least 2 h and planned to be extubated in the operating room. Laparotomy and laparoscopy surgery will not be restricted. Patients are ineligible if they have suffered from pneumothorax, acute lung injury or acute respiratory distress syndrome within the last 3 months. Other exclusion criteria are: a diagnosis of heart failure (New York Heart Association classes; NYHA IV), chronic renal failure (glomerular filtration rate < 30 ml/min), serious hepatic diseases (e.g., hepatic failure), scheduled for reoperation or postoperative mechanical circulatory support, known pregnancy, participation in another interventional study, and with a body mass index (BMI) of > 30 kg/m2.
Randomization, blinding and bias minimization
Patients will be recruited from West China Hospital of Sichuan University. Consecutive male or female patients aged 18 years or older who will undergo abdominal surgery under general anesthesia are screened for study eligibility. Randomization will be performed using a computer-generated randomization list (SPSS 22.0) with an allocation rate of 1:1. The allocation is concealed in an opaque envelope and will be sent to the attending anesthetist by a blinded investigator.
Given the characteristics of the study, the attending anesthetist must know the intervention. Researchers, including the investigator in the operating room, the data collector and the data analyzer, will all be blinded to the randomization arm. All the surgeons, nurses and anesthetists in the post-anesthesia care unit (PACU) will not know the allocation. Postoperative visits and outcome assessment will be performed by a blinded investigator. Emergency unblinding is permissible if hypoxemia occurs (defined as pulse oxygen saturation (SpO2) < 92% or partial pressure of oxygen in arterial blood (PaO2) < 60 mmHg).
Standard procedures
The risk of PPCs will be assessed using the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score [31] before randomization (Table 1). An investigator assesses the individual risk of PPCs with the seven predictors of the ARISCAT risk score (age, SpO2, respiratory infection in the last month, preoperative anemia, duration of surgery, and whether an emergency procedure). The ARISCAT score will help to analyze the effect of FiO2 to intermediate-high-risk patients who obtain a score of more than 26. All patients receiving an assessment will be included and randomized.
All randomized participants will receive standard care and monitoring including a five-lead electrocardiogram, SpO2, blood pressure (invasive or noninvasive) and end-tidal carbon dioxide (ETCO2). The attending anesthetist responsible for the patient can choose the bispectral index (BIS), muscle-relaxant monitoring and cardiac-output monitoring techniques according to clinical routines.
There will be no restriction to the anesthetic regimen and individualized health care will be performed intraoperatively. Use of antiemetics and muscle-relaxant antagonists (mainly neostigmine) will be recorded in the case report form (CRF).
Intraoperative ventilatory management
Pre-oxygenation will be prescribed for 5 min at 100% FiO2 using a mask. In accordance with the group allocation, the participants will be randomized to receive a low (30%) or a high (80%) FiO2 throughout the whole period of intraoperative mechanical ventilation after tracheal intubation. The FiO2 level is implemented through adjusting the air-oxygen ratio when the total gas flow remains at 2 L/min. The FiO2 in our protocol refers to the actual fraction of inspired oxygen presented in the display panel on the anesthesthetic machine. Table 2 shows the ventilation settings.
Intraoperative ventilation in all participants will be performed via the lung-protective ventilation strategy. A recruitment maneuver with peak airway pressure (Paw) of 30 cmH2O for 30 s will be performed instantly after intubation, every 60 min after intubation and before extubation. Other settings are shown in Table 2. Ventilatory parameters, including tidal volume (VT), minute volume (MV), airway pressure (Paw), plateau pressure (Pplat), fresh gas flow, PEEP and FiO2, will be monitored.
After extubation, patients will be sent to the PACU or the ward where they will be oxygenated with 2 L/min, pure oxygen via a nasal tube over 24 h. At the same time, they will receive standard monitoring.
Intraoperative care
After induction, standard intraoperative care will be applied in both groups to reach a target of standard state (Table 3). Vasoactive drugs can be used in patients with unstable hemodynamics as appropriate.
Rescue strategies for intraoperative hypoxemia
Around 30% FiO2 has been proved to be safe in mechanically ventilated patients and rarely causes hypoxemia [21]. We designed a rescue strategy for patients in whom SpO2, measured by pulse oximetry, fell to less than 92% or PaO2 to less than 60 mmHg for more than 1 min.
