Abstract
Background
It is unclear which kind of interventional therapy is the best when reducing blood loss in patients prepared for total hip arthroplasty (THA). We performed this network meta-analysis to rank the best intervention arm for blood loss control in THA patients.
Methods
We searched electronic databases about randomized controlled trials (RCTs) to compare three treatments (topical tranexamic acid (TXA), intravenous TXA, and topical fibrin sealant (FS)) versus placebo for the people prepared for THA. Traditional and network meta-analyses were performed. The quality assessment was conducted using Cochrane Collaboration’s tool. The network meta-analysis was conducted using Stata 13.0 software.
Results
Finally, a total of 32 RCTs were included in this network meta-analysis. Topical TXA, intravenous TXA, and topical FS significantly decreased the need for transfusion and total blood loss when compared with placebo. And intravenous TXA ranks the first hemostasis agent for reducing the need for transfusion and total blood loss. There was no significant difference between these three treatments (intravenous TXA, topical TXA, and topical FS) in the occurrence of deep venous thrombosis (DVT).
Conclusion
Intravenous TXA may be the best way to reduce the need for transfusion and total blood loss. More direct studies that focused on topical TXA versus FS are needed in the future.
Similar content being viewed by others
Introduction
Total hip arthroplasty (THA) is associated with considerable blood loss, which can lead to a need for transfusion. It is reported that perioperative blood loss in THA can be as much as 700–2000 ml, and subsequently, 16 to 37% of patients need blood transfusion [1, 2]. Blood transfusion has several serious complications, such as virus transmission and immunological reaction [3, 4]. What is more, the economic burden caused by blood transfusion will be increased correspondingly. Substantial blood loss was mainly caused by the osteotomy of the femoral and surgical trauma and fibrinolysis. In order to reduce blood loss, several strategies have been managed to inhibit the fibrinolysis and surgical bleeding. Fibrin sealant (FS) is composed of fibrinogen and thrombin that mainly derived from human blood products [5, 6]. When those components mixed, fibrin formed and crosslinked directly with tissue collagen [7]. Tranexamic acid (TXA) is a synthetic amino acid, and its structure is analogous to lysine that can competitively inhibit plasminogen and reduce fibrinolysis locally [8]. There are two main administration routes to the management of TXA: topical TXA and intravenous TXA [9]. Clinical studies and meta-analysis found that both the topical and intravenous TXA can reduce blood loss without sacrificing the safety [10]. And several studies have identified the efficacy and safety of FS for reducing perioperative blood loss in THA. In the current clinical practice, which hemostasis agents were the most effective was in debate. In addition, the meta-analysis comparing topical versus intravenous TXA in THA was limited. The purpose of this network meta-analysis was to compare the efficacy and safety of the three treatments (FS, topical TXA, and intravenous TXA) for patients prepared for THA. Our intention was to provide hierarchies of the need for transfusion, total blood loss, and incidence of deep venous thrombosis.
Methods
Criteria for considering studies
We only included RCTs which compared the need for transfusion, total blood loss, blood loss in drainage, and occurrence of DVT of the three main interventions (FS, topical TXA, and intravenous TXA) in people prepared for unilateral THA. Studies were included in the systematic review if they met the criteria: (1) primary unilateral THA; (2) RCTs; (3) intervention including FS, topical TXA, intravenous TXA, and control group; and (4) at least included one of the following outcomes: total blood loss, need for transfusion, and occurrence of deep venous thrombosis (DVT).
Trials were excluded if they (1) were meetings, letters, and protocols; (2) had repeated data or without insufficient data for meta-analysis; and (3) were retrospective design and prospective cohort studies.
