Abstract
Background
This systematic review aims to summarize the clinical studies on the use of scaffolds in the repair of bony defects.
Methods
The relevant articles were searched through PubMed database. The following keywords and search terms were used: “scaffolds,” “patient,” “clinic,” “bone repair,” “bone regeneration,” “repairing bone defect,” “repair of bone,” “osteanagenesis,” “osteanaphysis,” and “osteoanagenesis.” The articles were screened according to inclusion and exclusion criteria, performed by two reviewers.
Results
A total of 373 articles were obtained using PubMed database. After screening, 20 articles were identified as relevant for the purpose of this systematic review. We collected the data of biological scaffolds and synthetic scaffolds. There are eight clinical studies of biological scaffolds included collagen, gelatin, and cellular scaffolds for bone healing. In addition, 12 clinical studies of synthetic scaffolds on HAp, TCP, bonelike, and their complex scaffolds for repairing bone defects were involved in this systematic review.
Conclusions
There are a lot of clinical evidences showed that application of scaffolds had a good ability to facilitate bone repair and osteogenesis. However, the ideal and reliable guidelines are insufficiently applied and the number and quality of studies in this field remain to be improved.
Similar content being viewed by others
Background
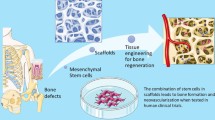
Healing of bone fractures and reconstruction of critical-sized bone defects represent a significant challenge. Autologous bone is the gold standard methods for the treatment of healing bone defects [1] due to stable structure, little immunogenicity [2], and natural osteogenic capacity [3,4,5]. However, the harvesting procedure has a high complication rate of 10–40%, including hemorrhage, nerve, and vascular lesions and postoperative pain [6]. Allograft bone, as bone graft substitute, shows good osteoconductive power and biomechanical characteristics and especially avoids the occurrence of complications [7]. However, the amount and quality of bone that can be harvested is limited, which restricts its use in large defects [8]. The disadvantages of bone autograft and allograft implantation have necessitated the development of alternative methods for bone repair [9].
A series of bone repair and transplantation substitutes have been derived with the development of material science and technology. In the past decades, cell- and gene-activating material, also known as bone-tissue engineering material, is the third generation bone-repair material. Tissue engineering material has been made into the extracellular matrix scaffold. The progenitor cell can proliferate and differentiate along scaffolds for better imitating the living situation of the surrounding tissue [10]. Tissue engineering scaffolds for bone regeneration have desirable characteristics of biocompatibility, non-toxicity, low cost, and non-carcinogenicity, with excellent osteoconductive and osteoinductive properties [11].
Biological scaffolds include corals, natural polymers, and demineralized bone matrix such as collagen sponge, gel foam, and cellular scaffold. Synthetic scaffolds include porous metals, synthetic polymers, and calcium phosphates (CaPs). Collagen contributes to mineral deposition, vascular ingrowth, and growth factor for bone regeneration [12]. CaPs ceramics is one of the most popular bone substitutes because its chemical composition resembles to bone mineral [13,14,15]. This feature enhances appropriate vascularization and stem cell proliferation and guides bone regeneration without causing any local or systemic toxicity [11]. Among the CaPs materials, hydroxyapatite (HAp) and β-tricalcium phosphate (β-TCP) are ideal substrates due to their excellent osteoconductive properties [16, 17].
Currently, one of the most advanced methods in tissue engineering is to transplant porous scaffolds with cell- and bone-stimulating agents into patients to form a complete bone transplanting. Tissue engineering scaffolds with osteoinductor were utilized for better bone regeneration by inducing bone cells to adhesion and proliferation. Mesenchymal stem cells (MSCs) can be well described and standardized, osteogenic differentiation from which is spontaneously into osteoblasts in vitro when compared to other mesenchyme tissues [18]. Bone morphogenetic protein (BMP), which combined with extracellular receptor, ultimately promote gene expression and induce mesenchymal stem cells to differentiate into osteoblasts [19, 20]. In addition, they enhance bone collagen synthesis and stimulate adjacent bone cells to grow [21, 22]. The periosteum is highly vascularized which can provide the cortical blood supply [23,24,25] and has been demonstrated to be an important factor in healing long bone fractures [26, 27].