Endotracheal-tube displacement, airway-secretion blockage, bronchospasm, pneumothorax, and hemodynamic change would all be checked for. After excluding these as underlying causes, a rescue recruitment maneuver with Paw 30 cmH2O for 30 s will be implemented [12, 16, 32]. If this were to fail, FiO2 and ventilation settings would be altered until acquiring the required oxygenation (SpO2 ≥ 92% or PaO2 ≥ 60 mmHg).
Outcome measurements
The primary outcome is the occurrence of pulmonary complications within the first 7 days postoperatively. The definition of PPCs follows the ARISCAT study (respiratory infection, respiratory failure, bronchospasm, atelectasis, pleural effusion, pneumothorax or aspiration pneumonitis.) [4].
The secondary outcomes include the occurrence of PPCs in the postoperative 30 days; SSI, postoperative nausea and vomiting (PONV) in the postoperative 7 days; the severity grade of pulmonary complications in the postoperative 7 and 30 days (Table 4); and death rate in the postoperative 7 and 30 days.
Pulmonary complications will be scored with a grade scale ranging from 0 to 5 adapted from Kroenke et al., Hulzebos et al., Fernandez-Bustamante et al. and Canet et al. [4, 5, 33, 34]. Grade 0 in the scale represents no PPCs, grades 1–4 represent increasing severity levels of pulmonary complications, and grade 5 represents death before discharge. SSI will be defined with the criteria from the Centers for Disease Control and Prevention (CDC) [35].
Tertiary outcomes in the first 7 and 30 days postoperatively are as follows:
-
1.
Sepsis: the infection-centric systemic response which needs to meet two or more criteria of the systemic inflammatory response syndrome (SIRS) [36]
-
2.
Septic shock: defined as a composite of sepsis-induced response, perfusion abnormalities, and hypotension despite adequate fluid resuscitation [36]
-
3.
Myocardial ischemia [37]
-
4.
Heart failure [37]
-
5.
Urinary system infection [37]
Acute kidney injury: defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria [38]
-
6.
Anastomotic fistula
-
7.
Reintubation
-
8.
Unplanned admission to the ICU
-
9.
Hospital length of stay postoperatively
Data collection and follow-up
The study will be conducted in the operating room and visits are restricted during the screening, hospitalization and follow-up periods. The primary and secondary outcomes will be measured on postoperative days 1, 2, 3, 5 and 7 or at discharge by interview. On postoperative day 30, participants will be contacted by phone (Fig. 2). Demographic and baseline data will be collected preoperatively, which include age, sex, weight, BMI, ASA physical status, ARISCAT risk score, smoking status, pulmonary status (chronic pbstructive pulmonary disease (COPD), atelectasis, asthma respiratory infection within the last 3 months, use of ventilatory support), routine laboratory tests (hemoglobin, white blood cell count, platelet count, neutrophil count) and medical history.
Both intraoperative surgery- and anesthesia-associated data will be recorded, including type of surgery, surgical incision or approach, duration of surgery and ventilation, blood loss, transfusion of blood products, fluid balance (calculated by subtracting the measurable fluid losses from measurable fluid intake during anesthesia), drugs during anesthesia (e.g., anesthetics and antiemetics), adjustment of ventilatory parameters or FiO2, hypoxemia events, the need for rescue strategy, number of emergency recruitment maneuvers, and unplanned admission to the ICU.
Postoperative visits will be conducted daily and clinical data required to assess PPC grade include body temperature, lung auscultation, symptoms (e.g., cough, expectoration and dyspnea), chest-imaging manifestations, and laboratory tests. Surgical incision assessment, PONV and other outcomes will also be measured and collected daily according to the evaluation criterion mentioned above.
The Data and Safety Monitoring Board (DSMB) composed of five independent individuals is set up to supervise the overall conduct of the study (the screening, recruitment and adherence to the protocol). The DSMB is responsible for checking and ensuring the completeness and validity of data recording. The interim analysis will be conducted when the first 120 participants are recruited and have been visited to completion. The DSMB has access to patient allocation, but the results of the interim analysis will be treated as strictly confidential.
Study drop-out
Participants have the right to withdraw from the study at any time without any consequences for further treatment. Investigators have the right to terminate the study at any time in consideration of the best interests of the participants. Both situations will be recorded in the CRF and discussed.