Search methods and study selection
We searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Google Scholar, and Web of Science from inception to August 2018. Keywords and MeSH terms including “total hip arthroplasty”; “total hip replacement”; “THA”; “THR”; “Arthroplasty, Replacement, Hip”[Mesh]; “fibrin glue”; “fibrin sealant”; “fibrin tissue adhesive”; “Fibrin Tissue Adhesive[Mesh]”; and “tranexamic acid” were used in the search strategy. We also viewed a systematic review and meta-analysis for any omissive papers. Two independent authors selected the included studies based on the title and abstract. Any disagreement about whether included or not was resolved by a discussion or consulted to a senior reviewer.
Data collection and quality assessment
Two reviewers (Zhihu Zhao, Xinlong Ma) used a standardized form to extract data from the included studies. Information included study, sample size, comparators, study design, male patients, mean age, bone cement (cemented or uncemented), and dose of interventions. Meanwhile, we collected data about final outcomes: need for transfusion, total blood loss, and the occurrence of DVT. When relevant data was missing or needed to be identified, attempts were made to connect with the corresponding author by e-mail.
Cochrane risk of bias tool was used to assess the risk of bias. A total of seven domains were assessed and classified as low, unclear, and high risk of bias according to the suggestion of Cochrane risk of bias tool.
Data analysis
Data were recorded into Microsoft® Excel (Microsoft Corporation, Redmond, WA, USA) by two reviewers (Zhihu Zhao and Xinlong Ma). If there are differences between reviewers, re-review the literature to resolve. For continuous data (total blood loss), the mean difference (MD) with 95% confidence interval (CI) was used for direct comparisons. For network meta-analysis, MD with 95% credible intervals (CrI) was calculated by Stata software. Dichotomous data (need for transfusion and the occurrence of DVT) were used for odds ratio (OR) with 95% CI or 95% CrI to express indirect comparisons. Anna Chaimani model for network meta-analysis was used as previously described [11, 12]. Briefly, we calculate the inconsistency factor (IF) and its 95% confidence interval (IF) to evaluate the consistency of each closed loop. When the lower limit of the 95% confidence interval is equal to 0, it was considered to be consistent. Otherwise, there is a significant inconsistency in the closed loop.
Results
Study identification and selection
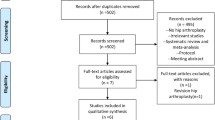
The literature search strategy process was shown in Fig. 1. Initially, we identified a total of 822 papers from electronic databases, and no additional records identified from other sources. After the duplicates were removed, a total of 406 papers were going to the next process. After scanning the titles and abstracts of these papers, 374 papers were excluded. In total, 32 studies were included in the meta-analysis [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44].
Study characteristics and risk of bias in included studies
As illustrated in Table 1, all the included studies provide detailed information about the general characteristics of the studies. A total of 31 clinical trials with 2476 patients were finally included in the meta-analysis. The sample size ranged from 20 to 102, and the age of the patients ranged from 45.4 to 73. Risk of bias summary and risk of bias graph can be seen in Figs. 2 and 3, respectively.
Effects of interventions on the need for transfusion
The network of four interventions on the need for transfusion was shown in Fig. 4. Table 2 provided hierarchies of effect size on the need for transfusion. Ranking graph of the distribution of probabilities on the need for transfusion was shown in Fig. 5. The direct and indirect comparisons indicated IV TXA, T TXA, and FS significantly decreased the need for transfusion compared with the control group. Based on SUCRA, control (0.97) ranked the first, the second was FS (0.66), the third was T TXA (0.23), and the last was IV TXA group (0.14).
Effects of interventions on the total blood loss
A total of 1287 THAs (IV TXA = 401, T TXA = 386, FS = 500, control = 562) were included for the analyses of total blood loss. The network of comparisons on total blood loss was shown in Fig. 6. Table 3 provided hierarchies of effect size on total blood loss. Figure 7 showed the ranking graph of the total blood loss between these treatments. The direct and indirect meta-analyses indicated IV TXA, T TXA, and FS significantly decreased total blood loss compared with the control group. Based on SUCRA value, Control (0.97) ranked the first, the second was FS (0.44), the third was T TXA (0.32), and the last was IV TXA group (0.27).