To our knowledge, there have been several systematic reviews of scaffold materials, animal study, preclinical study, and carrier in MSCs for bone repair [11, 12, 28,29,30]. While little systematic review of bone-repair scaffolds were related to the clinical application. To our knowledge, this is the first report of a systematic review regarding on the clinical studies for scaffolds of bone defects. Therefore, the main aim of this study was to examine and summarize clinical studies on the use of scaffolds in the treatment of bony defects.
Methods
The relevant articles were searched through PubMed database. The following keywords and search terms were used: “scaffolds,” “patient,” “clinic,” “bone repair,” “bone regeneration,” “repairing bone defect,” “repair of bone,” “osteanagenesis,” “osteanaphysis,” and “osteoanagenesis.” The articles were screened according to inclusion and exclusion criteria, performed by two reviewers.
Search terms were selected according to guidelines on Table 1.
Inclusion criteria
-
1.
Studies on scaffolds used in bone repair and bone regeneration
-
2.
Clinical studies
Exclusion criteria
-
1.
Studies that used scaffolds in engineering of cartilage
-
2.
Studies in the field of maxillofacial or neurosurgical defects
-
3.
Studies that used scaffolds in the treatment of periodontal and alveolar defects
-
4.
Studies only in vitro
-
5.
Animals studies
-
6.
Articles in any language other than English
-
7.
Unpublished literature
Any dispute about whether an article fits the inclusion criteria, such as study type, scaffold function, treatment efficacy, and safety, was resolved by discussion.
Results
A total of 373 articles were reviewed, and 20 articles were identified as relevant for the purpose of this systematic literature review. The studies included have been summarized in Fig. 1. There are eight clinical trials on the use of biological scaffolds including collagen scaffolds, complex cellular scaffolds, and gel foam scaffolds in Table 2. Biological scaffolds usually have good osteogenesis, biocompatibility, and security. Four studies assessed the use of collagen bone scaffolds with osteoinductor [18,19,20, 31], which is performed by Calori et al. [31], and compared the efficacy of recombinant bone morphogenetic protein 7 (rhBMP-7) and platelet-rich plasma (PRP) (both in collagen scaffolds) in the treatment of persistent fracture non-unions in 120 cases. A lower median clinical and radiographic healing time were observed in the rhBMP-7 group than the PRP group. Jager et al. [18] treated ten patients with volumetric bone deficiencies in a study that used porous collagen I as a scaffold with MSCs and bone marrow aspirate in a 3-year follow-up. The remaining two studies [19, 20] evaluated the safety and efficacy of the use of an absorbable collagen sponge impregnated with recombinant bone morphogenetic protein (rhBMP-2). The study demonstrated that rhBMP-2 is a safe bone-stimulating agent, which can significantly reduce the frequency of bone-grafting procedures for the treatment of type-III open tibial fractures. Jager et al. [32] investigated the potency of bone marrow aspiration concentrate (BMAC) to augment bone grafting and support bone healing in 39 patients of volumetric bone deficiencies. The result showed that all patients appeared new bone formation in radiographs during follow-up. Two studies on clinic involved with cellular scaffolds [33, 34]. Cuthbert et al. [33] reported that the complex cellular scaffolds with induced membrane (IM) were used for treating critical size defects of eight patients. They concluded that the constitution of IM like periosteum and had a cellular composition and molecular profile, which facilitated large defect repair. Another study [34] evaluated new bone formation after the application of BMAC and recorded possible complications in 101 bone defect patients. The majority of patients were not observed to have infections, excessive new bone formation, and induction of tumor formation, morbidity, and complications within the 24-month follow-up period. Philip et al. [35] showed that majority of ribs treated with gel foam scaffolds re-grew to normal morphology within 3–6 months of costectomy compared to those without scaffold. Although biological scaffolds have good bone formation performance, the weak mechanical strength is the main reason for not as a solo scaffold.