Any AEs and treatment side-effects will be sent to the DSMB which will discussed whether the participant should drop out accordingly.
Statistical considerations
The sample size required was estimated based on the investigative data in our medical center. The pilot study showed that PPCs (respiratory infection, respiratory failure, bronchospasm, atelectasis, pleural effusion, pneumothorax or aspiration pneumonitis) occurred in 50.4% patients received 80% FiO2 after abdominal surgery (sample size: 100). And assuming a round 50% rate of PPCs in the high-FiO2 (80%) group, we calculated that a total sample size of 252 patients (126 in each group) will have 80% power to detect a relative risk reduction of 35% in PPCs between groups, at a two-sided alpha level of 0.05 and 5% drop-out. We will conduct a sample size reassessment after recruiting half of the patients for safety consideration.
All statistics will be analyzed by SPSS 22.0 statistical software (IBM Corporation, Chicago, IL, USA) through the intention-to-treat principle, which covers all randomized patients receiving surgery. Participants with adjusted FiO2 values are still treated as the low-FiO2 population when analyzed. In a descriptive analysis of the population, mean and standard deviation (SD) will be used for normally distributed variables, medians and interquartile ranges used for non-normally distributed variables and percentages used for categorical variables. Stratified description will be used as appropriate.
There will be a baseline comparison of age, gender, BMI, type of surgery, surgical approach, duration of surgery and ARISCAT score between groups and logistic regression analysis will be performed if an imbalance between groups exists. The Student’s t test will be used for continuous, normally distributed variables and the Mann-Whitney U test will be used for continuous, non-normally distributed data. The primary and secondary outcomes will be compared using the χ2 test or Fisher’s exact test, while multiple logistic-regression analysis will be used to identify hazards. A two-sided P value < 0.05 is considered statistically significant.
A custom-made folder is made to store the participants’ data, which consists of documents and forms. Only blinded researchers have access to the folder. Only when the study is complete will the investigators obtain the data.
Discussion
The optimal intraoperative FiO2 remains highly debated. Many physicians consider excessive oxygen supplement a salutary practice which is now widely applied in routine practice due to its simplicity and ease of availability [39]. Despite the controversy, the majority of published randomized trials comparing 30 and 80% FiO2, mainly in SSI and PONV, show that intraoperative high FiO2 decreases the risk of both [40,41,42]. Furthermore, new World Health Organization (WHO) recommendations on intraoperative and postoperative measures for SSI prevention in 2016 suggest that patients undergoing general anesthesia with endotracheal intubation for surgical procedures should receive 80% FiO2 intraoperatively [43]. What remains controversial is whether the intraoperative use of an elevated FiO2 is essential to all intubated patients without hypoxemia, although both 30 and 80% FiO2 provide similar oxygenation [21]. A multicenter observational trial collecting the ventilator data 1 h after induction showed that most ventilated patients (83%) in Japan were exposed to potentially preventable hyperoxia, especially in one-lung ventilation patients and the elderly [20].
The “benefit” of this pervasive liberal oxygen management has recently been questioned. Concerns on potential detrimental effects, such as impairing lung-capillary endothelial function and facilitating oxidative stress due to the use of high FiO2, were raised [44,45,46]. Endothelial activation may initiate progressive hyperoxic lung injury when persistently ventilated under hyperoxic conditions at 70% FiO2 [19]. In addition, excessive oxygen can lead to pulmonary endothelial-cell damage through mitochondrial fragmentation [47]. This can be explained by the formation of reactive oxygen species (ROS) and pro-inflammatory cytokines in endothelial cells which were found in an animal study [19, 46]. Romagnoli et al. demonstrated that protective ventilation with the lowest level of FiO2 to keep the SpO2 ≥ 95% reduces oxygen toxicity by generating less ROS production [45]. However, there is a contradictory view on the detrimental effects of high FiO2 on endothelial function in healthy volunteers [48]. Another interpretation is that high FiO2 may change pulmonary-gas exchange in surgical patients. Ventilation with high FiO2 (80–100%) increases the intrapulmonary shunt [49] and impairs gas exchange [50]. In addition, resorption atelectasis results from a phenomenon in which nitrogen is displaced by oxygen which can diffuse more rapidly into the blood. Resorption atelectasis can also promote pulmonary shunting and cause hypoxemia [51]. Ventilation for induction of anesthesia with 100% FiO2 leads to significantly larger atelectatic areas than with 60% FiO2 [52]. Atelectatic areas tend towards having a low ventilation/perfusion ratio. Hyperoxia is also an important factor contributing to the apoptosis of alveolar epithelial cells and lowers the level of surfactant proteins that indicate damage of the lung tissue [53]. The synergetic action of the above factors increases the risk of lung injury and pulmonary complications.