Effects of treatments on the DVT
A total of 476 patients were assigned to IV therapy and 297 to topical therapy, 556 patients were assigned to the FS group, and 713 patients were assigned to control therapy. The network of 4 comparisons (IV TXA, T TXA, FS, and control) on the occurrence of DVT was shown in Fig. 8. We also made a ranking graph of the distribution of probabilities on the occurrence of DVT in Fig. 9. Based on SUCRA, Co (0.67) ranked the first, the second was T TXA (0.56), the third was FS (0.52), and the last was IV TXA group (0.48).
Small-study effect and inconsistency test
Figure 10 shows that the funnel plot is symmetrical, indicating there is no publication bias in this network meta-analysis. Inconsistency test between direct and indirect comparisons revealed that the statistical inconsistency in the current meta-analysis was generally low because the CI values included zero.
Discussion
This is the first systematic review and network meta-analysis that provided hierarchies for the need for transfusion, total blood loss, and the occurrence of DVT comparing two main hemostasis agents (FS and TXA) after THA. All the included studies were RCTs, and the general characteristic was comparable that all patients were old patients prepared for unilateral THA. There were several strengths in this network meta-analysis: (1) comprehensive search strategy by two authors was used to increase the robustness of the search results; (2) traditional and network meta-analysis were both performed to exhibit the evidence for hemostasis in THA patients; (3) we used SUCRA to rank these interventions; and (4) only RCTs were included in this article.
The meta-analysis indicated that (1) FS, IV TXA, and topical TXA can reduce total blood loss and need for transfusion after THA; (2) for decreasing the need for transfusion, the ranking of treatments was IV TXA, topical TXA, FS, and control; (3) for reducing total blood loss, the ranking of treatments was topical TXA, IV TXA, FS, and control group; (4) direct comparison indicated that there is no significant difference between IV TXA and topical TXA; (5) direct comparison showed that FS, IV TXA, and topical TXA can decrease blood loss and the need for transfusion compared with the control group; and (6) there is no direct comparison between topical TXA or intravenous TXA and FS.
The results of current network meta-analysis indicated that IV or topical TXA is the most preferable hemostasis agent in THA. The efficacy of hemostasis was tested by the need for transfusion and total blood loss. Though the blood transfusion trigger is different between the included studies, the consistency test was performed and the included studies are consistent. There is a contradictory result for IV TXA versus topical administration TXA. As for the need for transfusion, IV TXA ranks the first, and for total blood loss, topical TXA ranks the first. Based on these results, a direct comparison was conducted between topical and intravenous TXA for THA. The results indicated that there is no significant difference between topical TXA and intravenous TXA in THA. These results were consistent with the previous meta-analysis. Until now, there is no evidence indicating that IV TXA is superior to topical TXA. Only one trial directly compared IV TXA with FS since there is no relevant data for blood loss and the need for transfusion for meta-analysis. Indirect data showed that whether IV TXA or topical TXA shows better hemostasis effects than FS. For TXA, there are no actual protocols that what dose is effective and safe. In the previous studies, intravenous 10 mg/kg, 15 mg/kg, or multiple doses are all been identified as effective and safe. The dose of topical TXA ranged from 1 to 3 g, and the administration routes included intra-articular and drain tube.
There is a previous meta-analysis comparing TXA with FS in total knee arthroplasty and found that there is no significant difference between the two agents. The meta-analysis including limited studies and non-RCTs will make the large heterogeneity for the final results. Another factor that affects the alternative choice for hemostasis agent is the price. FS is considerably costlier than TXA. The therapeutic dose of TXA (10 mg/kg) will cost about 8€, while FS will cost between 450€ and 675.00€. FS was manufactured from human plasma products, and in common with other blood-derived products, there is a risk of transmission of disease but concern may remain relating to unknown vectors.