Therefore, due to the above reason, synthetic scaffolds of tissue engineering materials are used comprehensively, which performed good property of new bone formation and mechanical strength. The uses of synthetic scaffolds examined in clinical studies are summarized in Table 3. HAp, β-TCP, and their complex materials with bone-stimulating agents were used in the most of synthetic scaffolds. Six studies investigated the use of HAp and its complex scaffolds in bone defects. Morishita et al. [36] reported strong osteogenic ability of HAp scaffolds with MSCs after tumor curettage and found no adverse reactions in all three patients. Cells were isolated from bone marrow and seeded onto the porous HAp scaffolds in two related studies [37, 38]. Both studies showed abundant cellar formation along the implants after several months. Furthermore, Marcacci et al. [37] found no signs of pain, swelling, or infection at the implantation site and no major complications in the early or late postoperative periods. Yamasaki et al. [39] compared the effectiveness of the transplantation of bone-marrow-derived mononuclear cells (BMMNCs) plus interconnected porous calcium hydroxyapatite (IP-CHA) on early bone repair for osteonecrosis of the femoral head with those of without BMMNCs and found that the implantation of BMMNCs and IP-CHA appears to confer benefit in the repair of osteonecrosis and in the prevention of collapse. Sotome et al. [40] assessed the efficacy and safety of HAp/collagen scaffold in comparison to β-TCP and showed the porous HAp/collagen group had the highest grade of bone regeneration but also associated with higher incidence of adverse effects. The use of rhBMP-2 in the biphasic CaPs granules with or without internal fixation in patients of spondylolisthesis did not exceed grade 1 in Boden et al.’s study. However, statistically greater and quicker improvement in patient-derived clinical outcome was measured in the rhBMP-2 groups [41]. Five studies examined the use of β-TCP as a fundamental material and composition to manage bone defects in clinical studies. One study [42] combined a β-TCP scaffold with MSCs and showed that the addition of MSCs resulted in more trabecular remodeling in femoral defects. Ollivier et al. [43] showed that the addition of rhBMP-7 to a TCP scaffold is safe and efficient in the treatment of recalcitrant bone union. Three studies [44,45,46] in clinical studies examined the use of BoneSave, a porous bone graft substitute made of β-TCP and HAp ceramic. Kapur et al. [44] showed that 56.7% of cases achieved successful fusion in 45 posterolateral inter-transverse spinal patients. Two of studies involved impaction grafting of BoneSave and allograft, which is an effective method of dealing with loss of the acetabulum in short- and medium-term studies [45, 46]. A novel study about bonelike scaffold was studied [47]. The result indicated that bonelike can be an excellent bioactive scaffold and therefore regeneration of the defects was achieved in a rapid, controlled manner.
Discussion
In this systematic review, 4 studies of femoral or acetabular defects, 3 studies of tibial fractures, 2 studies of large bone defects, 2 bone tumors studies, 2 studies of spinal defects, 2 volumetric bone deficiencies studies, 1 long bone defect study, 1ribs study, 1 study of knees, 1 post-traumatic bone defects study, 1 various bone study were included in the systematic review. The common defect position and the important bone types were involved in this systematic review. All the mentioned results of studies achieved a favorable efficacy of bone regeneration and an increased heal rate of bone defects, which demonstrated the scaffolds for bone repair played a critical role of bone heal.