Indeed, supplemental oxygen results in hyperoxia, and is reported as an independent risk factor for ventilator-associated pneumonia in one observational study [54]. Liberal oxygen use is considered detrimental in mechanically ventilated patients in the aspect of lung function [55] and clinical outcomes [27]. The PROXI trial demonstrated that the incidence of PPCs, PONV and SSI after abdominal surgery were not significantly different in patients receiving 80 or 30% FiO2 [56]; nevertheless, the former suffers higher long-term mortality (23.3 vs 18.3%) [57]. Also one observational trial has suggested a dose-dependent manner in FiO2 and 30-day mortality. The incidence of PPCs has declined by half in the low-FiO2 group with a median of 31% (range 16–34%) [30].
Yet, no direct evidence has revealed the relationship between FiO2 in lung-protective ventilation and PPCs, and existing data reported that postoperative pulmonary function is better protected with a relative low FiO2 intraoperatively [58]. One systematic review showed that the included trials only focused on postoperative atelectasis, rather than on all forms of PPC [59]. Despite the PROXI trial demonstrating that PPCs did not differ after inhalation of 80 vs 30% oxygen, the results are worth discussing. The emergency surgery population was not excluded in the PROXI trial, emergency surgery being an independent risk factor for pulmonary complications [4]. Intubation time is also a key element for causing pneumonia and atelectasis. Moreover, the complication measures of PROXI lacked a standard and comprehensive judgment evaluation, which only assessed the three types of PPC (atelectasis, pneumonia and respiratory failure) according to the CDC criteria. And, above all, the ventilation strategy for patients is not specified, which plays a key role in the incidence of pulmonary complications. The iPROVE-O2 trial is an ongoing randomized controlled trial (ClinicalTrials.gov identifier: NCT02776046) comparing the efficacy of 80 and 30% FiO2 using an individualized, open-lung ventilatory strategy in reducing the incidence of SSI [60]. The major differences compared to the PROVIO trial are: the appearance of pulmonary complications as one of the secondary outcomes; individualized, open-lung ventilation as the ventilatory mode which is a combination of 8 ml/kg VT, recruitment maneuver and the optimal individualized PEEP. The recruitment maneuver will be performed by a PEEP-titration trial [61]. Undoubtedly, the individualized, open-lung ventilation strategy is more complex to implement clinically when compared to lung-protective ventilation [61].
The limitations of our study must be mentioned. Firstly, we conducted a pilot study to determine the incidence of PPCs in our medical center with reference to the sample size calculation. We hope that our results will provide the possible direction and reference for subsequent research into FiO2. Secondly, the study excludes the patients scheduled for some types of surgery because of the duration of the surgery. Thirdly, the oxygenation index and arterial oxygen pressure that may reflect the actual oxygenation state will not be measured during the perioperative period.
In the absence of an intraoperative lung-protective ventilation strategy, previous studies failed to identify a certain relationship between FiO2 and PPCs. We insist that lung-protective ventilation in both groups will reduce bias regarding ventilation-associated impact, and enhance lung protection. Conclusively, the PROVIO trial is the first clinical trial focusing on the effects of FiO2 added to lung-protective ventilation on PPCs. The results of the trial should support anesthetists in routine oxygen management during general anesthesia in an attempt to prevent PPCs.