There were several limitations for this meta-analysis: (1) the indirect comparison between FS and IV TXA was limited in total blood loss and the occurrence of DVT and thus may affect the precision of the final outcomes; (2) the follow-up in these studies was relatively short, and long-term follow-up was needed to identify the potential omitted complications; and (3) allocation concealment in some studies were limited and may cause heterogeneity between the studies.
In summary, our finding indicated that IV TXA was the most preferable hemostasis method for blood loss control in THA patients. And the use of IV TXA will not increase the occurrence of DVT. More direct evidence was needed to identify the optimal method for blood loss control in THA patients.
Abbreviations
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CI:
-
Confidence interval
- CrI:
-
Credible intervals
- DVT:
-
Deep venous thrombosis
- FS:
-
Fibrin sealant
- MDs:
-
Mean differences
- RCTs:
-
Randomized controlled trials
- THA:
-
Total hip arthroplasty
- TXA:
-
Tranexamic acid
References
Bierbaum BE, et al. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2–10.
Min JK, et al. The efficacy of bipolar sealer on blood loss in primary total hip arthroplasty: a meta-analysis. Medicine (Baltimore). 2016;95(19):e3435.
Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113(15):3406–17.
Hill GE, et al. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908–14.
Khan N, Troelsen A, Husted H. Prevention of post-operative anaemia in hip and knee arthroplasty--a systematic review. Dan Med J. 2015;62(12):A5170.
Maheshwari AV, et al. No additional benefit with use of a fibrin sealant to decrease peri-operative blood loss during primary total knee arthroplasty. J Arthroplast. 2014;29(11):2109–12.
Wang H, et al. Is fibrin sealant effective and safe in total knee arthroplasty? A meta-analysis of randomized trials. J Orthop Surg Res. 2014;9:36.
Park KJ, et al. Tranexamic acid reduces blood transfusions in revision total hip arthroplasty. J Arthroplast. 2016.
Sun X, Dong Q, Zhang YG. Intravenous versus topical tranexamic acid in primary total hip replacement: a systemic review and meta-analysis. Int J Surg. 2016;32:10–8.
Zhou XD, et al. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133(7):1017–27.
Jansen JP, et al. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11(5):956–64.
Chaimani A, et al. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654.
Benoni G, et al. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442–8.
Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397–401.
Clave A, et al. Efficacy of tranexamic acid on blood loss after primary cementless total hip replacement with rivaroxaban thromboprophylaxis: a case-control study in 70 patients. Orthop Traumatol Surg Res. 2012;98(5):484–90.
Ekback G, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124–30.
Garneti N, Field J. Bone bleeding during total hip arthroplasty after administration of tranexamic acid. J Arthroplast. 2004;19(4):488–92.
Husted H, et al. Tranexamic acid reduces blood loss and blood transfusions in primary total hip arthroplasty: a prospective randomized double-blind study in 40 patients. Acta Orthop Scand. 2003;74(6):665–9.
Ido K, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518–20.
Imai N, et al. Tranexamic acid for reduction of blood loss during total hip arthroplasty. J Arthroplast. 2012;27(10):1838–43.
Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314–9.
Kazemi SM, et al. The effect of tranexamic acid on reducing blood loss in cementless total hip arthroplasty under epidural anesthesia. Orthopedics. 2010;33(1):17.
Lee YC, et al. Effect of tranexamic acid on reducing postoperative blood loss in combined hypotensive epidural anesthesia and general anesthesia for total hip replacement. J Clin Anesth. 2013;25(5):393–8.
Lemay E, et al. Tranexamic acid reduces the need for allogenic red blood cell transfusions in patients undergoing total hip replacement. Can J Anaesth. 2004;51(1):31–7.
McConnell JS, et al. Reduction of blood loss in primary hip arthroplasty with tranexamic acid or fibrin spray. Acta Orthop. 2011;82(6):660–3.
Niskanen RO, Korkala OL. Tranexamic acid reduces blood loss in cemented hip arthroplasty: a randomized, double-blind study of 39 patients with osteoarthritis. Acta Orthop. 2005;76(6):829–32.