As we know, the complications in scaffold of bone regeneration fields are an important challenge for the orthopedic surgery because infectious complications are major threat to the process of patient recovery. Complex methods and long-term process were required especially for effective antibiotic therapy which is a foundation of therapy. In our research, we added the information related to complications and adverse event of clinical studies in Tables 2 and 3. Among the 8 studies of biological scaffolds, five studies presented the data of postoperative complication and adverse effect. Therein, major complications such as fracture, hematoma, pain, inflammation, and infection were the main reasons affecting the progress of postoperative recover. Among the 12 studies of synthetic scaffolds, seven mentioned these postoperative results of complication and adverse event. Five of the seven studies on complications indicated that there were no major postoperative complications and no signs of infections. Another two researches of the seven reported only several cases had complications including dislocation, pulmonary embolism, and fracture. In the BoneSave substitute, the common complications of donor site morbidity were involved in these studies. In general, these results demonstrated that the complications discovered in synthetic scaffolds were less than those of the biological scaffolds. This may be due to relatively poor antibacterial property and bio-compatibility of biological scaffolds.
Current autografts and allografts are considered as the gold standard treatment for bone defects and mostly harvested from the iliac crest. However, the disadvantages of donor site morbidity, disease transmission, and susceptible to infection limit its application. Therefore, tissue-engineered grafts had been driven to the investigation and development of synthetic and biological bone-tissue engineering applications. The third bone grafting material, which is the mixture of scaffolds, cell- and gene-activating grafts, is the new biological bone repair material.
An ideal biomaterial should stimulate or induce the differentiation and proliferation of stem cells and osteoblast cells to heal defect sites [30, 48]. In eight clinical studies of biological scaffolds, collagen, gel, and cellular scaffolds for bone healing were included in the review. Collagen is a natural polymer for biomedical application with resorbable properties [48] and showed sufficient osteanagenesis [18,19,20, 31]. Gelatin has many advantages that included biocompatibility, biodegradability, cost effectiveness, common availability, and more accessible functional groups, making it a suitable material for bone tissue applications [49]. The utility of cellular scaffolds also facilitated bone defect repair [33, 34]. Most of biological materials tend to have weak mechanical strength, so it is rarely used as a single bone regeneration scaffold in tissue engineering and usually combined with other materials of good mechanical strength for repairing bone defects. The composite scaffold of HAp/collagen showed the highest grade of bone regeneration [40].
Twelve clinical studies on synthetic scaffolds were involved with HAp, TCP, and their complexes for repairing bone defects. HAp is the most important inorganic component of bone tissue with widely available bioactive and bioresorbable trait [7]. Four studies [36,37,38,39] pointed out that HAp had strong osteogenic ability for bone healing, and adverse reaction and major complications were not seen. The BoneSave, a matrix of HAp and β-TCP, are ideal biphasic porous ceramic bone graft substitutes due to their excellent osseointegration properties, but concerns have been raised as to their ability to maintain their structural integrity under load [44,45,46].
Furthermore, several tissue engineering materials such as collagen I, TCP, or HAp are currently available clinically as bone substitutes and can be used as scaffolds in combination with the bone-stimulating agents to expedite bone healing. MSCs can be spontaneous differentiation into osteoblasts. The discovery of BMPs appears to be the most selective for expedite gene expression and osteoblasts differentiation [50]. Among this, rhBMP-2 and rhBMP-7 are used in a variety of complex orthopedic conditions. In several clinical studies [18, 19, 31, 41, 43], BMPs had the greatest efficacy as bone-stimulating agents for bone defects treatment. The periosteum provides the cortical blood supply in healing critical size defects. The technology of induced membrane (IM) serves as a conduit to contain cells or bone graft for bone regeneration [33].
Most of the systematic reviews in bone repair are related to animal experiments or preclinical trials. There is almost no systematic review for the clinical application of bone-repair scaffolds. In Crowley et al.’s review [12], only five studies about scaffolds for bone regeneration are related to clinical trials. Therein, three of these studies related to small numbers and four of the studies had no control group and all of the studies involved short follow-up time of several months and even weeks. In summary, it lacks of representative and convincing to demonstrate the clinical studies of bone-repair scaffolds. However, all the 20 articles included in our review were related to the clinical study of scaffolds for bone repair. Only four studies used small samples less than 10 numbers. Over half of the number had one or even more than one control group. The follow-up time also increased from a few months to more than 1 year in most of studies. All of the results reported positive results for clinical bone regeneration.