Trial status
The trial is ongoing from February 2018, and expected to complete in May 2019. The protocol version is 3.0 (issue date: 25 December 2018).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AE:
-
Adverse event
- ARISCAT:
-
Assess Respiratory Risk in Surgical Patients in Catalonia
- ASA:
-
American Society of Anesthesiologists
- BIS:
-
The bispectral index
- BMI:
-
Body mass index
- CDC:
-
The Centers for Disease Control and Prevention
- CONSORT:
-
Consolidated Standards of Reporting Trials
- COPD:
-
Chronic obstructive pulmonary disease
- CRF:
-
Case report form
- DSMB:
-
Data and Safety Monitoring Board
- ETCO2 :
-
End-tidal carbon dioxide
- FiO2 :
-
Inspiratory oxygen fraction
- Hb:
-
Hemoglobin
- HR:
-
Heart rate
- I:E:
-
Inspiratory to Expiratory ratio
- ICU:
-
Intensive care unit
- KDIGO:
-
Kidney Disease Improving Global Outcomes
- MAP:
-
Mean arterial pressure
- MV:
-
Minute volume
- NYHA:
-
New York Heart Association classes
- Pplat :
-
Plateau pressure
- PACU:
-
Post-anesthesia care unit
- PEEP:
-
Positive end-expiratory pressure
- PONV:
-
Postoperative nausea and vomiting
- PPCs:
-
Postoperative pulmonary complications
- PROVIO:
-
PROtective Ventilation with a low versus high Inspiratory Oxygen fraction trial
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- SIRS:
-
Systemic inflammatory response syndrome
- SPIRIT:
-
Standard Protocol Items: Recommendations for Interventional Trials
- SpO2 :
-
Pulse oxygen saturation
- SSI:
-
Surgical site infection
- VT :
-
Tidal volume
References
Pearse RM, Moreno RP, Bauer P, Pelosi P, Metnitz P, Spies C, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–65.
Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–41.
Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000;232:242–53.
Canet J, Gallart L, Gomar C, Paluzie G, Vallès J, Castillo J, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–50.
Fernandez-Bustamante A, Frendl G, Sprung J, Kor DJ, Subramaniam B, Martinez Ruiz R, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the Perioperative Research Network Investigators. JAMA Surg. 2017;152:157–66.
Gupta H, Gupta PK, Fang X, Miller WJ, Cemaj S, Forse RA, et al. Development and validation of a risk calculator predicting postoperative respiratory failure. Chest. 2011;140:1207–15.
Wolthuis EK, Choi G, Dessing MC, Bresser P, Lutter R, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology. 2008;108:46–54.
Hedenstierna G, Edmark L. The effects of anesthesia and muscle paralysis on the respiratory system. Intensive Care Med. 2005;31:1327–35.
Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102:838–54.
Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology. 2013;118:1307–21.
Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–37.
Güldner A, Kiss T, Serpa NA, Hemmes SN, Canet J, Spieth PM, et al. Intraoperative protective mechanical ventilation for prevention of postoperative pulmonary complications: a comprehensive review of the role of tidal volume, positive end-expiratory pressure, and lung recruitment maneuvers. Anesthesiology. 2015;123:692–713.
Yang D, Grant MC, Stone A, Wu CL, Wick EC. A meta-analysis of intraoperative ventilation strategies to prevent pulmonary complications: is low tidal volume alone sufficient to protect healthy lungs? Ann Surg. 2016;263:881–7.
Serpa NA, Cardoso SO, Manetta JA, Pereira VG, Espósito DC, Pasqualucci MO, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;58:1651–9.
Ladha K, Melo MFV, Mclean DJ, Wanderer JP, Grabitz SD, Kurth T, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ. 2015;351:h3646.
Costa LA, Hajjar LA, Volpe MS, Fukushima JT, Rr DSS, Osawa EA, et al. Effect of intensive vs moderate alveolar recruitment strategies added to lung-protective ventilation on postoperative pulmonary complications: a randomized clinical trial. JAMA. 2017;317:1422–32.
Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. 2004;32:2496–501.
Suzuki Y, Nishio K, Takeshita K, Takeuchi O, Watanabe K, Sato N, et al. Effect of steroid on hyperoxia-induced ICAM-1 expression in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L245–52.
Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, et al. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol. 2006;34:453–63.
Suzuki S, Mihara Y, Hikasa Y, Okahara S, Ishihara T, Shintani A, et al. Current ventilator and oxygen management during general anesthesia: a multicenter, cross-sectional observational study. Anesthesiology. 2018;129:67–76.
Staehr AK, Meyhoff CS, Henneberg SW, Christensen PL, Rasmussen LS. Influence of perioperative oxygen fraction on pulmonary function after abdominal surgery: a randomized controlled trial. BMC Res Notes. 2012;5:383.