Rajesparan K, et al. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776–83.
Singh J, et al. Effects of tranexamic acid on blood loss during total hip arthroplasty. J Orthop Surg (Hong Kong). 2010;18(3):282–6.
Yamasaki S, Masuhara K, Fuji T. Tranexamic acid reduces blood loss after cementless total hip arthroplasty-prospective randomized study in 40 cases. Int Orthop. 2004;28(2):69–73.
Alshryda S, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H). J Bone Joint Surg Am. 2013;95(21):1969–74.
Benoni G, et al. Tranexamic acid, given at the end of the operation, does not reduce postoperative blood loss in hip arthroplasty. Acta Orthop Scand. 2000;71(3):250–4.
Martin JG, et al. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplast. 2014;29(5):889–94.
Wei W, Wei B. Comparison of topical and intravenous tranexamic acid on blood loss and transfusion rates in total hip arthroplasty. J Arthroplast. 2014;29(11):2113–6.
Yue C, et al. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplast. 2014;29(12):2452–6.
Zhang Y, et al. What is the optimal approach for tranexamic acid application in patients with unilateral total hip arthroplasty? Orthopade. 2016;45(7):616–21.
Falez F, et al. Blood loss reduction in cementless total hip replacement with fibrin spray or bipolar sealer: a randomised controlled trial on ninety five patients. Int Orthop. 2013;37(7):1213–7.
Lassen MR, et al. A pilot study of the effects of Vivostat patient-derived fibrin sealant in reducing blood loss in primary hip arthroplasty. Clin Appl Thromb Hemost. 2006;12(3):352–7.
Mawatari M, et al. Effectiveness of autologous fibrin tissue adhesive in reducing postoperative blood loss during total hip arthroplasty: a prospective randomised study of 100 cases. J Orthop Surg (Hong Kong). 2006;14(2):117–21.
North WT, et al. Topical vs intravenous tranexamic acid in primary total hip arthroplasty: a double-blind, randomized controlled trial. J Arthroplast. 2016;31(5):1022–6.
Randelli F, et al. Effectiveness of fibrin sealant after cementless total hip replacement: a double-blind randomized controlled trial. Int J Immunopathol Pharmacol. 2013;26(1):189–97.
Wang GJ, et al. Fibrin sealant reduces perioperative blood loss in total hip replacement. J Long-Term Eff Med Implants. 2003;13(5):399–411.
Xie J, et al. Combined use of intravenous and topical tranexamic acid following cementless total hip arthroplasty: a randomised clinical trial. Hip Int. 2016;26(1):36–42.
Yi Z, et al. Tranexamic acid administration in primary total hip arthroplasty: a randomized controlled trial of intravenous combined with topical versus single-dose intravenous administration. J Bone Joint Surg Am. 2016;98(12):983–91.
Malhotra R, Kumar V, Garg B. The use of tranexamic acid to reduce blood loss in primary cementless total hip arthroplasty. European Journal of Orthopaedic Surgery & Traumatology. 2011;21(21):101–4.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 81501061, No.81401792, and No. 81572154) and the Traditional Chinese Medicine Administration of Tianjin, China (No.13123).
Funding
This study was funded by the National Natural Science Foundation of China (No. 81572154, 81401792 and No. 81501061).
Availability of data and materials
We state that the data will not be shared because all the raw data are present in the figures included in the article.
Author information
Authors and Affiliations
Contributions
ZZ, XM, and JM designed and conceived the experiment. JM and ZZ performed the experiments. ZZ and XM analyzed the data. ZZ, XM, and JM wrote the manuscript. All of the authors listed have reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
None
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhao, Z., Ma, J. & Ma, X. Comparative efficacy and safety of different hemostatic methods in total hip arthroplasty: a network meta-analysis. J Orthop Surg Res 14, 3 (2019). https://doi.org/10.1186/s13018-018-1028-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-018-1028-2