Conclusions
Tissue engineering materials are currently available clinically as bone substitutes and can be used as scaffolds in combination with the bone-stimulating agents to expedite bone healing, which has made great progress comparing to a decade ago. Application of scaffolds in clinical field showed a good ability to facilitate bone repair and osteogenesis. However, significant challenges still exist in clinical studies due to limitations and translational difficulties which prevent their implementation into clinical practice [51]. Currently, application of scaffolds on clinical field showed a good ability to facilitate bone repair and osteogenesis in our systematic review. This systematic review provided an ideal and reliable result for the further progression and development of clinical study, which will promote other researchers and readers in this tissue engineering fields to comprehensively understand the clinical results of scaffolds for bone regeneration and applied these achievements for the further clinical practice. In addition, the ideal and reliable guidelines need to be sufficiently applied and the number and quality of studies in this field remain need to be improved.
Abbreviations
- BMAC:
-
Bone marrow aspiration concentrate
- BMMNCs:
-
Bone-marrow-derived mononuclear cells
- CaPs:
-
Calcium phosphate
- CT:
-
Computed tomography
- IM:
-
Induced membrane
- IP-CHA:
-
Interconnected porous calcium hydroxyapatite
- MSC:
-
Mesenchymal stromal cell
- OHS:
-
Oxford hip score
- P:
-
Periosteum
- PRP:
-
Platelet-rich plasma
- rCPBS:
-
Resorbable calcium phosphate bone substitute
- rhBMP-2:
-
Recombinant human bone morphogenetic protein-2
- rhBMP-7:
-
Recombinant bone morphogenetic protein 7
- SAPS:
-
Self-reported satisfaction scale
- SF12:
-
Short-form
- TSRH:
-
Texas Scottish Rite Hospital
References
Pieske O, Wittmann A, Zaspel J, Loffler T, Rubenbauer B, Trentzsch H, et al. Autologous bone graft versus demineralized bone matrix in internal fixation of ununited long bones. J Trauma Manage Outcomes. 2009;3:11.
Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:18.
Nandi SK, Roy S, Mukherjee P, Kundu B, De DK, Basu D. Orthopaedic applications of bone graft & graft substitutes: a review. Indian J Med Res. 2010;132:15–30.
Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res. 1987:7–16.
Behnia H, Khojasteh A, Soleimani M, Tehranchi A, Atashi A. Repair of alveolar cleft defect with mesenchymal stem cells and platelet derived growth factors: a preliminary report. J Craniomaxillofac Surg. 2012;40:2–7.
Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–5.
Gupta MC, Maitra S. Bone grafts and bone morphogenetic proteins in spine fusion. Cell Tissue Bank. 2002;3:255–67.
Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187–208.
Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part a: current challenges in BMP delivery. Biotechnol Lett. 2009;31:1817–24.
Hench LL, Thompson I. Twenty-first century challenges for biomaterials. J R Soc Interface. 2010;7(Suppl 4):S379–91.
Oliveira HL, WLO DR, Cuevas-Suarez CE, NLV C, da Silva AF, Guim TN, et al. Histological evaluation of bone repair with hydroxyapatite: a systematic review. Calcif Tissue Int. 2017;
Crowley C, Wong JM, Fisher DM, Khan WS. A systematic review on preclinical and clinical studies on the use of scaffolds for bone repair in skeletal defects. Current Stem Cell Res Ther. 2013;8:243–52.
Prins HJ, Schulten EA, Ten Bruggenkate CM, Klein-Nulend J, Helder MN. Bone regeneration using the freshly isolated autologous stromal vascular fraction of adipose tissue in combination with calcium phosphate ceramics. Stem Cells Transl Med. 2016;5:1362–74.
Wernike E, Montjovent MO, Liu Y, Wismeijer D, Hunziker EB, Siebenrock KA, et al. Vegf incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. European Cells Mater. 2010;19:30–40.