Meyhoff CS. Perioperative hyperoxia: why guidelines, research and clinical practice collide. Br J Anaesth. 2019;122:289–91.
de Jonge S, Egger M, Latif A, Loke YK, Berenholtz S, Boermeester M, et al. Effectiveness of 80% vs 30-35% fraction of inspired oxygen in patients undergoing surgery: an updated systematic review and meta-analysis. Br J Anaesth. 2019;122:325–34.
Mattishent K, Thavarajah M, Sinha A, Peel A, Egger M, Solomkin J, et al. Safety of 80% vs 30-35% fraction of inspired oxygen in patients undergoing surgery: a systematic review and meta-analysis. Br J Anaesth. 2019;122:311–24.
Wetterslev J, Meyhoff CS, Jorgensen LN, Gluud C, Lindschou J, Rasmussen LS. The effects of high perioperative inspiratory oxygen fraction for adult surgical patients. Cochrane Database Syst Rev. 2015:CD008884. https://doi.org/10.1002/14651858.CD008884.pub2.
Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, De JE. Association between arterial hyperoxia and outcome in subsets of critical illness: a systematic review, metaanalysis, and meta-regression of cohort studies. Crit Care Med. 2015;43:1508–19.
Chu DK, Kim LH, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693–705.
Martin DS, Grocott MP. Oxygen therapy in anaesthesia: the yin and yang of O2. Br J Anaesth. 2013;111:867–71.
Ball L, Lumb AB, Pelosi P. Intraoperative fraction of inspired oxygen: bringing back the focus on patient outcome. Br J Anaesth. 2017;119:16–8.
Staehr-Rye AK, Meyhoff CS, Scheffenbichler FT, Vidal Melo MF, Gatke MR, Walsh JL, et al. High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. Br J Anaesth. 2017;119:140–9.
Mazo V, Sabaté S, Canet J, Gallart L, de Abreu MG, Belda J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219–31.
Spieth PM, Guldner A, Uhlig C, Bluth T, Kiss T, Schultz MJ, et al. Variable versus conventional lung protective mechanical ventilation during open abdominal surgery: study protocol for a randomized controlled trial. Trials. 2014;15:155.
Kroenke K, Lawrence VA, Theroux JF, Tuley MR. Operative risk in patients with severe obstructive pulmonary disease. Arch Intern Med. 1992;152:967.
Hulzebos EH, Helders PJ, Favié NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery. JAMA. 2006;296:1851–9.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. JAMA. 1992;101:1644–55.
Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM Joint Taskforce on perioperative outcome measure. Eur J Anaesthesiol. 2015;32:88.
Wanner C, Tonelli M. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303–9.
Kabon B, Kurz A. Optimal perioperative oxygen administration. Curr Opin Anaesthesiol. 2006;19:11–8.
Greif R, Akça O, Horn EP, Kurz A, Sessler DI, Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161–7.
Goll V, Akça O, Greif R, Freitag H, Arkiliç CF, Scheck T, et al. Ondansetron is no more effective than supplemental intraoperative oxygen for prevention of postoperative nausea and vomiting. Anesth Analg. 2001;92:112–7.
Belda FJ, Aguilera L, Garcia de la Asuncion J, Alberti J, Vicente R, Ferrandiz L, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA. 2005;294:2035–42.
Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, De JS, De VF, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e288–303.
Martin DS, Mckenna HT, Morkane CM. Intraoperative hyperoxemia: an unnecessary evil? Anesth Analg. 2016;123:1643.
Romagnoli S, Becatti M, Bonicolini E, Fiorillo C, Zagli G. Protective ventilation with low fraction of inspired oxygen and radicals of oxygen production during general anaesthesia. Br J Anaesth. 2015;115:143–4.
Nagato AC, Bezerra FS, Lanzetti M, Lopes AA, Silva MA, Porto LC, et al. Time course of inflammation, oxidative stress and tissue damage induced by hyperoxia in mouse lungs. Int J Exp Pathol. 2012;93:269–78.
Ma C, Beyer AM, Durand M, Clough AV, Zhu D, Norwood Toro L, et al. Hyperoxia causes mitochondrial fragmentation in pulmonary endothelial cells by increasing expression of pro-fission proteins. Arterioscler Thromb Vasc Biol. 2018;38:622–35.
Larsen M, Ekeloef S, Kokotovic D, Schoupedersen AM, Lykkesfeldt J, Gögenür I. Effect of high inspiratory oxygen fraction on endothelial function in healthy volunteers: a randomized controlled crossover pilot study. Anesth Analg. 2017;125:1.