Wilson CE, van Blitterswijk CA, Verbout AJ, Dhert WJ, de Bruijn JD. Scaffolds with a standardized macro-architecture fabricated from several calcium phosphate ceramics using an indirect rapid prototyping technique. J Mater Sci Mater Med. 2011;22:97–105.
Kasten P, Vogel J, Luginbuhl R, Niemeyer P, Tonak M, Lorenz H, et al. Ectopic bone formation associated with mesenchymal stem cells in a resorbable calcium deficient hydroxyapatite carrier. Biomaterials. 2005;26:5879–89.
Vanecek V, Klima K, Kohout A, Foltan R, Jirousek O, Sedy J, et al. The combination of mesenchymal stem cells and a bone scaffold in the treatment of vertebral body defects. Eur Spine J. 2013;22:2777–86.
Jager M, Jelinek EM, Wess KM, Scharfstadt A, Jacobson M, Kevy SV, et al. Bone marrow concentrate: a novel strategy for bone defect treatment. Current Stem Cell Res Ther. 2009;4:34–43.
Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-a:2123–34.
Swiontkowski MF, Aro HT, Donell S, Esterhai JL, Goulet J, Jones A, et al. Recombinant human bone morphogenetic protein-2 in open tibial fractures: a subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am. 2006;88:1258–65.
Heppenstall RB, Brighton CT, Esterhai JL Jr, Muller G. Prognostic factors in nonunion of the tibia: an evaluation of 185 cases treated with constant direct current. J Trauma. 1984;24:790–5.
Finkemeier CG, Schmidt AH, Kyle RF, Templeman DC, Varecka TF. A prospective, randomized study of intramedullary nails inserted with and without reaming for the treatment of open and closed fractures of the tibial shaft. J Orthop Trauma. 2000;14:187–93.
Fan W, Crawford R, Xiao Y. Structural and cellular differences between metaphyseal and diaphyseal periosteum in different aged rats. Bone. 2008;42:81–9.
Squier CA, Ghoneim S, Kremenak CR. Ultrastructure of the periosteum from membrane bone. J Anat. 1990;171:233–9.
De Bari C, Dell’Accio F, Vanlauwe J, Eyckmans J, Khan IM, Archer CW, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–21.
Knothe UR, Springfield DS. A novel surgical procedure for bridging of massive bone defects. World J Surg Oncol. 2005;3:7.
Yu YY, Lieu S, Lu C, Colnot C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone. 2010;47:65–73.
Dahl M, Jorgensen NR, Horberg M, Pinholt EM. Carriers in mesenchymal stem cell osteoblast mineralization—state-of-the-art. J Craniomaxillofac Surg. 2014;42:41–7.
Delgado-Ruiz RA, Calvo Guirado JL, Romanos GE. Bone grafting materials in critical defects in rabbit calvariae. A systematic review and quality evaluation using arrive guidelines. Clin Oral Implants Res. 2015;
Kuttappan S, Mathew D, Nair MB. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering—a mini review. Int J Biol Macromol. 2016;93:1390–401.
Calori GM, Tagliabue L, Gala L, d'Imporzano M, Peretti G, Albisetti W. Application of rhbmp-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury. 2008;39:1391–402.
Jager M, Herten M, Fochtmann U, Fischer J, Hernigou P, Zilkens C, et al. Bridging the gap: bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J Orthop Res 2011;29:173-180.
Cuthbert RJ, Churchman SM, Tan HB, McGonagle D, Jones E, Giannoudis PV. Induced periosteum a complex cellular scaffold for the treatment of large bone defects. Bone. 2013;57:484–92.
Hendrich C, Franz E, Waertel G, Krebs R, Jager M. Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop Rev. 2009;1:e32.
Philip SJ, Kumar RJ, Menon KV. Morphological study of rib regeneration following costectomy in adolescent idiopathic scoliosis. Eur Spine J. 2005;14:772–6.
Morishita T, Honoki K, Ohgushi H, Kotobuki N, Matsushima A, Takakura Y. Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients’ mesenchymal stem cells. Artif Organs. 2006;30:115–8.
Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–55.
Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–6.
Yamasaki T, Yasunaga Y, Ishikawa M, Hamaki T, Ochi M. Bone-marrow-derived mononuclear cells with a porous hydroxyapatite scaffold for the treatment of osteonecrosis of the femoral head: a preliminary study. J Bone Joint Surg British. 2010;92:337–41.
Sotome S, Ae K, Okawa A, Ishizuki M, Morioka H, Matsumoto S, et al. Efficacy and safety of porous hydroxyapatite/type 1 collagen composite implantation for bone regeneration: a randomized controlled study. J Orthop Sci. 2016;21:373–80.
Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 volvo award in clinical studies. Spine. 2002;27:2662–73.
Sponer P, Filip S, Kucera T, Brtkova J, Urban K, Palicka V, et al. Utilizing autologous multipotent mesenchymal stromal cells and beta-tricalcium phosphate scaffold in human bone defects: a prospective, controlled feasibility trial. Biomed Res Int. 2016;2016:2076061.
Ollivier M, Gay AM, Cerlier A, Lunebourg A, Argenson JN, Parratte S. Can we achieve bone healing using the diamond concept without bone grafting for recalcitrant tibial nonunions? Injury. 2015;46:1383–8.
Kapur RA, Amirfeyz R, Wylde V, Blom AW, Nelson IW, Hutchinson J. Clinical outcomes and fusion success associated with the use of bonesave in spinal surgery. Arch Orthop Trauma Surg. 2010;130:641–7.
Whitehouse MR, Dacombe PJ, Webb JC, Blom AW. Impaction grafting of the acetabulum with ceramic bone graft substitute mixed with femoral head allograft: high survivorship in 43 patients with a median follow-up of 7 years: a follow-up report. Acta Orthop. 2013;84:365–70.
Blom AW, Wylde V, Livesey C, Whitehouse MR, Eastaugh-Waring S, Bannister GC, et al. Impaction bone grafting of the acetabulum at hip revision using a mix of bone chips and a biphasic porous ceramic bone graft substitute. Acta Orthop. 2009;80:150–4.
Gutierres M, Hussain NS, Lopes MA, Afonso A, Cabral AT, Almeida L, et al. Histological and scanning electron microscopy analyses of bone/implant interface using the novel bonelike synthetic bone graft. J Orthop Res. 2006;24:953–8.
Jacinto-Tinajero JC, Ascencio D, Marquina B, Barrios-Payan J, Gutierrez MC, Lim MG, et al. Induction of bone formation in abdominal implants constituted by collagen sponges embedded with plant-based human transforming growth factor family proteins in ectopic dog model. J Exp Orthop. 2014;1:11.
Su K, Wang C. Recent advances in the use of gelatin in biomedical research. Biotechnol Lett. 2015;37:2139–45.
Lutwak L, Singer FR, Urist MR. Ucla conference: current concepts of bone metabolism. Ann Intern Med. 1974;80:630–44.
Shahrezaie M, Moshiri A. Effectiveness of tissue engineered three-dimensional bioactive graft on bone healing and regeneration: an in vivo study with significant clinical value. 2017.
Acknowledgements
None
Funding
This work was supported by the National Natural Science Foundation of China under Grant [number8156035, 81560377].
Availability of data and materials
As this paper is a systematic review, there are no patient data sets. Please contact the author for data requests if needed.
Author information
Authors and Affiliations
Contributions
LX, SL, PQ, and LD conceived and designed the manuscript. LX, SL, PQ, and SX analyzed and interpreted the data. LX, XL, ZT, and JZ provided materials and cases. SL and JL searched the literatures and collected the data. LX provided the financial foundation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zeng, JH., Liu, SW., Xiong, L. et al. Scaffolds for the repair of bone defects in clinical studies: a systematic review. J Orthop Surg Res 13, 33 (2018). https://doi.org/10.1186/s13018-018-0724-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-018-0724-2