Marntell S, Nyman G, Hedenstierna G. High inspired oxygen concentrations increase intrapulmonary shunt in anaesthetized horses. Vet Anaesth Analg. 2005;32:338–47.
Staffieri F, Monte VD, Marzo CD, Grasso S, Crovace A. Effects of two fractions of inspired oxygen on lung aeration and gas exchange in cats under inhalant anaesthesia. Vet Anaesth Analg. 2010;37:483–90.
Akça O, Podolsky A, Eisenhuber E, Panzer O, Hetz H, Lampl K, et al. Comparable postoperative pulmonary atelectasis in patients given 30% or 80% oxygen during and 2 hours after colon resection. Anesthesiology. 1999;91:991–8.
Edmark L, Kostovaaherdan K, Enlund M, Hedenstierna G. Optimal oxygen concentration during induction of general anesthesia. Anesthesiology. 2003;98:28–33.
Jin Y, Peng LQ, Zhao AL. Hyperoxia induces the apoptosis of alveolar epithelial cells and changes of pulmonary surfactant proteins. Eur Rev Med Pharmacol Sci. 2018;22:492–7.
Six S, Jaffal K, Ledoux G, Jaillette E, Wallet F, Nseir S. Hyperoxemia as a risk factor for ventilator-associated pneumonia. Crit Care. 2016;20:195.
Rachmale S. The authors respond to: Practice of excessive FIO2 and effect on pulmonary outcomes in mechanically ventilated patients with acute lung injury. Respir Care. 2013;58:83–4.
Meyhoff CS, Wetterslev J, Jorgensen LN, Henneberg SW, Høgdall C, Lundvall L, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543–50.
Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS. Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomized clinical trial. Anesth Analg. 2012;115:849–54.
Zoremba M, Dette F, Hunecke T, Braunecker S, Wulf H. The influence of perioperative oxygen concentration on postoperative lung function in moderately obese adults. Eur J Anaesthesiol. 2010;27:501–7.
Hovaguimian F, Lysakowski C, Elia N, Tramèr MR. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2013;119:303–16.
Ferrando C, Soro M, Unzueta C, Canet J, Tusman G, Suarez-Sipmann F, et al. Rationale and study design for an individualised perioperative open-lung ventilatory strategy with a high versus conventional inspiratory oxygen fraction (iPROVE-O2) and its effects on surgical site infection: study protocol for a randomised controlled trial. BMJ Open. 2017;7:e016765.
Acosta J, Aguilar G, Alberola MJ, Alcón A, Alonso JM, Alonso MD, et al. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med. 2018;6:193–203.
Acknowledgements
We thank the patients for participating in the trial dissemination. We also thank the authors of the primary studies and our colleagues supporting the study.
Confidentiality
The personal information of the patients will be confidential at all periods of the trial. Data will be handled according to Chinese law and archived for at least 5 years. Meanwhile, the database will be anonymized and kept for 5 years. Then, the data will be destroyed according to the hospital standards concerning the destruction of confidential information.
Funding
This research received a funding grant from the 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (project number: 2018HXFH052). The 1·3·5 project will in no way intervene in any aspect of the trial, including its design, data collection, analysis or presentation.
Author information
Authors and Affiliations
Contributions
X-FL, X-YY and HaiY provided substantial contributions to the study conception and design. X-FL and HaiY drafted the protocol and edited the manuscript. DJ, HongY, Y-LJ, J-LJ and L-LH participated in the study design. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study has been approved by The Ethical Committee of the West China Hospital of Sichuan University (2018 approval No. 8) and informed consent will be obtained from all study patients before participating. Our trial was registered at http://www.chictr.org.cn (ChiCTR1800014901). We will obtain informed consent in written form from all patients who meet all the inclusion criteria and none of the exclusion criteria before arrival in the operating room.
The results of the PROVIO trial will be published in peer-reviewed journals focused on perioperative medicine and presented at national and international conferences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Checklist for the protocol of a clinical trial. (DOCX 25 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, XF., Jiang, D., Jiang, YL. et al. PROtective Ventilation with a low versus high Inspiratory Oxygen fraction (PROVIO) and its effects on postoperative pulmonary complications: protocol for a randomized controlled trial. Trials 20, 619 (2019). https://doi.org/10.1186/s13063-019-3668-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-019-3668-x